Chapter 13 Solutions SUDHIR KUMAR MAINGI PGT CHEMISTRY











































- Slides: 92

Chapter 13 Solutions SUDHIR KUMAR MAINGI PGT (CHEMISTRY) K. V. 1 AFS PATHANKOT 1

Solutions • Homogeneous mixtures. üComposition may vary from one sample to another. üAppears to be one substance, though really contains multiple materials. • Most homogeneous materials we encounter are actually solutions. üE. g. , air and lake water. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 2

Solutions, Continued • Solute is the dissolved substance. üSeems to “disappear. ” ü“Takes on the state” of the solvent. • Solvent is the substance solute dissolves in. üDoes not appear to change state. • When both solute and solvent have the same state, the solvent is the component present in the highest percentage. • Solutions in which the solvent is water are called aqueous solutions. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 3

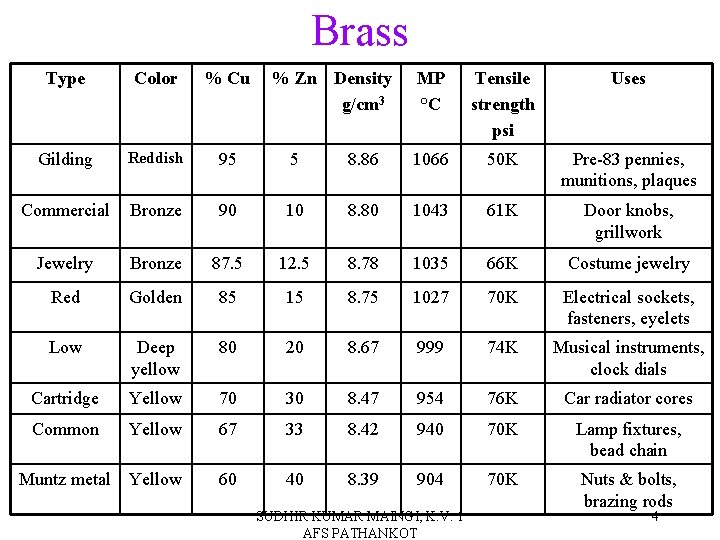
Brass Type Color % Cu % Zn Density g/cm 3 MP °C Tensile strength psi Uses Gilding Reddish 95 5 8. 86 1066 50 K Pre-83 pennies, munitions, plaques Commercial Bronze 90 10 8. 80 1043 61 K Door knobs, grillwork Jewelry Bronze 87. 5 12. 5 8. 78 1035 66 K Costume jewelry Red Golden 85 15 8. 75 1027 70 K Electrical sockets, fasteners, eyelets Low Deep yellow 80 20 8. 67 999 74 K Musical instruments, clock dials Cartridge Yellow 70 30 8. 47 954 76 K Car radiator cores Common Yellow 67 33 8. 42 940 70 K Lamp fixtures, bead chain Muntz metal Yellow 60 40 8. 39 904 70 K Nuts & bolts, brazing rods SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 4

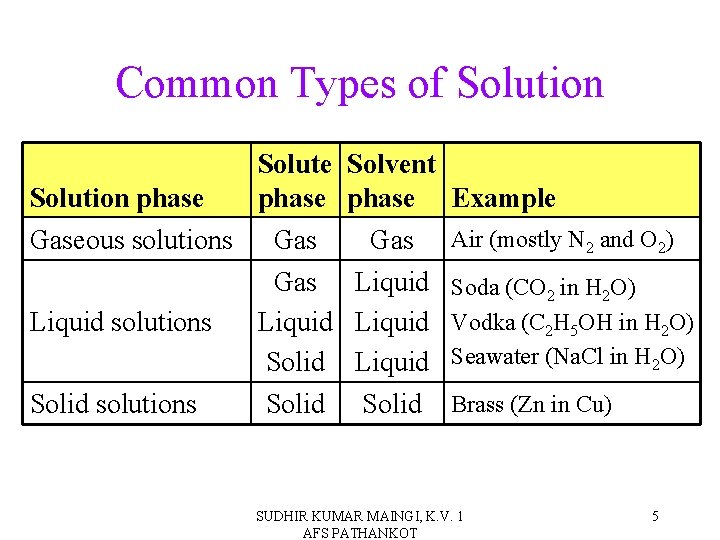
Common Types of Solution Solute Solution phase Gaseous solutions Gas Liquid solutions Liquid Solid solutions Solid Solvent phase Gas Liquid Solid Example Air (mostly N 2 and O 2) Soda (CO 2 in H 2 O) Vodka (C 2 H 5 OH in H 2 O) Seawater (Na. Cl in H 2 O) Brass (Zn in Cu) SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 5

Solubility • When one substance (solute) dissolves in another (solvent) it is said to be soluble. üSalt is soluble in water. üBromine is soluble in methylene chloride. • When one substance does not dissolve in another it is said to be insoluble. üOil is insoluble in water. • The solubility of one substance in another depends on two factors: nature’s tendency towards mixing and the types of intermolecular attractive forces. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 6

Will It Dissolve? • Chemist’s rule of thumb: Like dissolves like • A chemical will dissolve in a solvent if it has a similar structure to the solvent. • When the solvent and solute structures are similar, the solvent molecules will attract the solute particles at least as well as the solute particles to each other. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 7

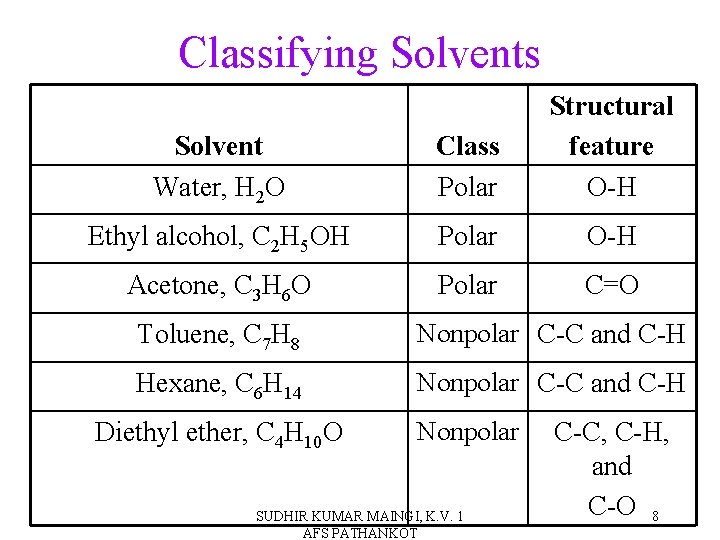
Classifying Solvents Solvent Water, H 2 O Class Polar Structural feature O-H Ethyl alcohol, C 2 H 5 OH Polar O-H Acetone, C 3 H 6 O Polar C=O Toluene, C 7 H 8 Nonpolar C-C and C-H Hexane, C 6 H 14 Nonpolar C-C and C-H Diethyl ether, C 4 H 10 O Nonpolar SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT C-C, C-H, and C-O 8

Will It Dissolve in Water? • Ions are attracted to polar solvents. ü Many ionic compounds dissolve in water. Ø Generally, if the ions total charges < 4. • Polar molecules are attracted to polar solvents. ü Table sugar, ethyl alcohol, and glucose all dissolve well in water. Ø Have either multiple OH groups or little CH. • Nonpolar molecules are attracted to nonpolar solvents. ü b-carotene (C 40 H 56) is not water soluble; it dissolves in fatty (nonpolar) tissues. • Many molecules have both polar and nonpolar structures —whether they will dissolve in water depends on the kind, number, and location of polar and nonpolar structural features in the molecule. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 9

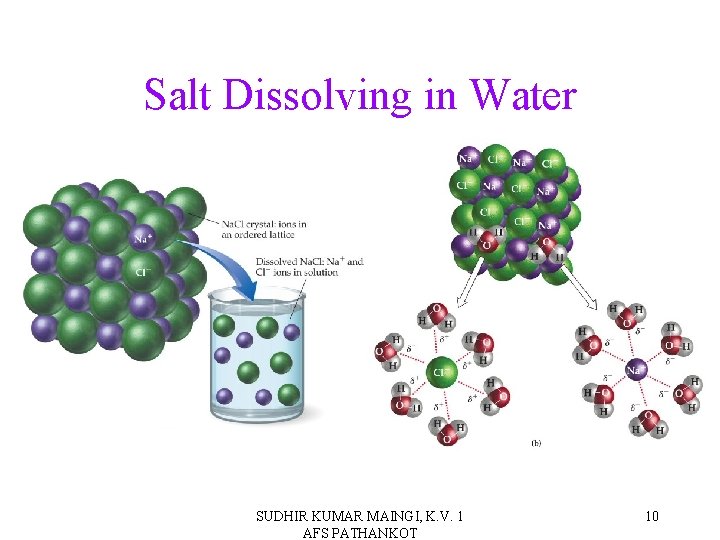
Salt Dissolving in Water SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 10

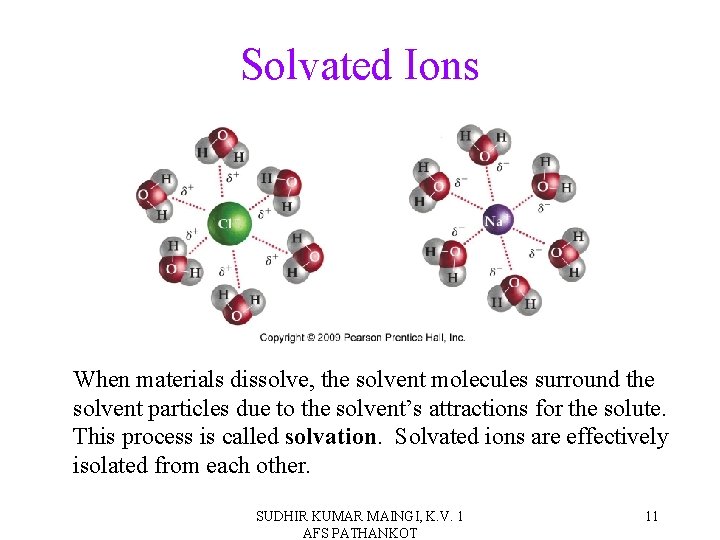
Solvated Ions When materials dissolve, the solvent molecules surround the solvent particles due to the solvent’s attractions for the solute. This process is called solvation. Solvated ions are effectively isolated from each other. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 11

Practice—Decide if Each of the Following Will Be Significantly Soluble in Water. • • • potassium iodide, KI soluble. octane, C 8 H 18 insoluble. methanol, CH 3 OH soluble. copper, Cu insoluble. cetyl alcohol, CH 3(CH 2)14 CH 2 OH insoluble. iron(III) sulfide, Fe 2 S 3 insoluble. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 12

Solubility • There is usually a limit to the solubility of one substance in another. üGases are always soluble in each other. üTwo liquids that are mutually soluble are said to be miscible. ØAlcohol and water are miscible. ØOil and water are immiscible. • The maximum amount of solute that can be dissolved in a given amount of solvent is called solubility. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 13

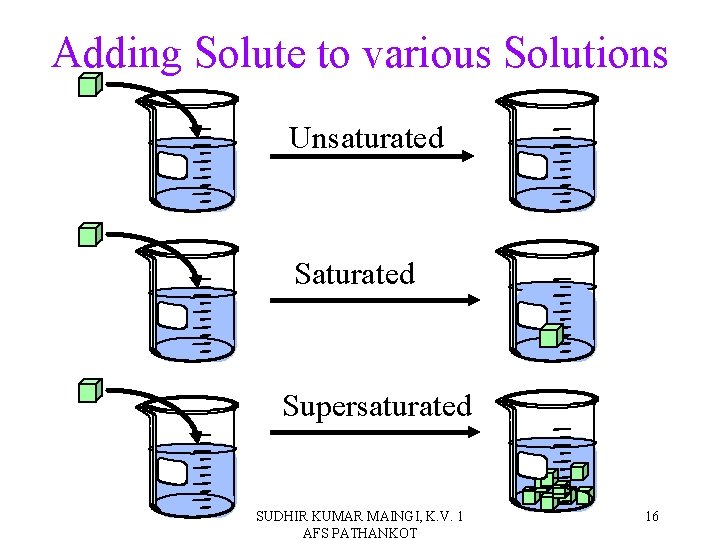
Descriptions of Solubility • Saturated solutions have the maximum amount of solute that will dissolve in that solvent at that temperature. • Unsaturated solutions can dissolve more solute. • Supersaturated solutions are holding more solute than they should be able to at that temperature. üUnstable. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 14

Supersaturated Solution A supersaturated solution has more dissolved solute than the solvent can hold. When disturbed, all the solute above the saturation level comes out of solution. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 15

Adding Solute to various Solutions Unsaturated Supersaturated SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 16

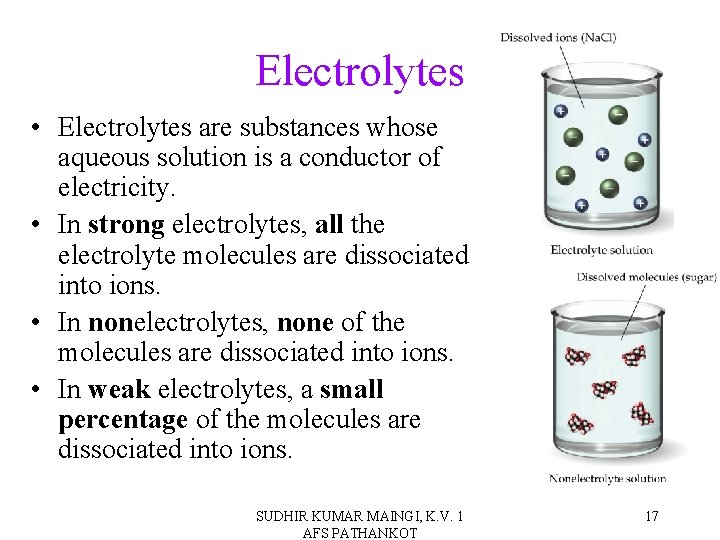
Electrolytes • Electrolytes are substances whose aqueous solution is a conductor of electricity. • In strong electrolytes, all the electrolyte molecules are dissociated into ions. • In nonelectrolytes, none of the molecules are dissociated into ions. • In weak electrolytes, a small percentage of the molecules are dissociated into ions. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 17

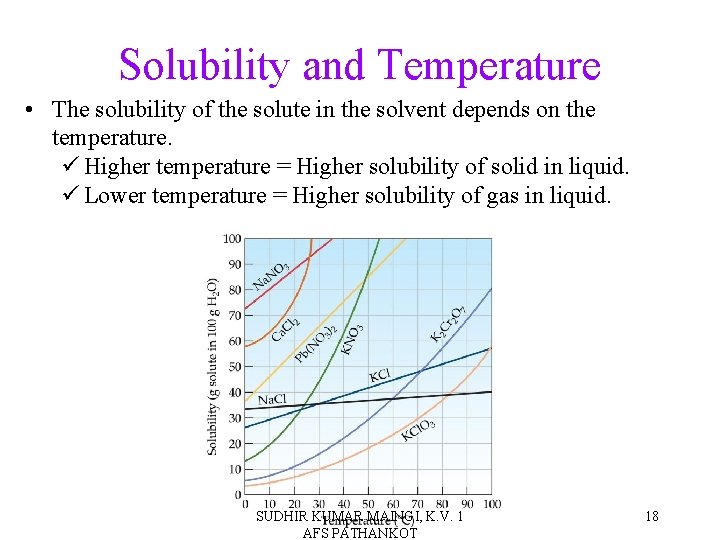
Solubility and Temperature • The solubility of the solute in the solvent depends on the temperature. ü Higher temperature = Higher solubility of solid in liquid. ü Lower temperature = Higher solubility of gas in liquid. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 18

Changing Temperature = Changing Solubility • When a solution is saturated, it is holding the maximum amount of solute it can at that temperature. • If the temperature is changed, the solubility of the solute changes. üIf a solution contains 71. 3 g of NH 4 Cl in 100 g of water at 90 C, it will be saturated. üIf the temperature drops to 20 C, the saturation level of NH 4 Cl drops to 37. 2 g. üTherefore, 24. 1 g of NH 4 Cl will precipitate. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 20

Purifying Solids: Recrystallization • When a solid precipitates from a solution, crystals of the pure solid form by arranging the particles in a crystal lattice. • Formation of the crystal lattice tends to reject impurities. • To purify a solid, chemists often make a saturated solution of it at high temperature; when it cools, the precipitated solid will have much less impurity than before. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 21

Solubility of Gases: Effect of Temperature • Many gases dissolve in water. üHowever, most have very limited solubility. • The solubility of a gas in a liquid decreases as the temperature increases. üBubbles seen when tap water is heated (before the water boils) are gases that are dissolved, coming out of the solution. üOpposite of solids. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 22

Solubility of Gases: Effect of Pressure • The solubility of a gas is directly proportional to its partial pressure. üHenry’s law. üThe solubility of solid is not effected by pressure. • The solubility of a gas in a liquid increases as the pressure increases. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 23

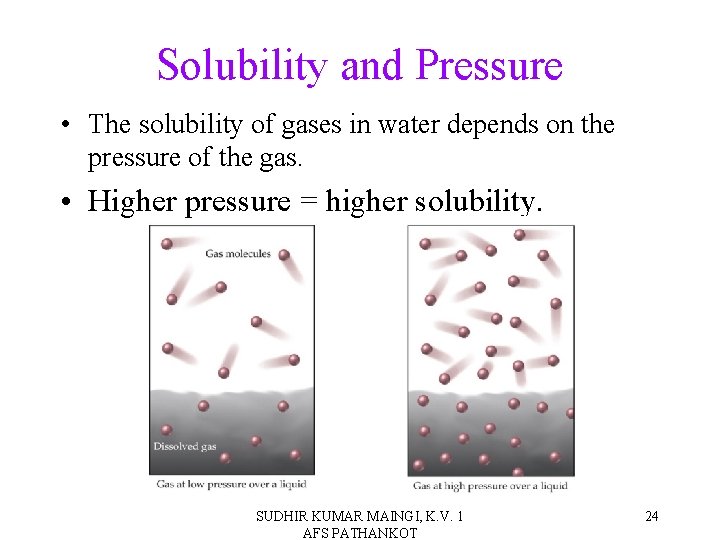
Solubility and Pressure • The solubility of gases in water depends on the pressure of the gas. • Higher pressure = higher solubility. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 24

Solubility and Pressure, Continued When soda pop is sealed, the CO 2 is under pressure. Opening the container lowers the pressure, which decreases the solubility of CO 2 and causes bubbles to form. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 25

Solution Concentrations SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 26

Describing Solutions • Solutions have variable composition. • To describe a solution, you need to describe both the components and their relative amounts. • Dilute solutions have low amounts of solute per amount of solution. • Concentrated solutions have high amounts of solute per amount of solution. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 27

Concentrations—Quantitative Descriptions of Solutions • A more precise method for describing a solution is to quantify the amount of solute in a given amount of solution. • Concentration = Amount of solute in a given amount of solution. üOccasionally amount of solvent. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 28

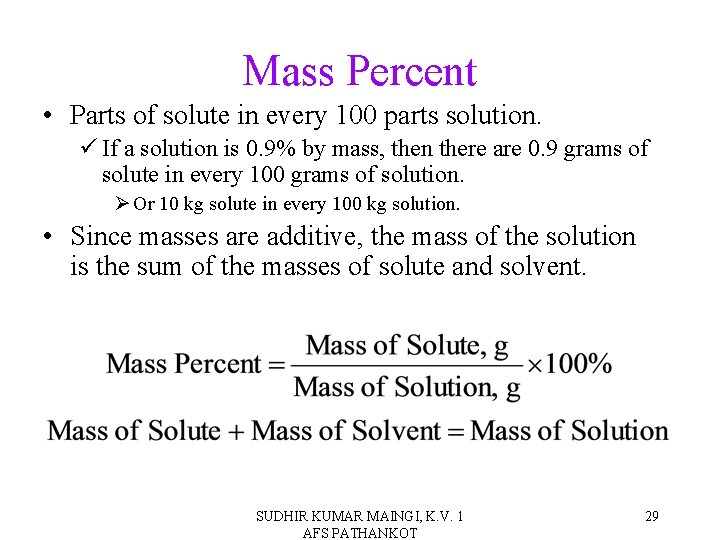
Mass Percent • Parts of solute in every 100 parts solution. ü If a solution is 0. 9% by mass, then there are 0. 9 grams of solute in every 100 grams of solution. Ø Or 10 kg solute in every 100 kg solution. • Since masses are additive, the mass of the solution is the sum of the masses of solute and solvent. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 29

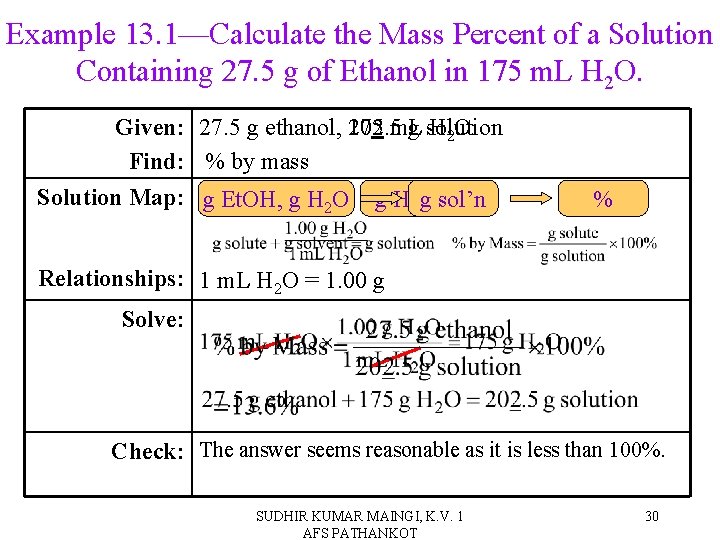
Example 13. 1—Calculate the Mass Percent of a Solution Containing 27. 5 g of Ethanol in 175 m. L H 2 O. Given: 27. 5 g ethanol, 202. 5 175 m. L g solution H 2 O Find: % by mass Solution Map: m. L g Et. OH, g sol’n H 2 O g H 2 O % Relationships: 1 m. L H 2 O = 1. 00 g Solve: Check: The answer seems reasonable as it is less than 100%. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 30

Practice—Calculate the Mass Percent of a Solution that Has 10. 0 g of I 2 Dissolved in 150. 0 g of Ethanol. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 40

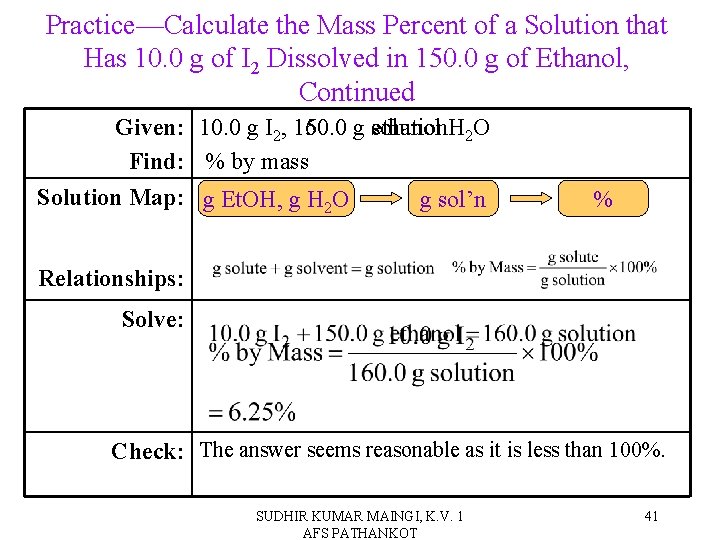
Practice—Calculate the Mass Percent of a Solution that Has 10. 0 g of I 2 Dissolved in 150. 0 g of Ethanol, Continued Given: 10. 0 g I 2, 160. 0 150. 0 g solution ethanol H 2 O Find: % by mass Solution Map: g Et. OH, g H 2 O g sol’n % Relationships: Solve: Check: The answer seems reasonable as it is less than 100%. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 41

Using Concentrations as Conversion Factors • Concentrations show the relationship between the amount of solute and the amount of solvent. ü 12% by mass sugar (aq) means 12 g sugar 100 g solution. • The concentration can then be used to convert the amount of solute into the amount of solution or visa versa. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 42

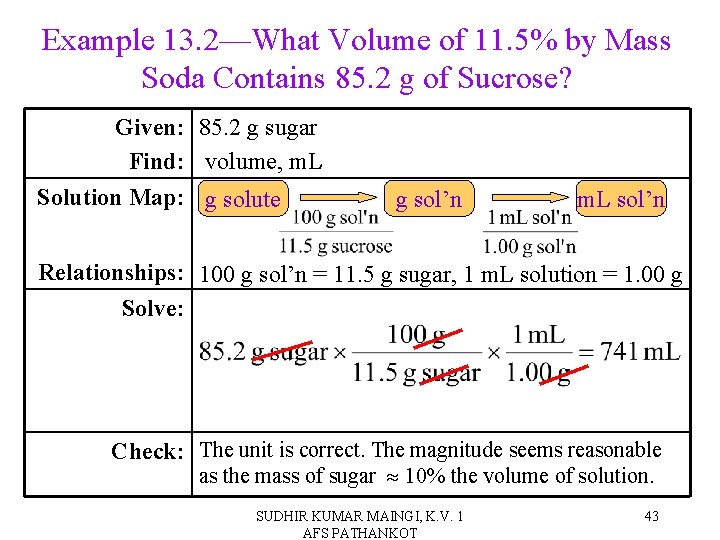
Example 13. 2—What Volume of 11. 5% by Mass Soda Contains 85. 2 g of Sucrose? Given: 85. 2 g sugar Find: volume, m. L Solution Map: g solute g sol’n m. L sol’n Relationships: 100 g sol’n = 11. 5 g sugar, 1 m. L solution = 1. 00 g Solve: Check: The unit is correct. The magnitude seems reasonable as the mass of sugar 10% the volume of solution. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 43

Practice—Milk Is 4. 5% by Mass Lactose. Determine the Mass of Lactose in 175 g of Milk. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 51

Practice—Milk Is 4. 5% by Mass Lactose. Determine the Mass of Lactose in 175 g of Milk, Continued. Given: 175 g milk 175 g solution Find: g lactose Equivalence: 4. 5 g lactose 100 g solution Solution Map: g solution g Lactose Apply Solution Map: Check Answer: Units are correct. Number makes sense because lactose is a component of the mixture, therefore, its amount should be less. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 52

Preparing a Solution • Need to know amount of solution and concentration of solution. • Calculate the mass of solute needed. üStart with amount of solution. üUse concentration as a conversion factor. Ø 5% by mass Þ 5 g solute 100 g solution. ü“Dissolve the grams of solute in enough solvent to total the total amount of solution. ” SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 53

Example—How Would You Prepare 250. 0 g of 5. 00% by Mass Glucose Solution (Normal Glucose)? Given: Find: Equivalence: Solution Map: 250. 0 g solution g glucose 5. 00 g glucose 100 g solution g glucose Apply Solution Map: Answer: Dissolve 12. 5 g of glucose in enough water to total 250. 0 g. SUDHIR KUMAR MAINGI, K. V. 1 54 AFS PATHANKOT

Practice—How Would You Prepare 450. 0 g of 15. 0% by Mass Aqueous Ethanol? SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 55

Practice—How Would You Prepare 450. 0 g of 15. 0% by Mass Aqueous Ethanol? , Continued Given: Find: Equivalence: Solution map: 450. 0 g solution g ethanol (Et. OH) 15. 0 g Et. OH 100 g solution g Et. OH Apply solution map: Answer: Dissolve 67. 5 g of ethanol in enough water to total 450. 0 g. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 56

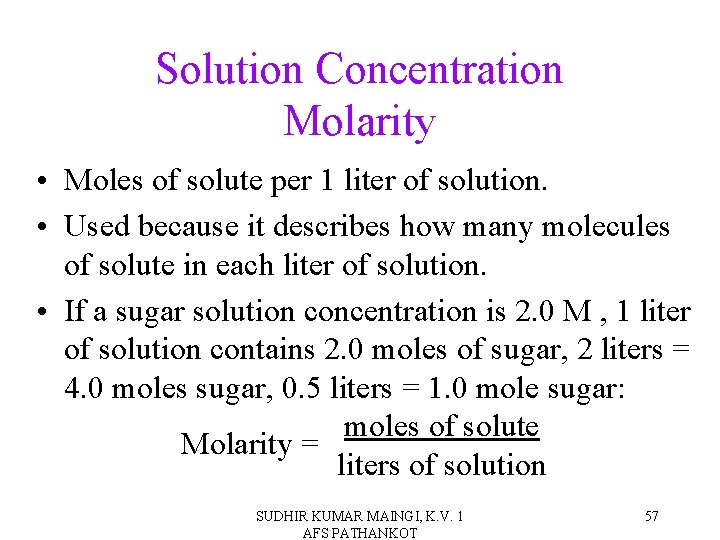
Solution Concentration Molarity • Moles of solute per 1 liter of solution. • Used because it describes how many molecules of solute in each liter of solution. • If a sugar solution concentration is 2. 0 M , 1 liter of solution contains 2. 0 moles of sugar, 2 liters = 4. 0 moles sugar, 0. 5 liters = 1. 0 mole sugar: moles of solute Molarity = liters of solution SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 57

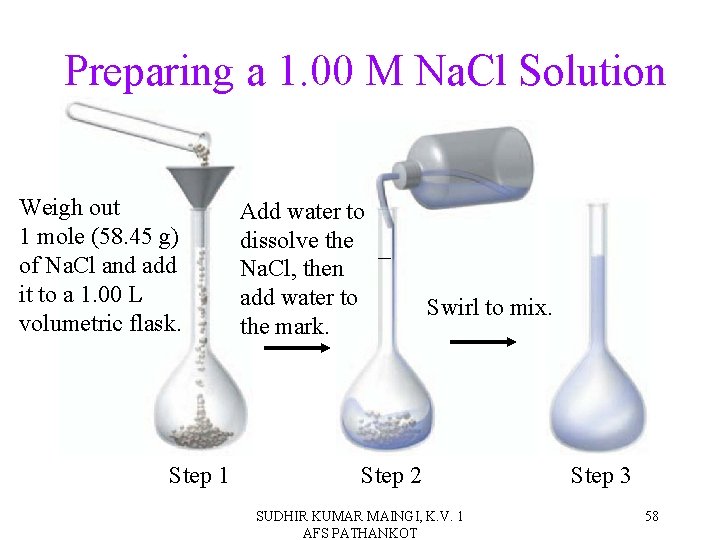
Preparing a 1. 00 M Na. Cl Solution Weigh out 1 mole (58. 45 g) of Na. Cl and add it to a 1. 00 L volumetric flask. Step 1 Add water to dissolve the Na. Cl, then add water to the mark. Swirl to mix. Step 2 SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT Step 3 58

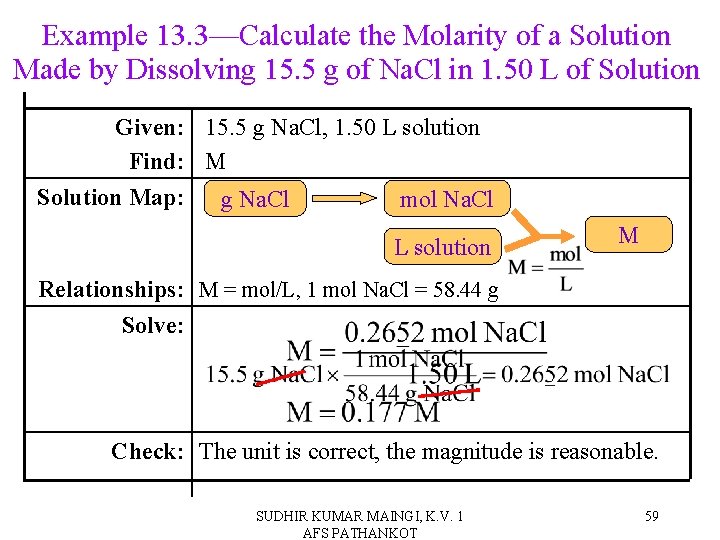
Example 13. 3—Calculate the Molarity of a Solution Made by Dissolving 15. 5 g of Na. Cl in 1. 50 L of Solution Given: 15. 5 g Na. Cl, 1. 50 L solution Find: M Solution Map: g Na. Cl mol Na. Cl L solution M Relationships: M = mol/L, 1 mol Na. Cl = 58. 44 g Solve: Check: The unit is correct, the magnitude is reasonable. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 59

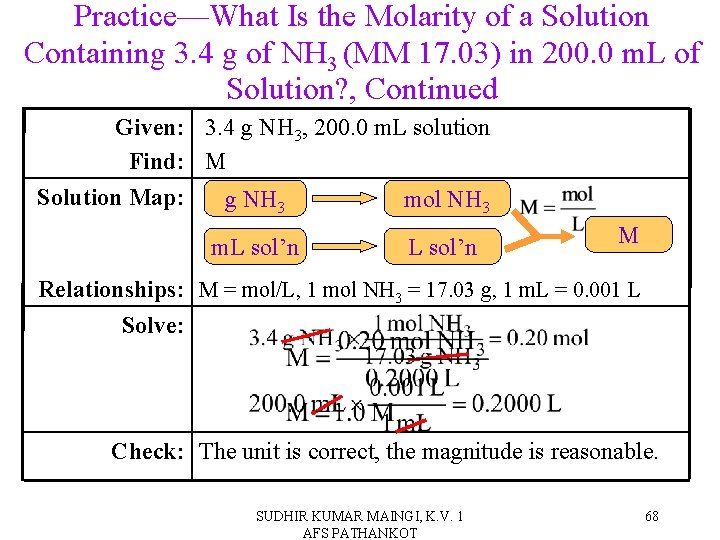
Practice—What Is the Molarity of a Solution Containing 3. 4 g of NH 3 (MM 17. 03) in 200. 0 m. L of Solution? SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 67

Practice—What Is the Molarity of a Solution Containing 3. 4 g of NH 3 (MM 17. 03) in 200. 0 m. L of Solution? , Continued Given: 3. 4 g NH 3, 200. 0 m. L solution Find: M Solution Map: g NH 3 mol NH 3 m. L sol’n M Relationships: M = mol/L, 1 mol NH 3 = 17. 03 g, 1 m. L = 0. 001 L Solve: Check: The unit is correct, the magnitude is reasonable. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 68

Using Concentrations as Conversion Factors • Concentrations show the relationship between the amount of solute and the amount of solvent. ü 0. 12 M sugar (aq) means 0. 12 mol sugar 1. 0 L solution. • The concentration can then be used to convert the moles of solute into the liters of solution, or visa versa. • Since we normally measure the amount of solute in grams, we will need to convert between grams and moles. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 69

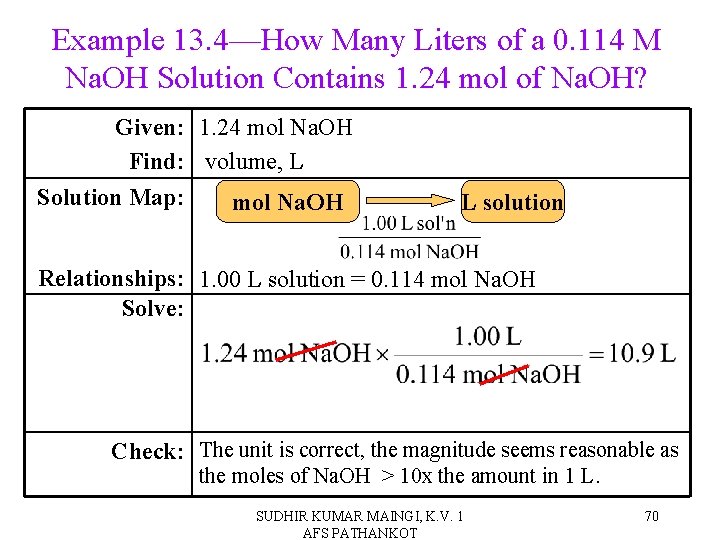
Example 13. 4—How Many Liters of a 0. 114 M Na. OH Solution Contains 1. 24 mol of Na. OH? Given: 1. 24 mol Na. OH Find: volume, L Solution Map: mol Na. OH L solution Relationships: 1. 00 L solution = 0. 114 mol Na. OH Solve: Check: The unit is correct, the magnitude seems reasonable as the moles of Na. OH > 10 x the amount in 1 L. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 70

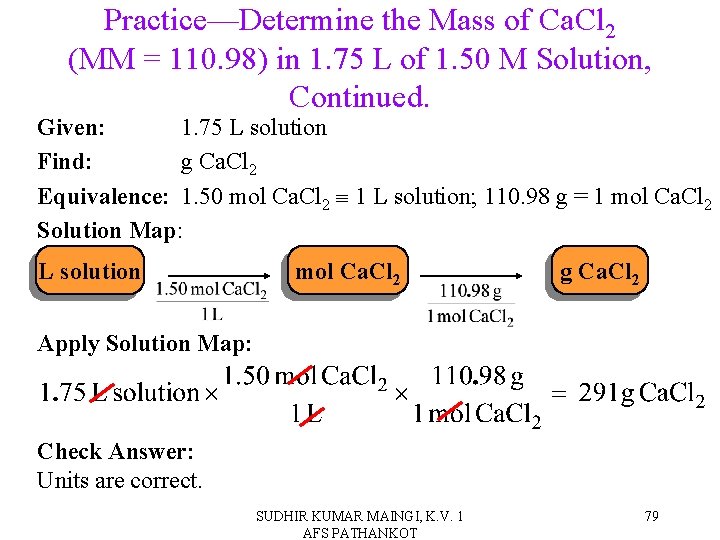
Practice—Determine the Mass of Ca. Cl 2 (MM = 110. 98) in 1. 75 L of 1. 50 M Solution. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 78

Practice—Determine the Mass of Ca. Cl 2 (MM = 110. 98) in 1. 75 L of 1. 50 M Solution, Continued. Given: 1. 75 L solution Find: g Ca. Cl 2 Equivalence: 1. 50 mol Ca. Cl 2 1 L solution; 110. 98 g = 1 mol Ca. Cl 2 Solution Map: L solution mol Ca. Cl 2 g Ca. Cl 2 Apply Solution Map: Check Answer: Units are correct. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 79

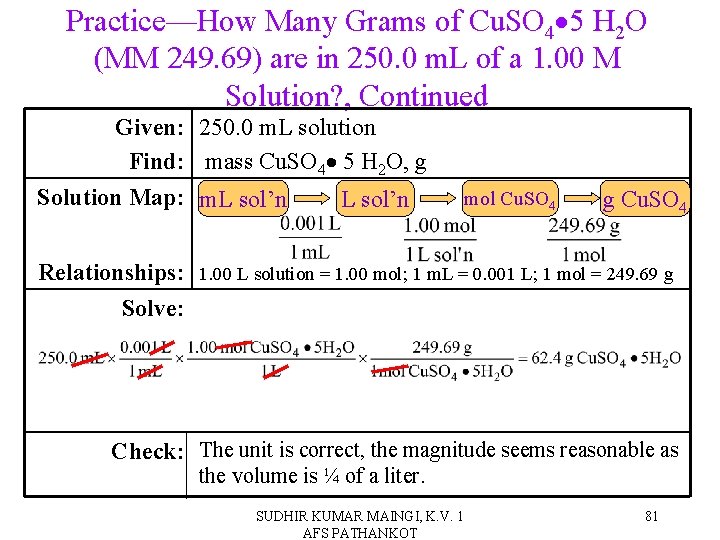
Practice—How Many Grams of Cu. SO 4 5 H 2 O (MM 249. 69) are in 250. 0 m. L of a 1. 00 M Solution? SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 80

Practice—How Many Grams of Cu. SO 4 5 H 2 O (MM 249. 69) are in 250. 0 m. L of a 1. 00 M Solution? , Continued Given: 250. 0 m. L solution Find: mass Cu. SO 4 5 H 2 O, g Solution Map: m. L sol’n Relationships: Solve: mol Cu. SO 4 g Cu. SO 4 1. 00 L solution = 1. 00 mol; 1 m. L = 0. 001 L; 1 mol = 249. 69 g Check: The unit is correct, the magnitude seems reasonable as the volume is ¼ of a liter. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 81

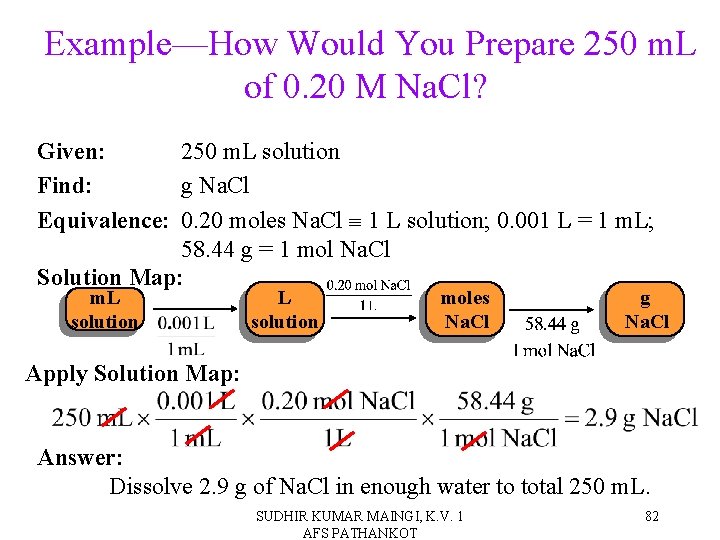
Example—How Would You Prepare 250 m. L of 0. 20 M Na. Cl? Given: 250 m. L solution Find: g Na. Cl Equivalence: 0. 20 moles Na. Cl 1 L solution; 0. 001 L = 1 m. L; 58. 44 g = 1 mol Na. Cl Solution Map: m. L solution moles Na. Cl g Na. Cl Apply Solution Map: Answer: Dissolve 2. 9 g of Na. Cl in enough water to total 250 m. L. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 82

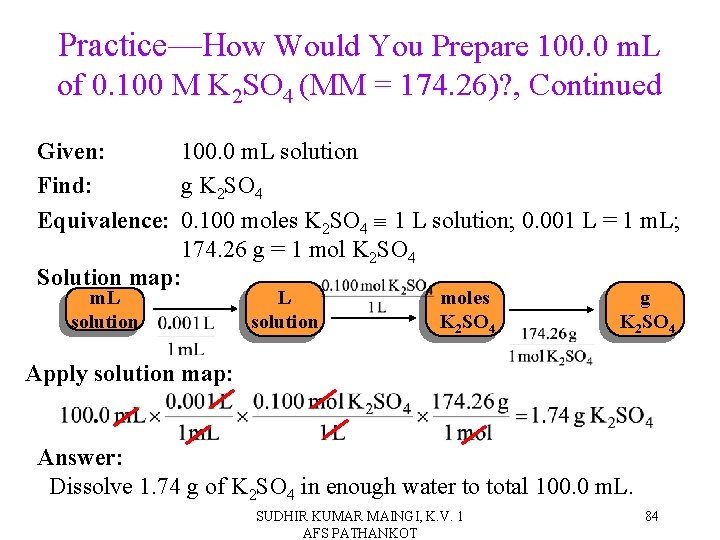
Practice—How Would You Prepare 100. 0 m. L of 0. 100 M K 2 SO 4 (MM = 174. 26)? SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 83

Practice—How Would You Prepare 100. 0 m. L of 0. 100 M K 2 SO 4 (MM = 174. 26)? , Continued Given: 100. 0 m. L solution Find: g K 2 SO 4 Equivalence: 0. 100 moles K 2 SO 4 1 L solution; 0. 001 L = 1 m. L; 174. 26 g = 1 mol K 2 SO 4 Solution map: m. L solution moles K 2 SO 4 g K 2 SO 4 Apply solution map: Answer: Dissolve 1. 74 g of K 2 SO 4 in enough water to total 100. 0 m. L. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 84

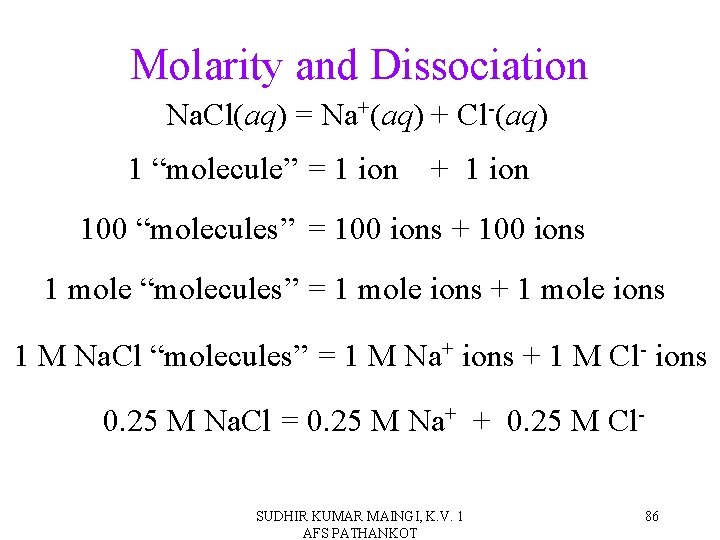
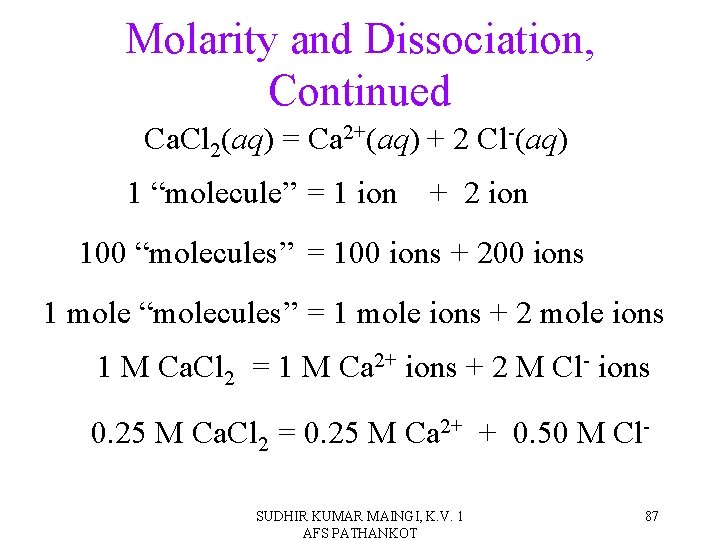
Molarity and Dissociation • When strong electrolytes dissolve, all the solute particles dissociate into ions. • By knowing the formula of the compound and the molarity of the solution, it is easy to determine the molarity of the dissociated ions. Simply multiply the salt concentration by the number of ions. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 85

Molarity and Dissociation Na. Cl(aq) = Na+(aq) + Cl-(aq) 1 “molecule” = 1 ion + 1 ion 100 “molecules” = 100 ions + 100 ions 1 mole “molecules” = 1 mole ions + 1 mole ions 1 M Na. Cl “molecules” = 1 M Na+ ions + 1 M Cl- ions 0. 25 M Na. Cl = 0. 25 M Na+ + 0. 25 M Cl. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 86

Molarity and Dissociation, Continued Ca. Cl 2(aq) = Ca 2+(aq) + 2 Cl-(aq) 1 “molecule” = 1 ion + 2 ion 100 “molecules” = 100 ions + 200 ions 1 mole “molecules” = 1 mole ions + 2 mole ions 1 M Ca. Cl 2 = 1 M Ca 2+ ions + 2 M Cl- ions 0. 25 M Ca. Cl 2 = 0. 25 M Ca 2+ + 0. 50 M Cl. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 87

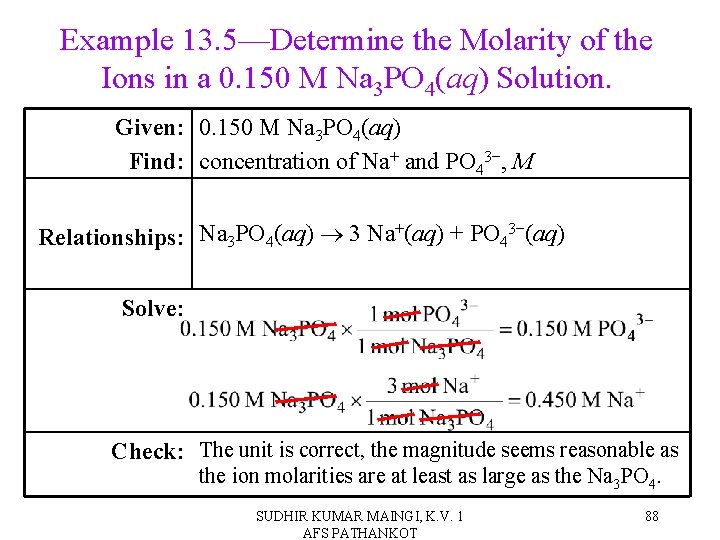
Example 13. 5—Determine the Molarity of the Ions in a 0. 150 M Na 3 PO 4(aq) Solution. Given: 0. 150 M Na 3 PO 4(aq) Find: concentration of Na+ and PO 43−, M Relationships: Na 3 PO 4(aq) 3 Na+(aq) + PO 43−(aq) Solve: Check: The unit is correct, the magnitude seems reasonable as the ion molarities are at least as large as the Na 3 PO 4. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 88

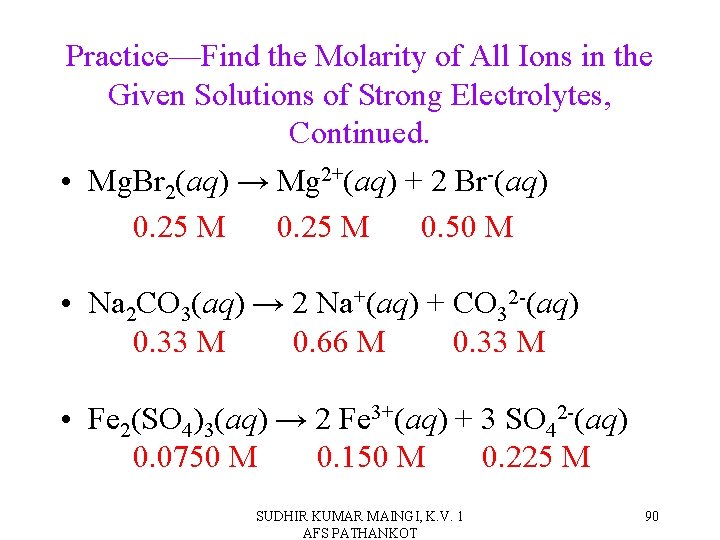
Practice—Find the Molarity of All Ions in the Given Solutions of Strong Electrolytes. • 0. 25 M Mg. Br 2(aq). • 0. 33 M Na 2 CO 3(aq). • 0. 0750 M Fe 2(SO 4)3(aq). SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 89

Practice—Find the Molarity of All Ions in the Given Solutions of Strong Electrolytes, Continued. • Mg. Br 2(aq) → Mg 2+(aq) + 2 Br-(aq) 0. 25 M 0. 50 M • Na 2 CO 3(aq) → 2 Na+(aq) + CO 32 -(aq) 0. 33 M 0. 66 M 0. 33 M • Fe 2(SO 4)3(aq) → 2 Fe 3+(aq) + 3 SO 42 -(aq) 0. 0750 M 0. 150 M 0. 225 M SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 90

Dilution • Dilution is adding extra solvent to decrease the concentration of a solution. • The amount of solute stays the same, but the concentration decreases. • Dilution Formula: Concstart solnx Volstart soln = Concfinal solnx Volfinal sol • Concentrations and volumes can be most units as long as they are consistent. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 91

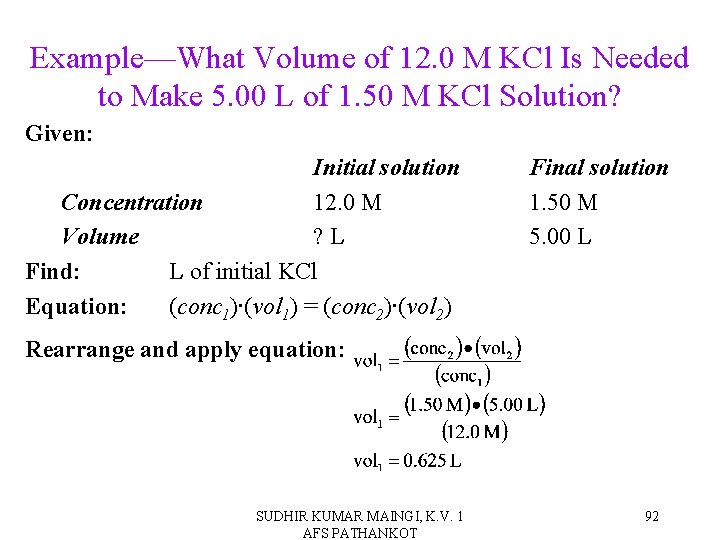
Example—What Volume of 12. 0 M KCl Is Needed to Make 5. 00 L of 1. 50 M KCl Solution? Given: Initial solution Concentration 12. 0 M Volume ? L Find: L of initial KCl Equation: (conc 1)∙(vol 1) = (conc 2)∙(vol 2) Final solution 1. 50 M 5. 00 L Rearrange and apply equation: SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 92

Making a Solution by Dilution M 1 x V 1 = M 2 x V 2 M 1 = 12. 0 M V 1 = ? L M 2 = 1. 50 M V 2 = 5. 00 L Dilute 0. 625 L of 12. 0 M solution to 5. 00 L. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 93

Example—Dilution Problems • What is the concentration of a solution made by diluting 15 m. L of 5. 0% sugar to 135 m. L? M 1 = 5. 0 % M 2 = ? % V 1 = 15 m. L V 2 = 135 m. L (5. 0%)(15 m. L) = M 2 x (135 m. L) M 2 = 0. 55% • How would you prepare 200 m. L of 0. 25 M Na. Cl solution from a 2. 0 M solution? M 1 = 2. 0 M V 1 = ? m. L M 2 = 0. 25 M V 2 = 200 m. L (2. 0 M) x V 1 = (0. 25 M)(200 m. L) V 1 = 25 m. L Dilute 25 m. L of 2. 0 M Na. Cl solution to 200 m. L. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 94

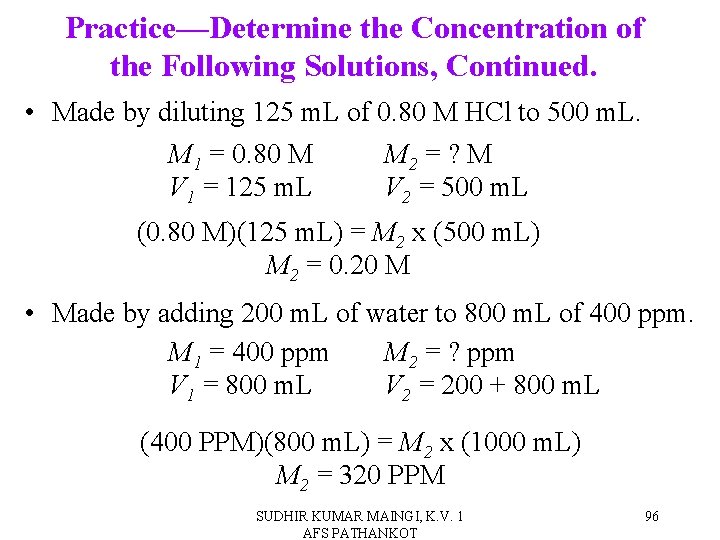
Practice—Determine the Concentration of the Following Solutions. • Made by diluting 125 m. L of 0. 80 M HCl to 500 m. L. • Made by adding 200 m. L of water to 800 m. L of 400 ppm. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 95

Practice—Determine the Concentration of the Following Solutions, Continued. • Made by diluting 125 m. L of 0. 80 M HCl to 500 m. L. M 1 = 0. 80 M M 2 = ? M V 1 = 125 m. L V 2 = 500 m. L (0. 80 M)(125 m. L) = M 2 x (500 m. L) M 2 = 0. 20 M • Made by adding 200 m. L of water to 800 m. L of 400 ppm. M 1 = 400 ppm M 2 = ? ppm V 1 = 800 m. L V 2 = 200 + 800 m. L (400 PPM)(800 m. L) = M 2 x (1000 m. L) M 2 = 320 PPM SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 96

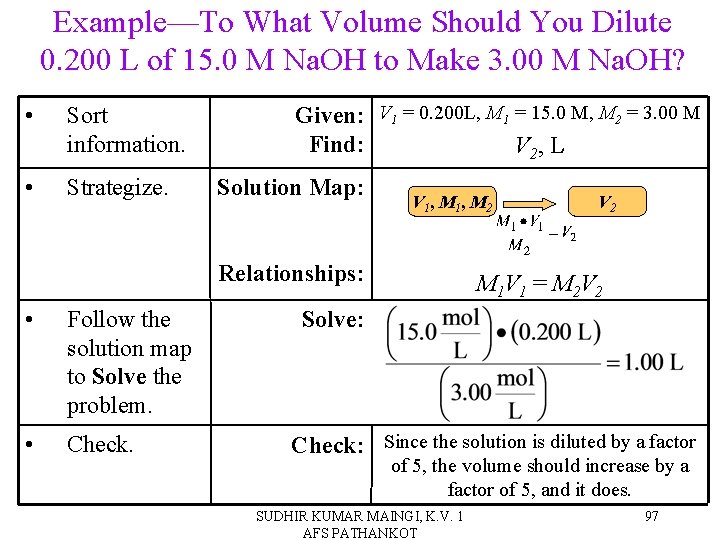
Example—To What Volume Should You Dilute 0. 200 L of 15. 0 M Na. OH to Make 3. 00 M Na. OH? • Sort information. • Strategize. Given: V 1 = 0. 200 L, M 1 = 15. 0 M, M 2 = 3. 00 M Find: V 2, L Solution Map: V 1 , M 2 Relationships: • Follow the solution map to Solve the problem. • Check. V 2 M 1 V 1 = M 2 V 2 Solve: Check: Since the solution is diluted by a factor of 5, the volume should increase by a factor of 5, and it does. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 97

Practice Question 1—How Would You Prepare 400 m. L of a 4. 0% Solution From a 12% Solution? Practice Question 2—How Would You Prepare 250 m. L of a 3. 0% Solution From a 7. 5% Solution? SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 98

Practice Question 1—How Would You Prepare 400 ML of a 4. 0% Solution From a 12% Solution? , Continued M 1 = 12 % M 2 = 4. 0 % (12%) x V 1 = (4. 0%)(400 m. L) V 1 = 133 m. L V 1 = ? m. L V 2 = 400 m. L Dilute 133 m. L of 12% solution to 400 m. L. Practice Question 2—How Would You Prepare 250 ML of a 3. 0% Solution From a 7. 5% Solution? , Continued (7. 5%) x V 1 = (3. 0%)(250 m. L) M 1 = 7. 5 % M 2 = 3. 0 % V 1 = 100 m. L V 1 = ? m. L V 2 = 250 m. L Dilute 100 m. L of 7. 5% solution to 250 m. L. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 99

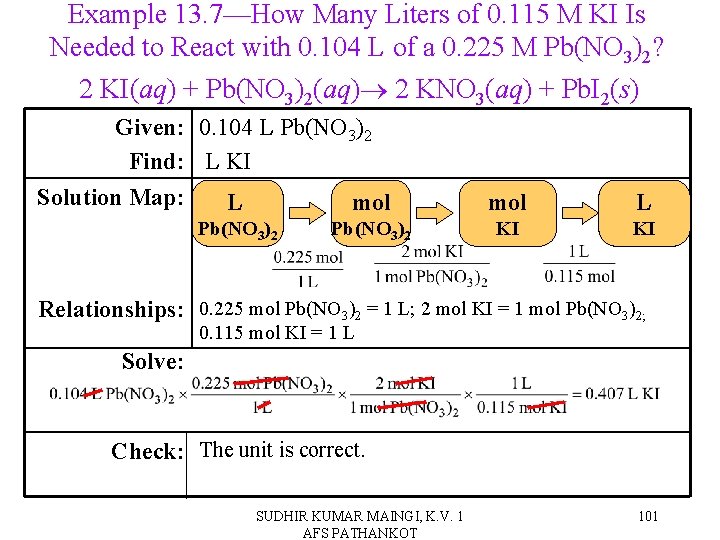
Solution Stoichiometry • We know that the balanced chemical equation tells us the relationship between moles of reactants and products in a reaction. ü 2 H 2(g) + O 2(g) → 2 H 2 O(l) implies that for every 2 moles of H 2 you use, you need 1 mole of O 2 and will make 2 moles of H 2 O. • Since molarity is the relationship between moles of solute and liters of solution, we can now measure the moles of a material in a reaction in solution by knowing its molarity and volume. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 100

Example 13. 7—How Many Liters of 0. 115 M KI Is Needed to React with 0. 104 L of a 0. 225 M Pb(NO 3)2? 2 KI(aq) + Pb(NO 3)2(aq) 2 KNO 3(aq) + Pb. I 2(s) Given: 0. 104 L Pb(NO 3)2 Find: L KI Solution Map: L mol Pb(NO 3)2 mol L KI KI Relationships: 0. 225 mol Pb(NO 3)2 = 1 L; 2 mol KI = 1 mol Pb(NO 3)2; 0. 115 mol KI = 1 L Solve: Check: The unit is correct. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 101

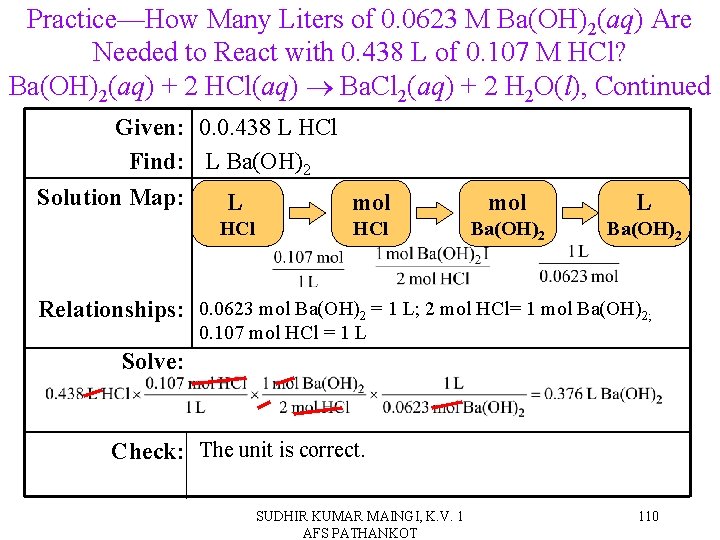
Practice—How Many Liters of 0. 0623 M Ba(OH)2(aq) Are Needed to React with 0. 438 L of 0. 107 M HCl? Ba(OH)2(aq) + 2 HCl(aq) Ba. Cl 2(aq) + 2 H 2 O(l) SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 109

Practice—How Many Liters of 0. 0623 M Ba(OH)2(aq) Are Needed to React with 0. 438 L of 0. 107 M HCl? Ba(OH)2(aq) + 2 HCl(aq) Ba. Cl 2(aq) + 2 H 2 O(l), Continued Given: 0. 0. 438 L HCl Find: L Ba(OH)2 Solution Map: L mol HCl mol L Ba(OH)2 Relationships: 0. 0623 mol Ba(OH)2 = 1 L; 2 mol HCl= 1 mol Ba(OH)2; 0. 107 mol HCl = 1 L Solve: Check: The unit is correct. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 110

Why Do We Do That? • We spread salt on icy roads and walkways to melt the ice. • We add antifreeze to car radiators to prevent the water from boiling or freezing. ü Antifreeze is mainly ethylene glycol. • When we add solutes to water, it changes the freezing point and boiling point of the water. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 111

Colligative Properties • The properties of the solution are different from the properties of the solvent. • Any property of a solution whose value depends only on the number of dissolved solute particles is called a colligative property. üIt does not depend on what the solute particle is. • The freezing point, boiling point, and osmotic pressure of a solution are colligative properties. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 112

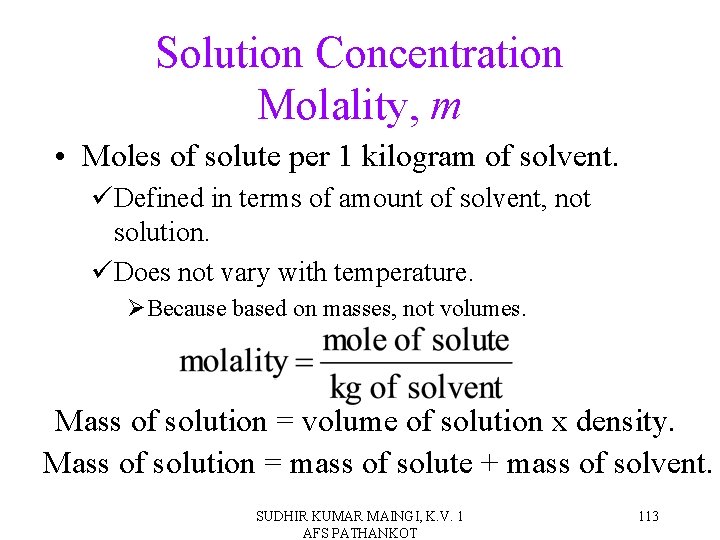
Solution Concentration Molality, m • Moles of solute per 1 kilogram of solvent. üDefined in terms of amount of solvent, not solution. üDoes not vary with temperature. ØBecause based on masses, not volumes. Mass of solution = volume of solution x density. Mass of solution = mass of solute + mass of solvent. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 113

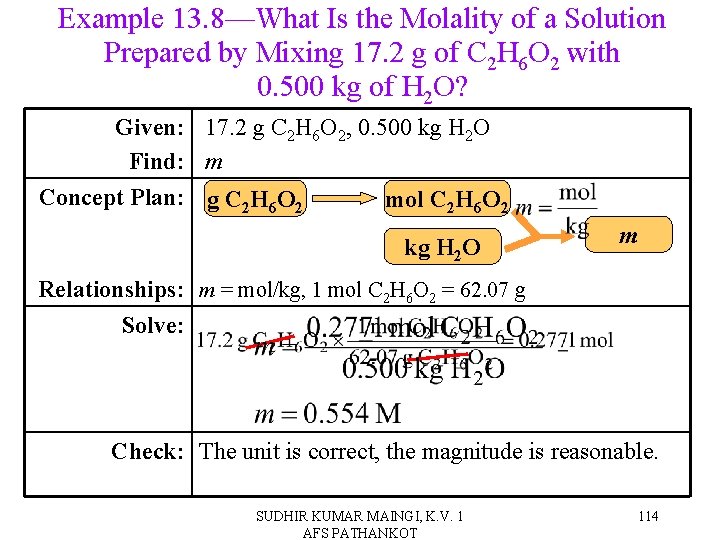
Example 13. 8—What Is the Molality of a Solution Prepared by Mixing 17. 2 g of C 2 H 6 O 2 with 0. 500 kg of H 2 O? Given: 17. 2 g C 2 H 6 O 2, 0. 500 kg H 2 O Find: m Concept Plan: g C 2 H 6 O 2 mol C 2 H 6 O 2 kg H 2 O m Relationships: m = mol/kg, 1 mol C 2 H 6 O 2 = 62. 07 g Solve: Check: The unit is correct, the magnitude is reasonable. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 114

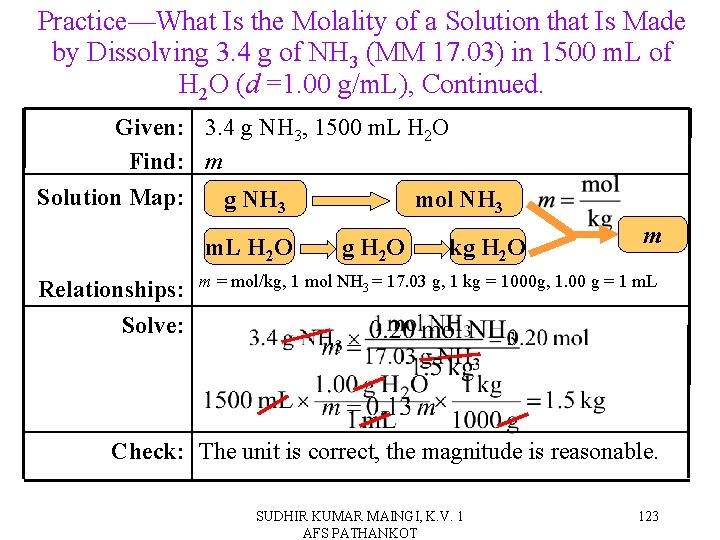
Practice—What Is the Molality of a Solution that Is Made by Dissolving 3. 4 g of NH 3 (MM 17. 03) in 1500 m. L of H 2 O (d =1. 00 g/m. L). SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 122

Practice—What Is the Molality of a Solution that Is Made by Dissolving 3. 4 g of NH 3 (MM 17. 03) in 1500 m. L of H 2 O (d =1. 00 g/m. L), Continued. Given: 3. 4 g NH 3, 1500 m. L H 2 O Find: m Solution Map: g NH 3 mol NH 3 m. L H 2 O Relationships: Solve: g H 2 O kg H 2 O m m = mol/kg, 1 mol NH 3 = 17. 03 g, 1 kg = 1000 g, 1. 00 g = 1 m. L Check: The unit is correct, the magnitude is reasonable. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 123

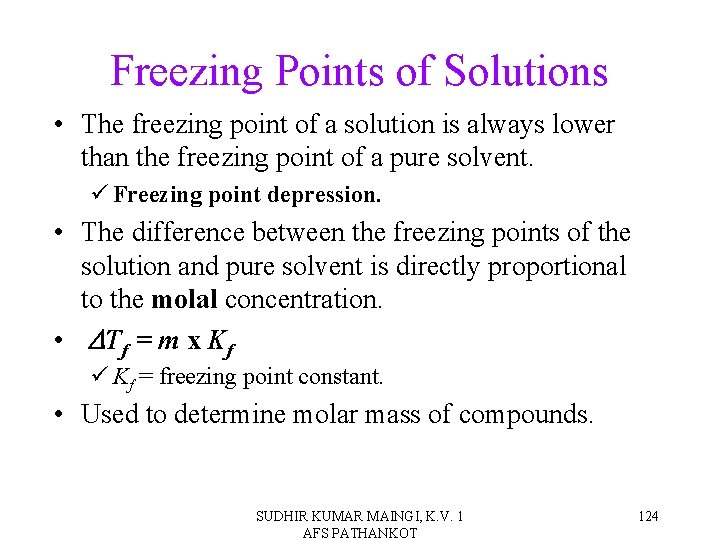
Freezing Points of Solutions • The freezing point of a solution is always lower than the freezing point of a pure solvent. ü Freezing point depression. • The difference between the freezing points of the solution and pure solvent is directly proportional to the molal concentration. • DTf = m x Kf ü Kf = freezing point constant. • Used to determine molar mass of compounds. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 124

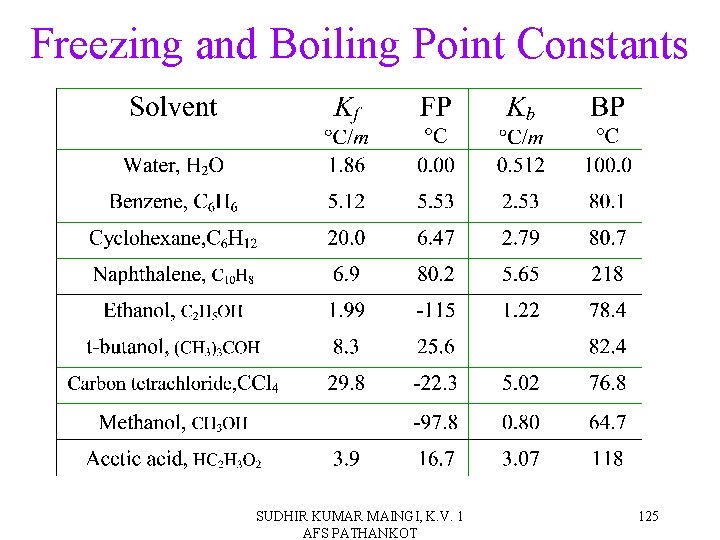
Freezing and Boiling Point Constants SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 125

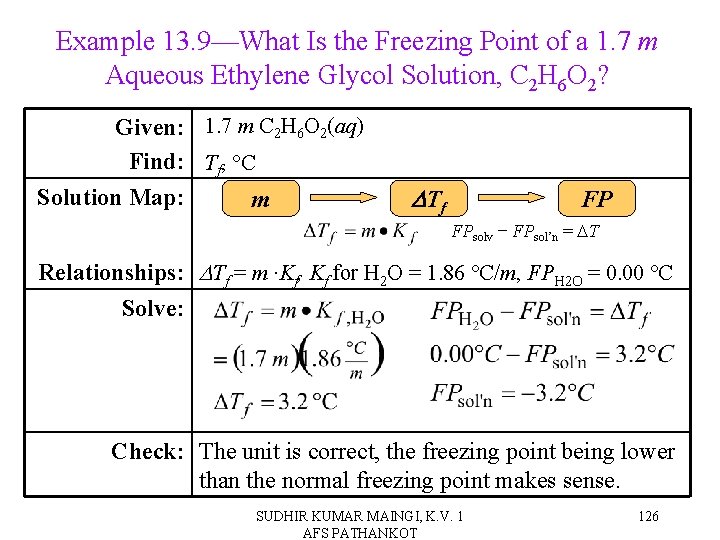
Example 13. 9—What Is the Freezing Point of a 1. 7 m Aqueous Ethylene Glycol Solution, C 2 H 6 O 2? Given: 1. 7 m C 2 H 6 O 2(aq) Find: Tf, °C Solution Map: m DTf FP FPsolv − FPsol’n = DT Relationships: DTf = m ∙Kf, Kf for H 2 O = 1. 86 °C/m, FPH 2 O = 0. 00 °C Solve: Check: The unit is correct, the freezing point being lower than the normal freezing point makes sense. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 126

Practice—What Is the Freezing Point of a Solution that Has 0. 20 moles of Sulfur Dissolved in 0. 10 kg of Cyclohexane? (FPcyclohexane = 6. 5 C, Kf = 20. 0 C/m) SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 134

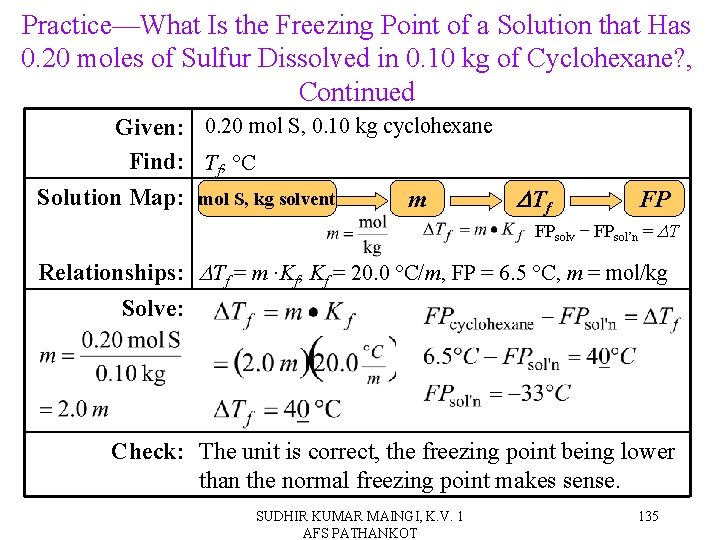
Practice—What Is the Freezing Point of a Solution that Has 0. 20 moles of Sulfur Dissolved in 0. 10 kg of Cyclohexane? , Continued Given: 0. 20 mol S, 0. 10 kg cyclohexane Find: Tf, °C Solution Map: mol S, kg solvent m DTf FP FPsolv − FPsol’n = DT Relationships: DTf = m ∙Kf, Kf = 20. 0 °C/m, FP = 6. 5 °C, m = mol/kg Solve: Check: The unit is correct, the freezing point being lower than the normal freezing point makes sense. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 135

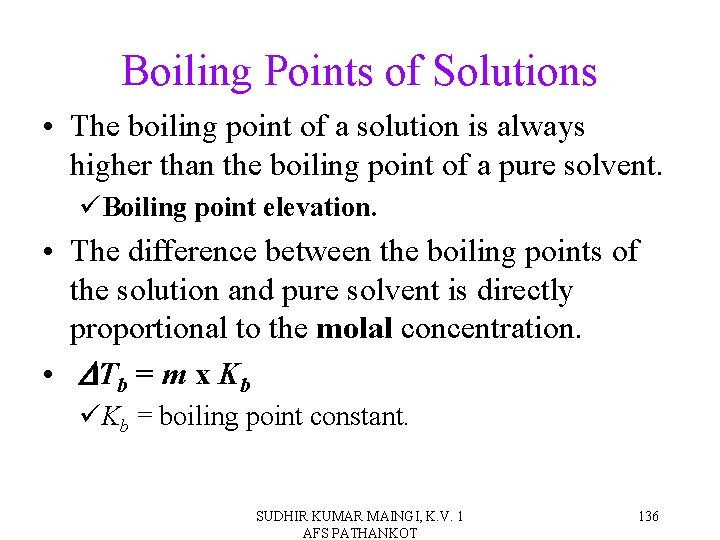
Boiling Points of Solutions • The boiling point of a solution is always higher than the boiling point of a pure solvent. üBoiling point elevation. • The difference between the boiling points of the solution and pure solvent is directly proportional to the molal concentration. • DTb = m x Kb üKb = boiling point constant. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 136

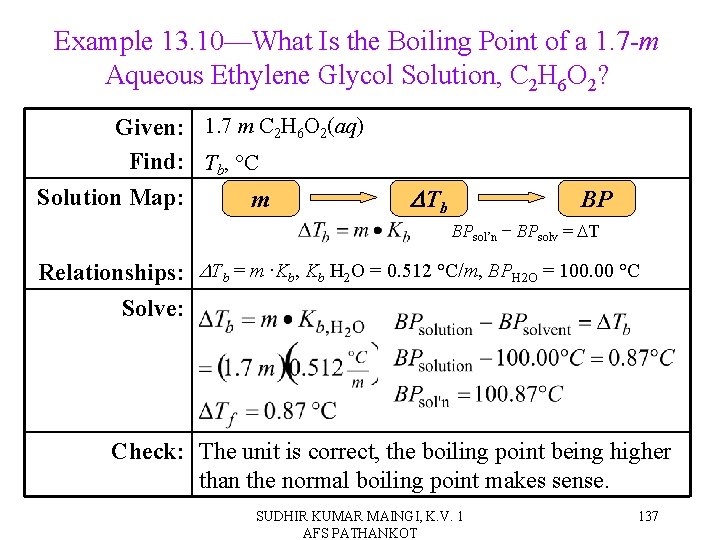
Example 13. 10—What Is the Boiling Point of a 1. 7 -m Aqueous Ethylene Glycol Solution, C 2 H 6 O 2? Given: 1. 7 m C 2 H 6 O 2(aq) Find: Tb, °C Solution Map: m DTb BP BPsol’n − BPsolv = DT Relationships: DTb = m ∙Kb, Kb H 2 O = 0. 512 °C/m, BPH 2 O = 100. 00 °C Solve: Check: The unit is correct, the boiling point being higher than the normal boiling point makes sense. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 137

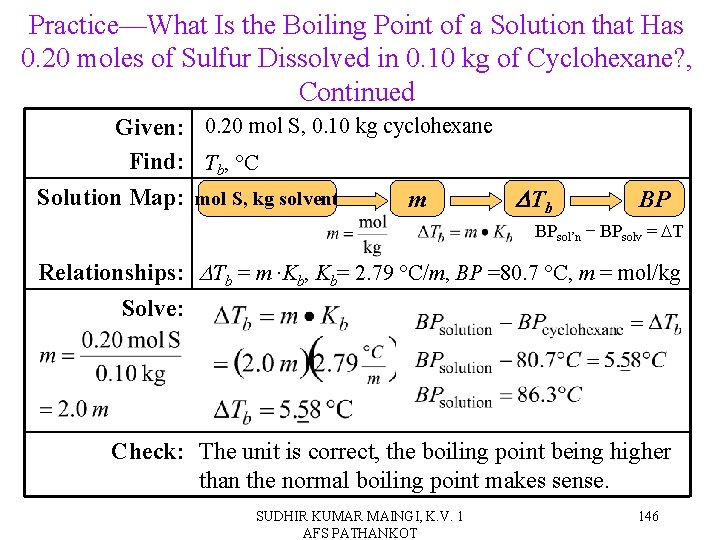
Practice—What Is the Boiling Point of a Solution that Has 0. 20 moles of Sulfur Dissolved in 0. 10 kg of Cyclohexane? (BPcyclohexane = 80. 7 C, Kb= 2. 79 C/m) SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 145

Practice—What Is the Boiling Point of a Solution that Has 0. 20 moles of Sulfur Dissolved in 0. 10 kg of Cyclohexane? , Continued Given: 0. 20 mol S, 0. 10 kg cyclohexane Find: Tb, °C Solution Map: mol S, kg solvent m DTb BP BPsol’n − BPsolv = DT Relationships: DTb = m ∙Kb, Kb= 2. 79 °C/m, BP =80. 7 °C, m = mol/kg Solve: Check: The unit is correct, the boiling point being higher than the normal boiling point makes sense. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 146

Osmosis and Osmotic Pressure • Osmosis is the process in which solvent molecules pass through a semipermeable membrane that does not allow solute particles to pass. ü Solvent flows to try to equalize concentration of solute on both sides. ü Solvent flows from side of low concentration to high concentration. • Osmotic pressure is pressure that is needed to prevent osmotic flow of solvent. • Isotonic, hypotonic, and hypertonic solutions. ü Hemolysis. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 147

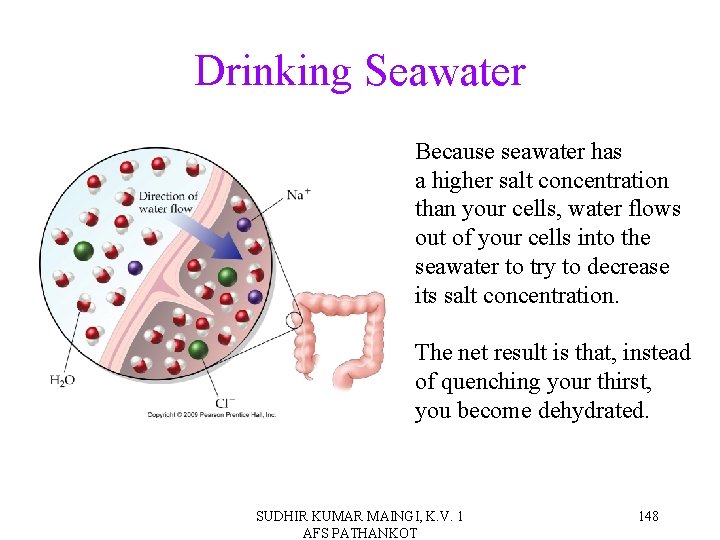
Drinking Seawater Because seawater has a higher salt concentration than your cells, water flows out of your cells into the seawater to try to decrease its salt concentration. The net result is that, instead of quenching your thirst, you become dehydrated. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 148

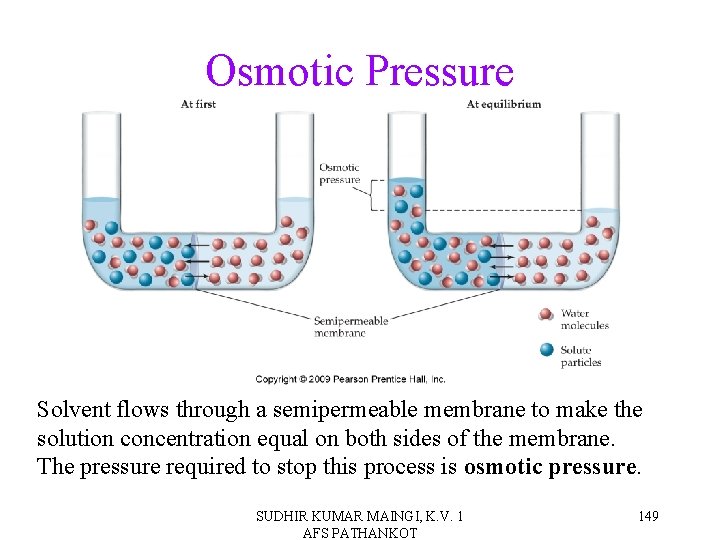
Osmotic Pressure Solvent flows through a semipermeable membrane to make the solution concentration equal on both sides of the membrane. The pressure required to stop this process is osmotic pressure. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 149

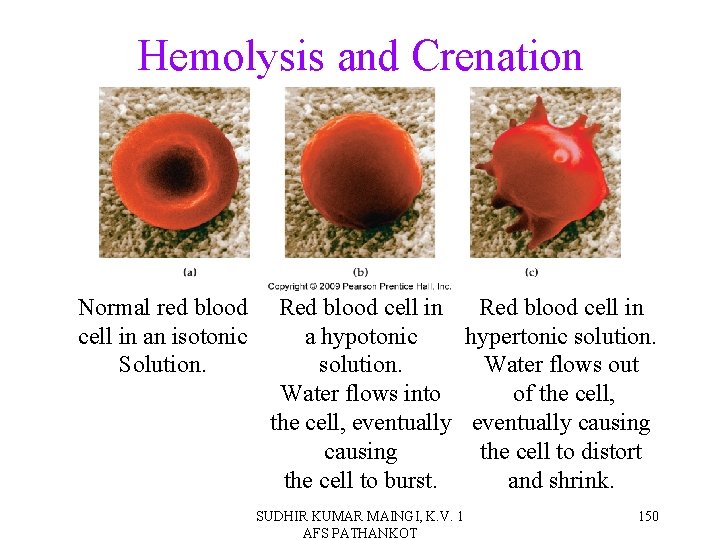
Hemolysis and Crenation Normal red blood cell in an isotonic Solution. Red blood cell in a hypotonic hypertonic solution. Water flows out Water flows into of the cell, eventually causing the cell to distort the cell to burst. and shrink. SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 150

Thank you SUDHIR KUMAR MAINGI, K. V. 1 AFS PATHANKOT 151