Vasodilators in PAH Kailash Kumar Goyal Definition of



























- Slides: 87

Vasodilators in PAH Kailash Kumar Goyal

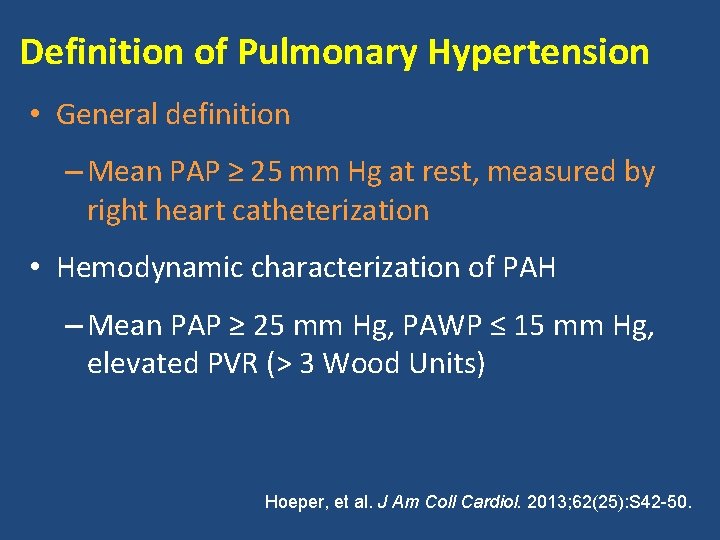
Definition of Pulmonary Hypertension • General definition – Mean PAP ≥ 25 mm Hg at rest, measured by right heart catheterization • Hemodynamic characterization of PAH – Mean PAP ≥ 25 mm Hg, PAWP ≤ 15 mm Hg, elevated PVR (> 3 Wood Units) Hoeper, et al. J Am Coll Cardiol. 2013; 62(25): S 42 -50.

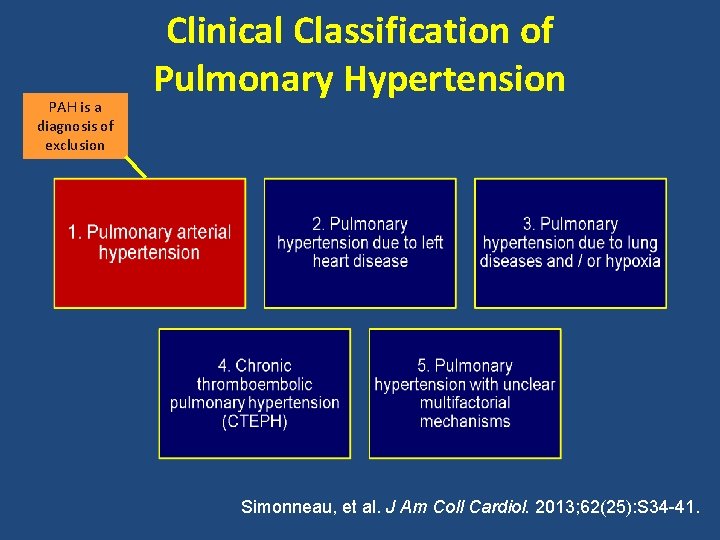
PAH is a diagnosis of exclusion Clinical Classification of Pulmonary Hypertension Simonneau, et al. J Am Coll Cardiol. 2013; 62(25): S 34 -41.

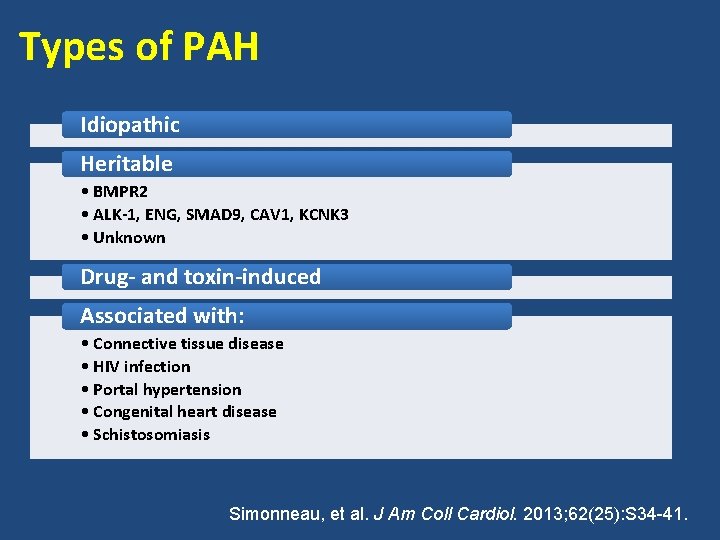
Types of PAH Idiopathic Heritable • BMPR 2 • ALK-1, ENG, SMAD 9, CAV 1, KCNK 3 • Unknown Drug- and toxin-induced Associated with: • Connective tissue disease • HIV infection • Portal hypertension • Congenital heart disease • Schistosomiasis Simonneau, et al. J Am Coll Cardiol. 2013; 62(25): S 34 -41.

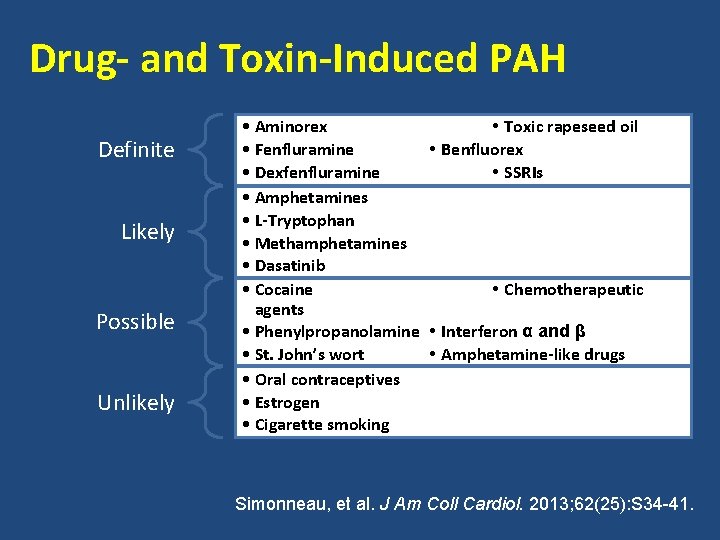
Drug- and Toxin-Induced PAH Definite Likely Possible Unlikely • Aminorex • Toxic rapeseed oil • Fenfluramine • Benfluorex • Dexfenfluramine • SSRIs • Amphetamines • L-Tryptophan • Methamphetamines • Dasatinib • Cocaine • Chemotherapeutic agents • Phenylpropanolamine • Interferon α and β • St. John’s wort • Amphetamine-like drugs • Oral contraceptives • Estrogen • Cigarette smoking Simonneau, et al. J Am Coll Cardiol. 2013; 62(25): S 34 -41.

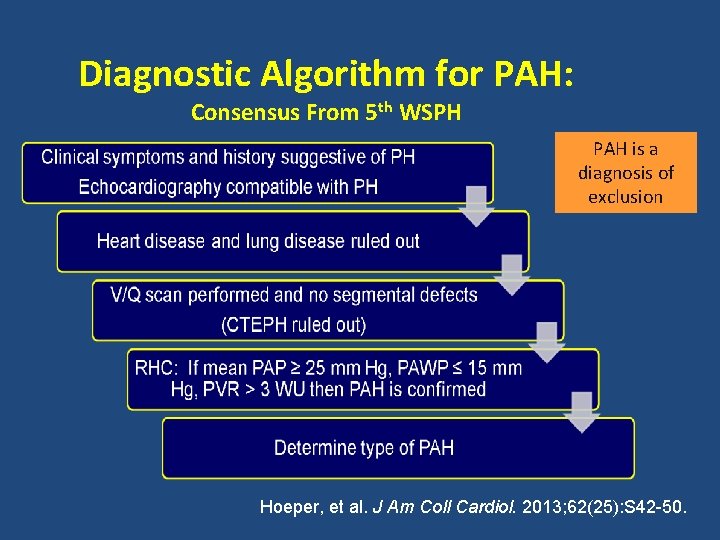
Diagnostic Algorithm for PAH: Consensus From 5 th WSPH PAH is a diagnosis of exclusion Hoeper, et al. J Am Coll Cardiol. 2013; 62(25): S 42 -50.

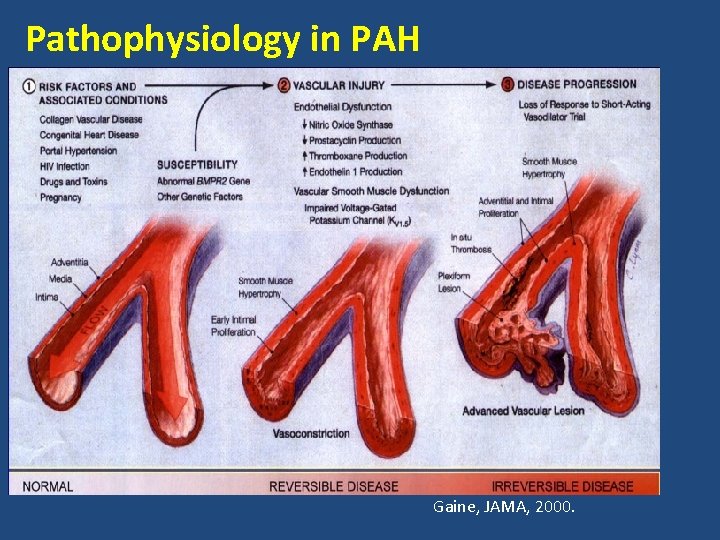
Pathophysiology in PAH Gaine, JAMA, 2000.

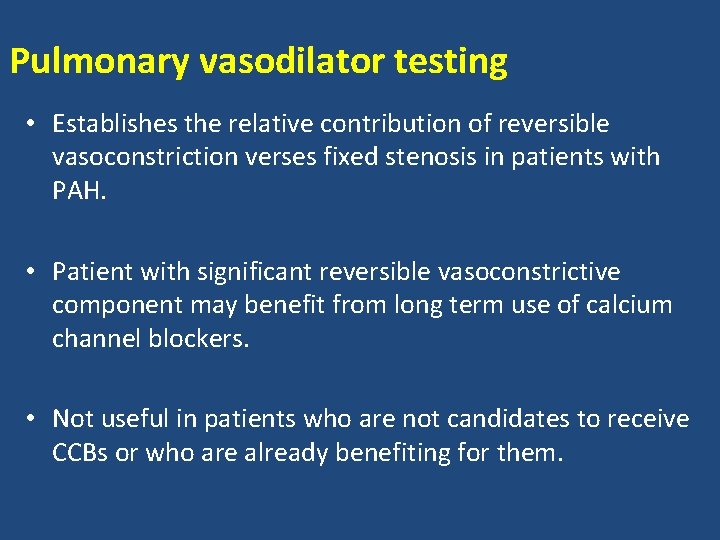
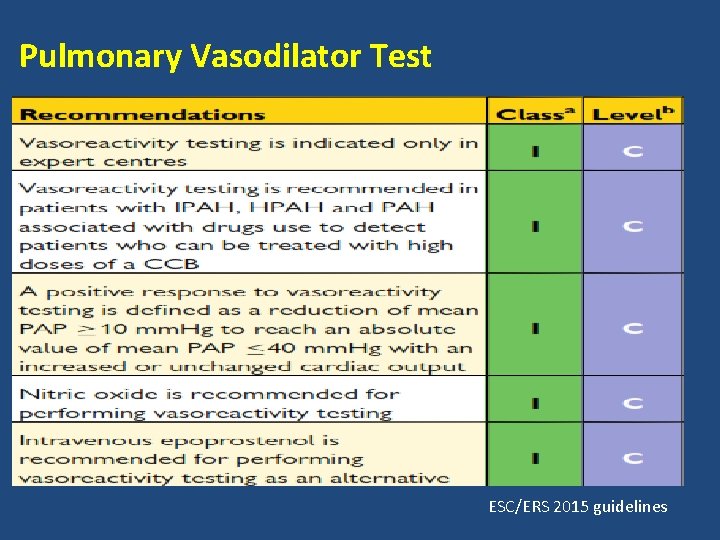
Pulmonary vasodilator testing • Establishes the relative contribution of reversible vasoconstriction verses fixed stenosis in patients with PAH. • Patient with significant reversible vasoconstrictive component may benefit from long term use of calcium channel blockers. • Not useful in patients who are not candidates to receive CCBs or who are already benefiting for them.

Criteria for a positive test • Multiple criteria for a positive pulmonary vasodilator test have been suggested. • The latest criterion improve the specificity thereby avoiding adverse effects of CCB treatment in patients not likely to respond to CCBs. • According to Sitbon et al, the sensitivity, specificity, positive and negative predictive values using the new criteria are 69, 87, 78 and 81 percent respectively.

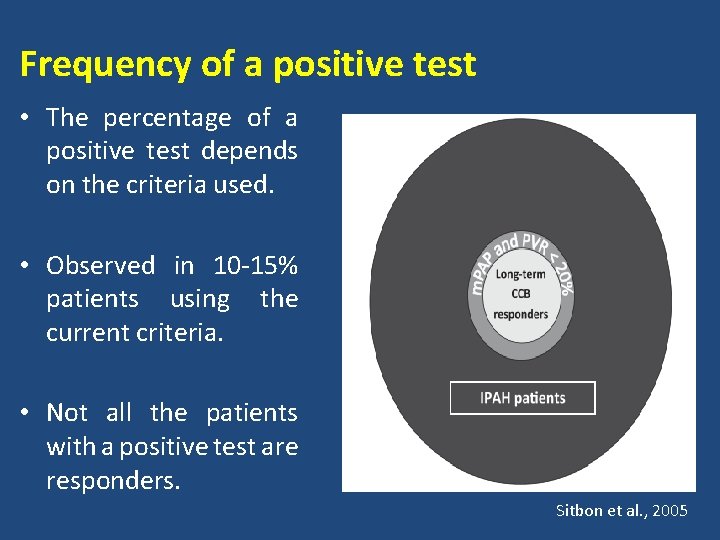
Frequency of a positive test • The percentage of a positive test depends on the criteria used. • Observed in 10 -15% patients using the current criteria. • Not all the patients with a positive test are responders. Sitbon et al. , 2005


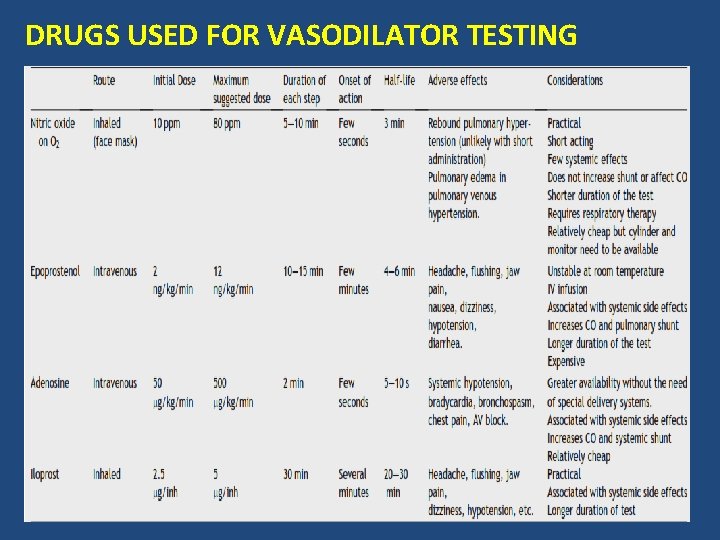
DRUGS USED FOR VASODILATOR TESTING

Pulmonary Vasodilator Test ESC/ERS 2015 guidelines

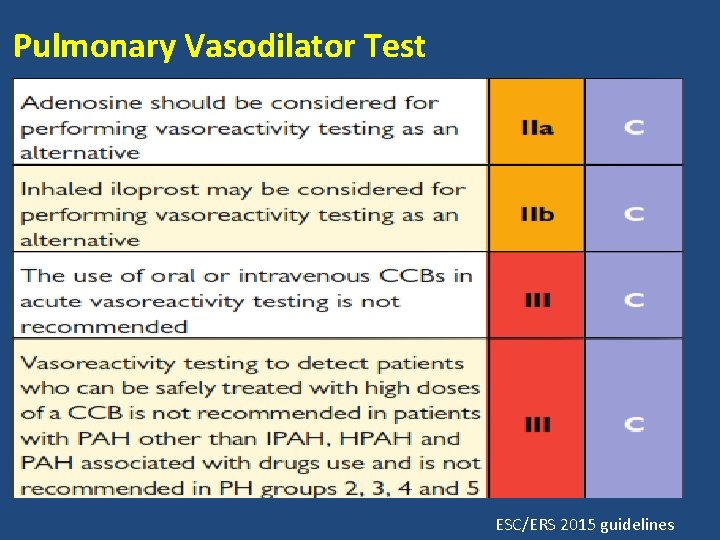
Pulmonary Vasodilator Test ESC/ERS 2015 guidelines

Limitations • Positive only in a minority of patients, predominantly those with IPAH and anorexigen induced PAH. • All positive patients may not be long term CCBs responders. • Potential adverse effects depending on the agent used. • Time consuming. • Cost.

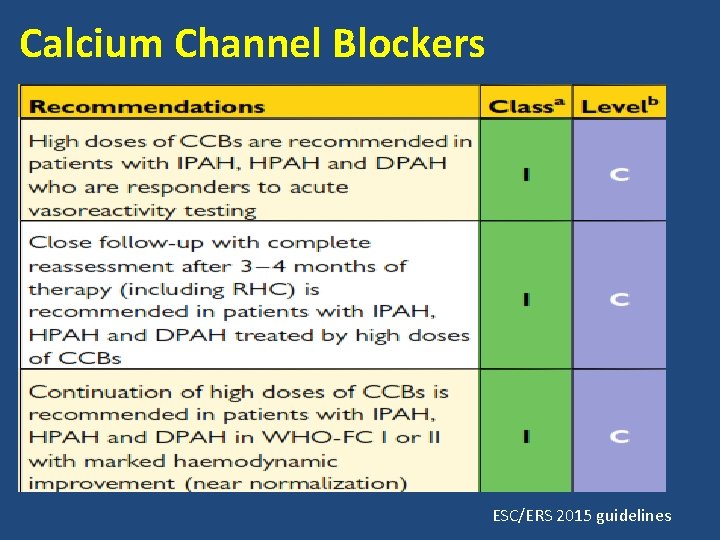
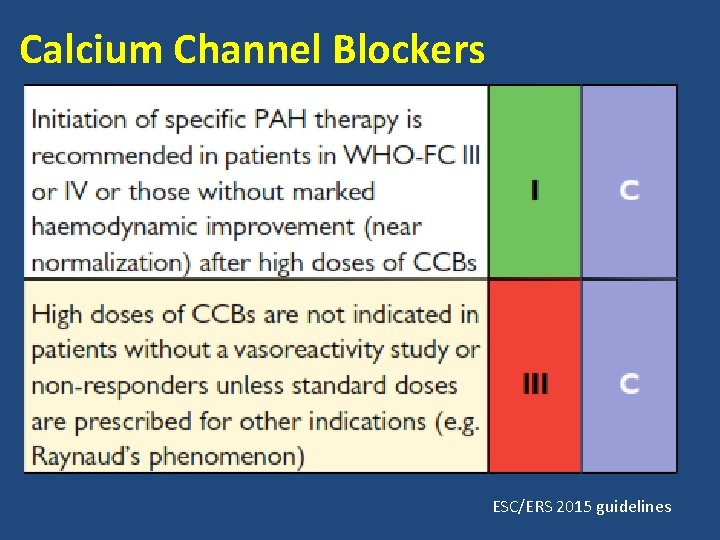
Calcium Channel Blockers

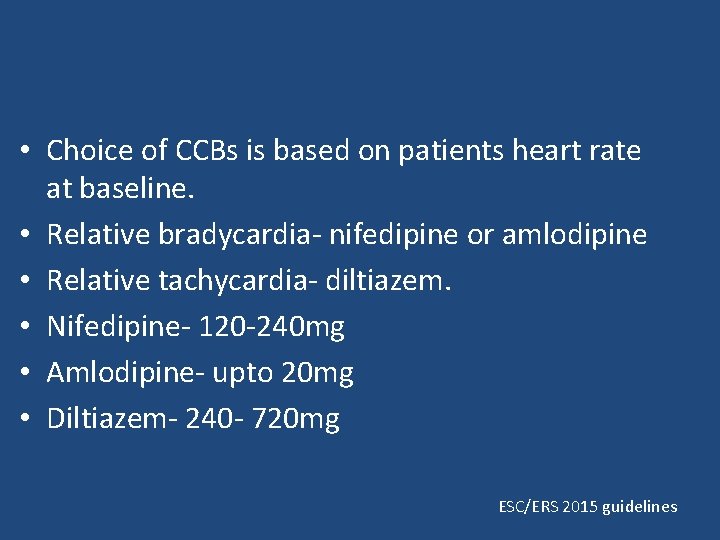
• Choice of CCBs is based on patients heart rate at baseline. • Relative bradycardia- nifedipine or amlodipine • Relative tachycardia- diltiazem. • Nifedipine- 120 -240 mg • Amlodipine- upto 20 mg • Diltiazem- 240 - 720 mg ESC/ERS 2015 guidelines

Calcium Channel Blockers ESC/ERS 2015 guidelines

Calcium Channel Blockers ESC/ERS 2015 guidelines

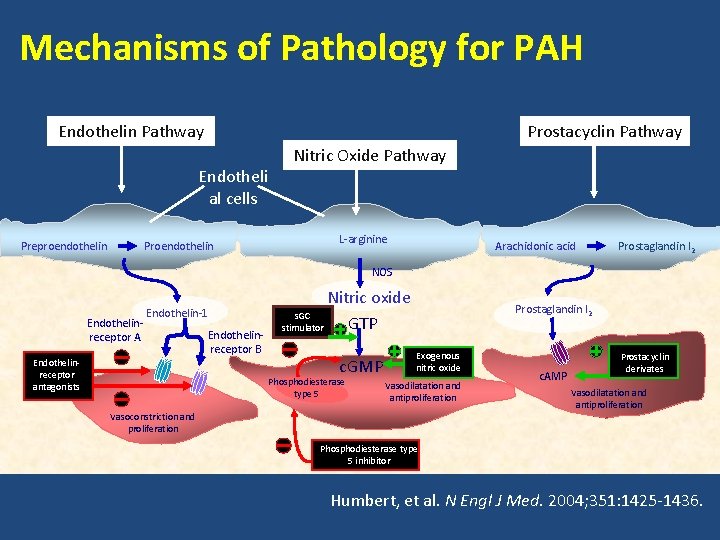
Mechanisms of Pathology for PAH Prostacyclin Pathway Endotheli al cells Preproendothelin Proendothelin Nitric Oxide Pathway L-arginine Arachidonic acid Prostaglandin I 2 NOS Endothelinreceptor A Endothelin-1 Endothelinreceptor antagonists Endothelinreceptor B Nitric oxide s. GC GTP stimulator c. GMP Phosphodiesterase type 5 Prostaglandin I 2 Exogenous nitric oxide Vasodilatation and antiproliferation Vasoconstriction and proliferation c. AMP Prostacyclin derivates Vasodilatation and antiproliferation Phosphodiesterase type 5 inhibitor Humbert, et al. N Engl J Med. 2004; 351: 1425 -1436.

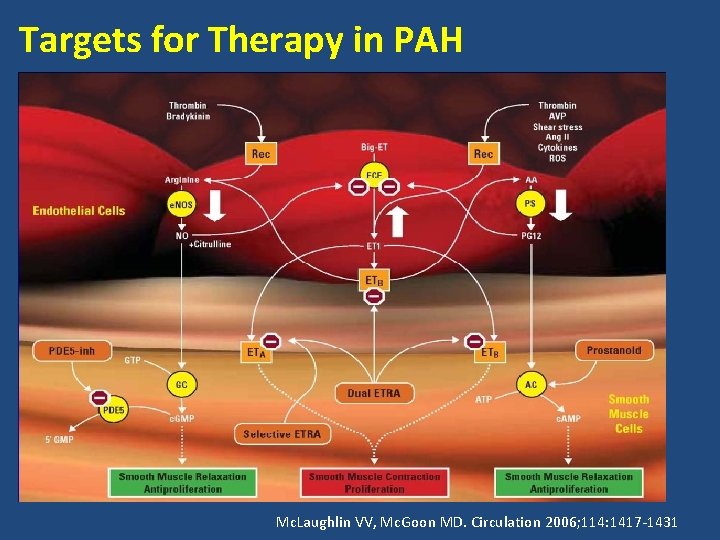
Targets for Therapy in PAH Mc. Laughlin VV, Mc. Goon MD. Circulation 2006; 114: 1417 -1431

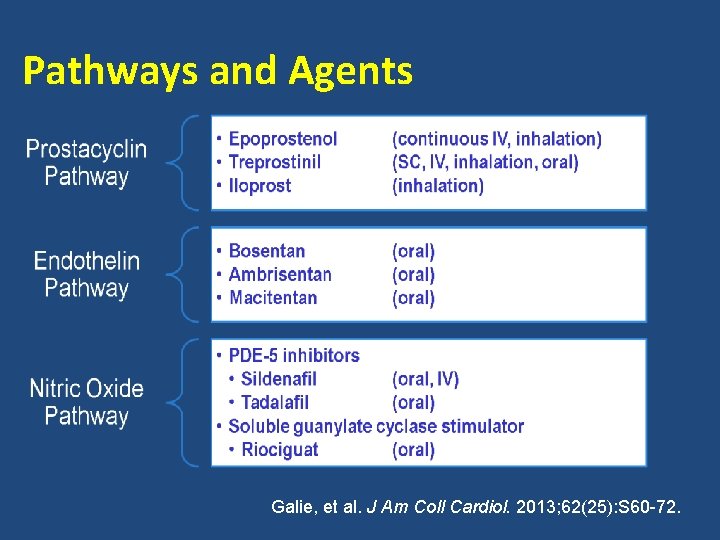
Pathways and Agents Galie, et al. J Am Coll Cardiol. 2013; 62(25): S 60 -72.

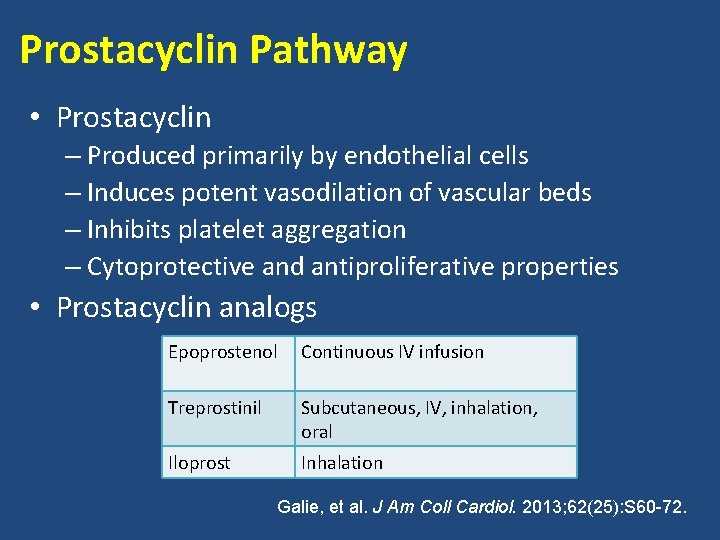
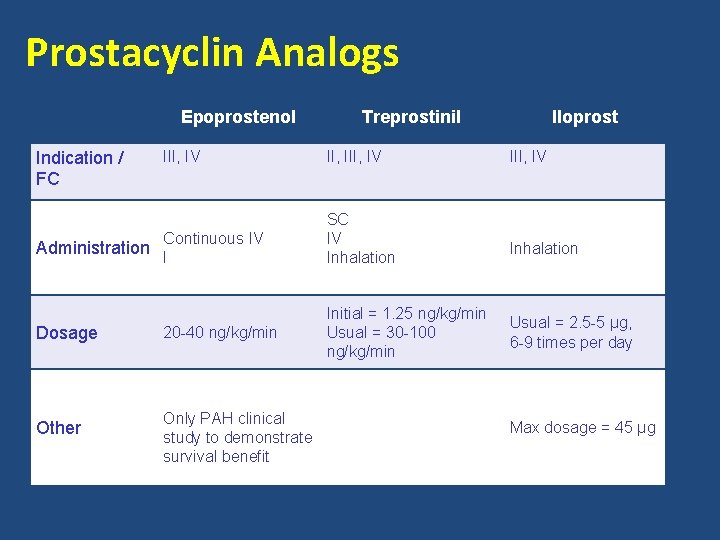
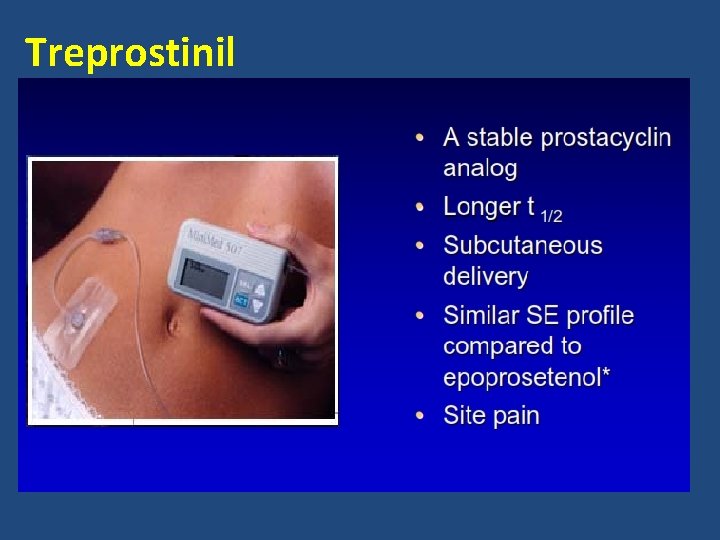
Prostacyclin Pathway • Prostacyclin – Produced primarily by endothelial cells – Induces potent vasodilation of vascular beds – Inhibits platelet aggregation – Cytoprotective and antiproliferative properties • Prostacyclin analogs Epoprostenol Continuous IV infusion Treprostinil Subcutaneous, IV, inhalation, oral Iloprost Inhalation Galie, et al. J Am Coll Cardiol. 2013; 62(25): S 60 -72.

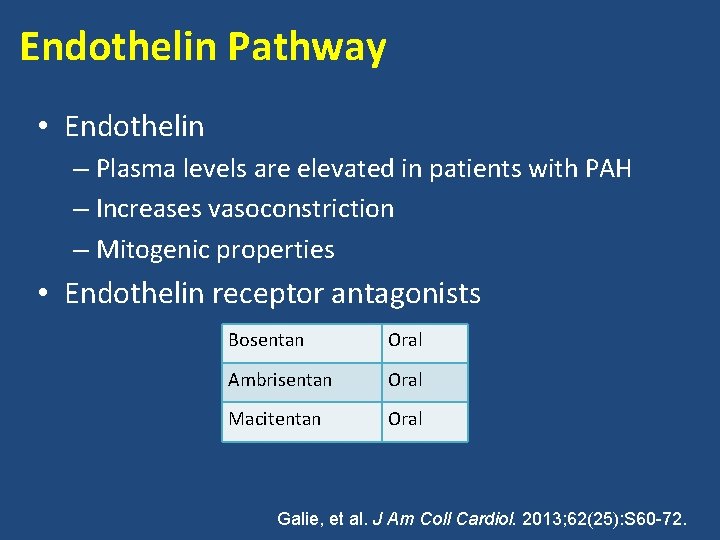
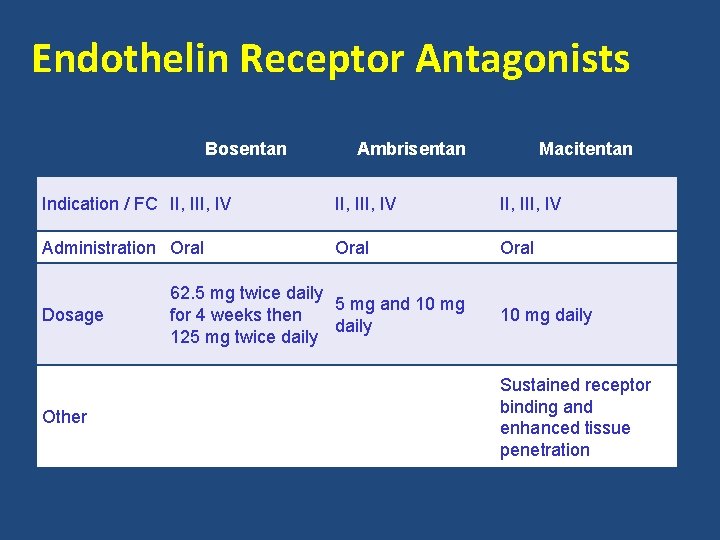
Endothelin Pathway • Endothelin – Plasma levels are elevated in patients with PAH – Increases vasoconstriction – Mitogenic properties • Endothelin receptor antagonists Bosentan Oral Ambrisentan Oral Macitentan Oral Galie, et al. J Am Coll Cardiol. 2013; 62(25): S 60 -72.

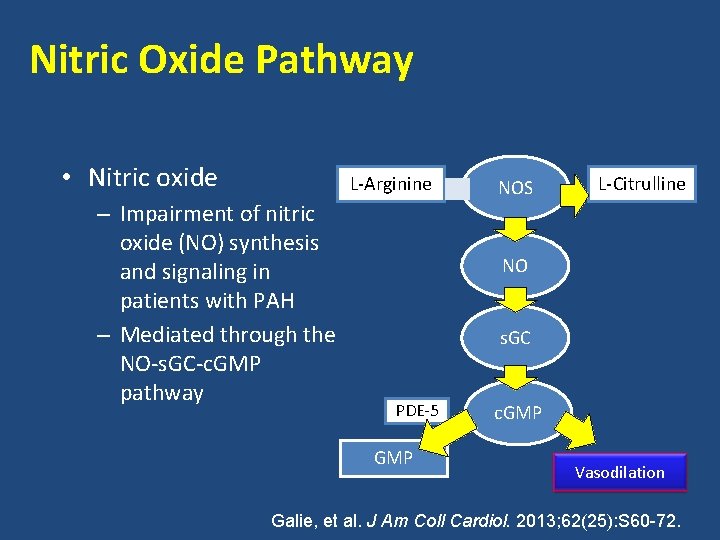
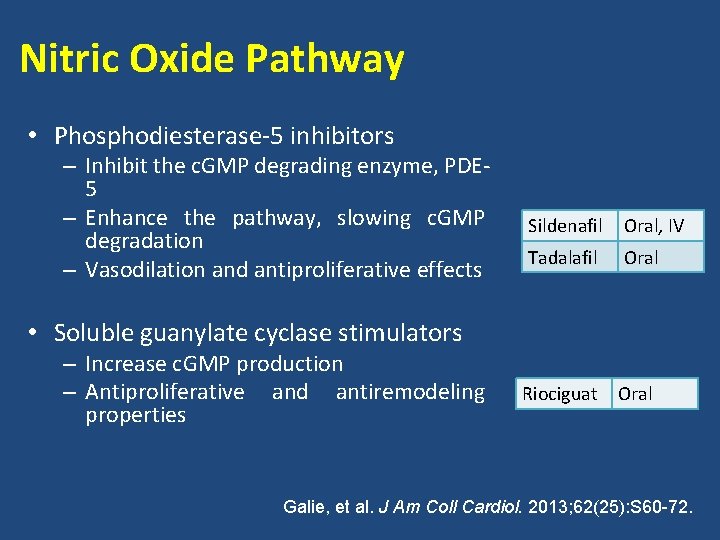
Nitric Oxide Pathway • Nitric oxide L-Arginine – Impairment of nitric oxide (NO) synthesis and signaling in patients with PAH – Mediated through the NO-s. GC-c. GMP pathway NOS L-Citrulline NO s. GC PDE-5 GMP c. GMP Vasodilation Galie, et al. J Am Coll Cardiol. 2013; 62(25): S 60 -72.

Nitric Oxide Pathway • Phosphodiesterase-5 inhibitors – Inhibit the c. GMP degrading enzyme, PDE 5 – Enhance the pathway, slowing c. GMP degradation – Vasodilation and antiproliferative effects Sildenafil Oral, IV Tadalafil Oral Riociguat Oral • Soluble guanylate cyclase stimulators – Increase c. GMP production – Antiproliferative and antiremodeling properties Galie, et al. J Am Coll Cardiol. 2013; 62(25): S 60 -72.

Trial design: What is Optimum ? • End Points – Mortality • Vital importance in a fatal disease like PAH • Effect of drug on survival • Since the incidence of disease is low – limit applicability – Morbidity end points • Various – hospitalization, decrease in exercise capacity – 6 MWD • • Easy & inexpensive Most commonly used primary end point Carries prognostic significance Clinically relevant improvement in 6 MWD not established – Composite - Time to Clinical Worsening • Has been used as secondary end point in majority of studies • Has variously included death, hospitalization, clinical worsening, decrease in 6 MWD • Should be included as the primary end point & uniformly defined JACC; 2009, Vol. 54, No. 1, Suppl S; S 97– 107


• Follow up – Most trials – 12 -24 wk duration – Longer follow up is required – to bring out significance of hard end points like mortality • Head to head comparisons – Between drugs of same and different group is lacking

Prostacyclin Analogs Epoprostenol Indication / FC III, IV Continuous IV SC IV Inhalation 20 -40 ng/kg/min Initial = 1. 25 ng/kg/min Usual = 30 -100 ng/kg/min Administration I Dosage Other Treprostinil Only PAH clinical study to demonstrate survival benefit Iloprost III, IV Inhalation Usual = 2. 5 -5 µg, 6 -9 times per day Max dosage = 45 µg

Epoprostenol • Short half life of 3 -5 minutes. • Stable at room temperature for only 8 hours. • Requires cooling and continuous administration by means of an infusion pump and a permanent tunnelled catheter. • Tested in three unblinded randomized controlled trials.



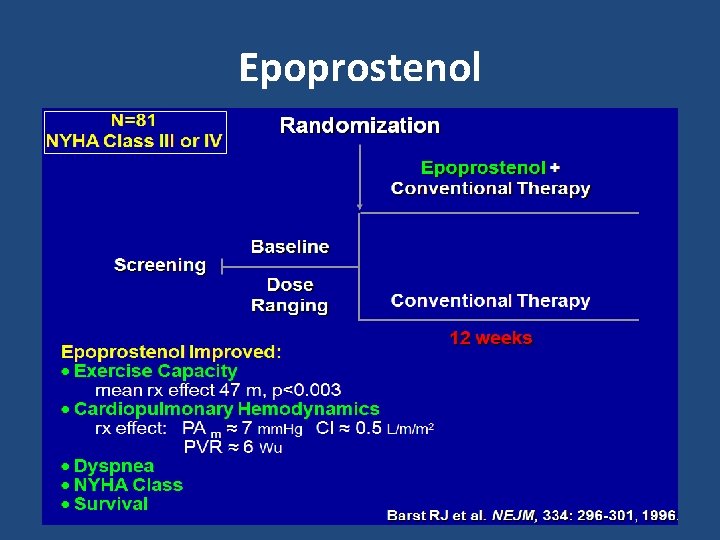
Epoprostenol

Epoprostenol

Treprostinil


Treprostinil Simonneau G. et al. Am J Resp Crit Care Med 2002; 165: 800 -804.

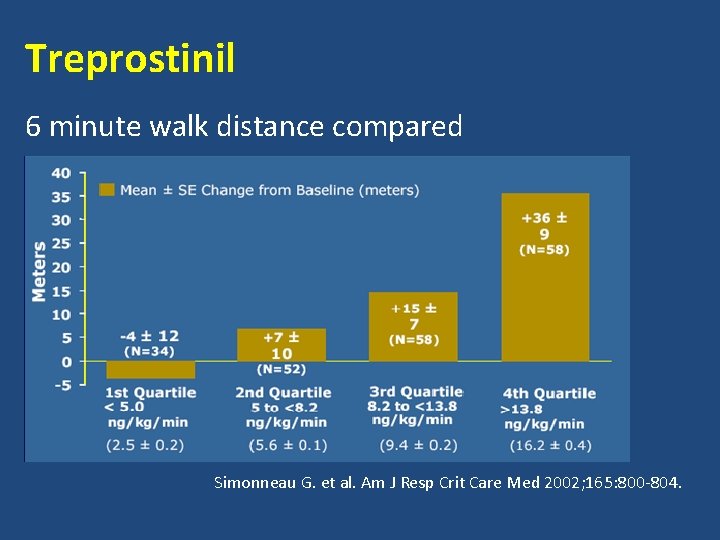
Treprostinil 6 minute walk distance compared Simonneau G. et al. Am J Resp Crit Care Med 2002; 165: 800 -804.

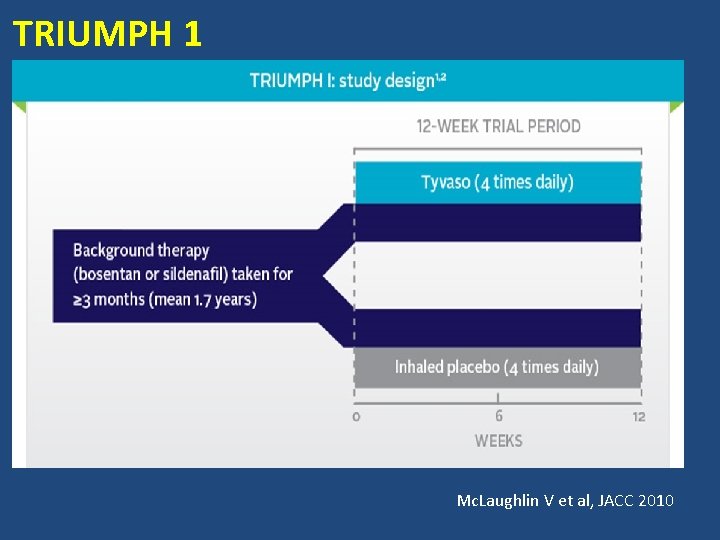
TRIUMPH 1 STUDY

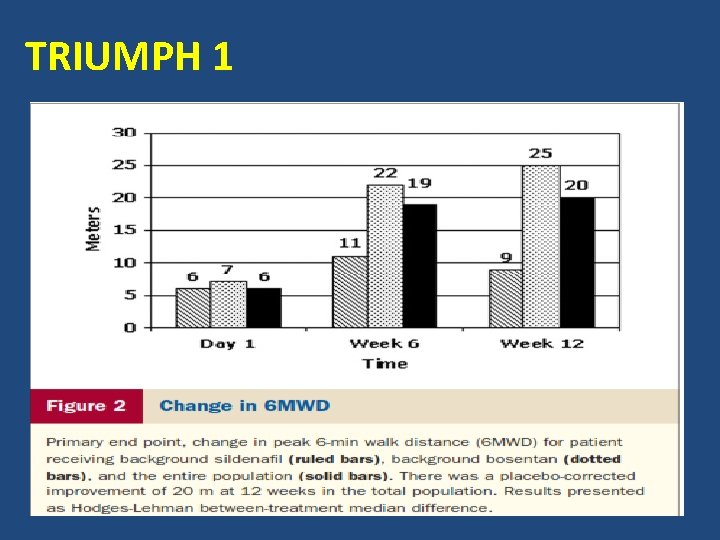
TRIUMPH 1 Mc. Laughlin V et al, JACC 2010

TRIUMPH 1

FREEDOM STUDIES

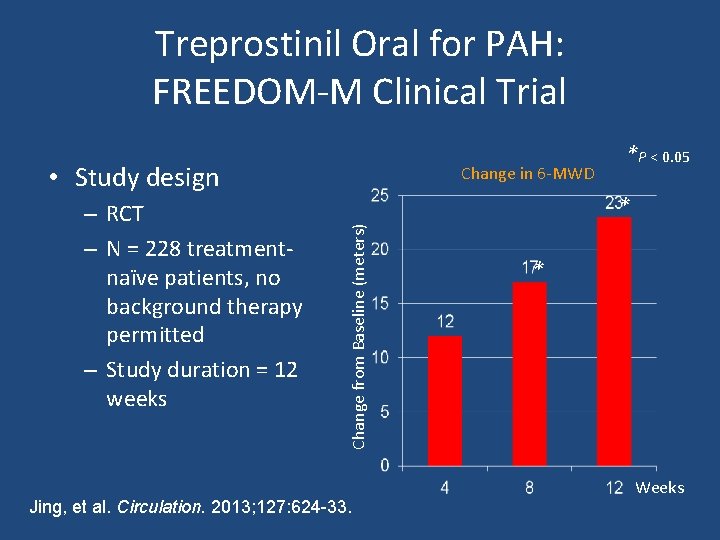
Treprostinil Oral for PAH: FREEDOM-M Clinical Trial • Study design * Change from Baseline (meters) – RCT – N = 228 treatmentnaïve patients, no background therapy permitted – Study duration = 12 weeks Change in 6 -MWD *P < 0. 05 Jing, et al. Circulation. 2013; 127: 624 -33. * Weeks

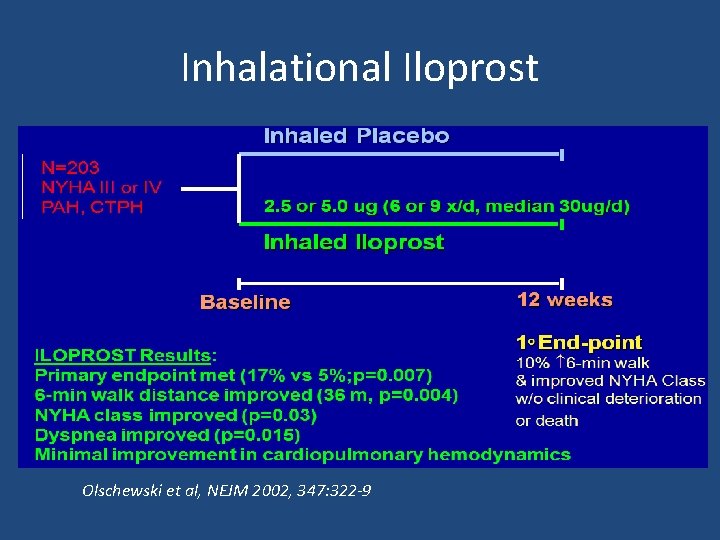
Iloprost

Inhalational Iloprost Olschewski et al, NEJM 2002, 347: 322 -9

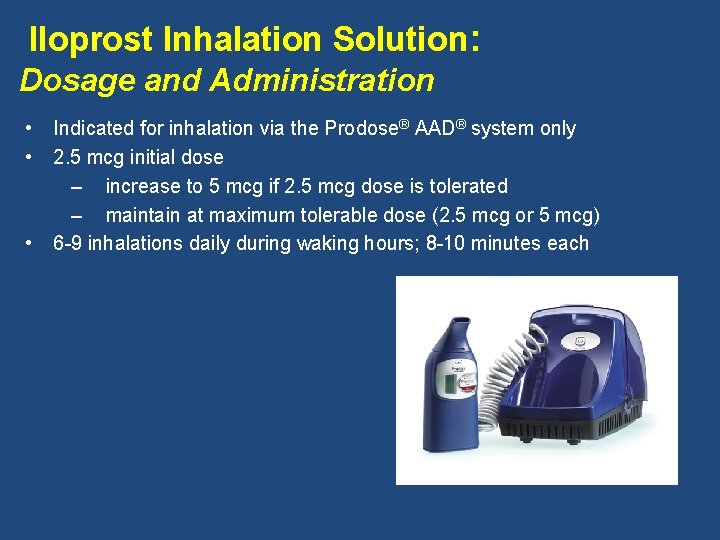
Iloprost Inhalation Solution: Dosage and Administration • • • Indicated for inhalation via the Prodose® AAD® system only 2. 5 mcg initial dose – increase to 5 mcg if 2. 5 mcg dose is tolerated – maintain at maximum tolerable dose (2. 5 mcg or 5 mcg) 6 -9 inhalations daily during waking hours; 8 -10 minutes each

Endothelin Receptor Antagonists Bosentan Ambrisentan Macitentan Indication / FC II, III, IV Administration Oral Dosage Other 62. 5 mg twice daily 5 mg and 10 mg for 4 weeks then daily 125 mg twice daily 10 mg daily Sustained receptor binding and enhanced tissue penetration


STUDY-351

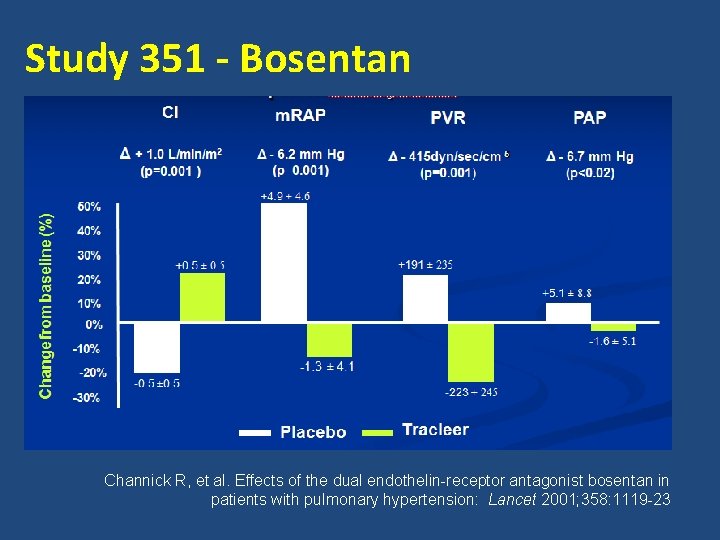
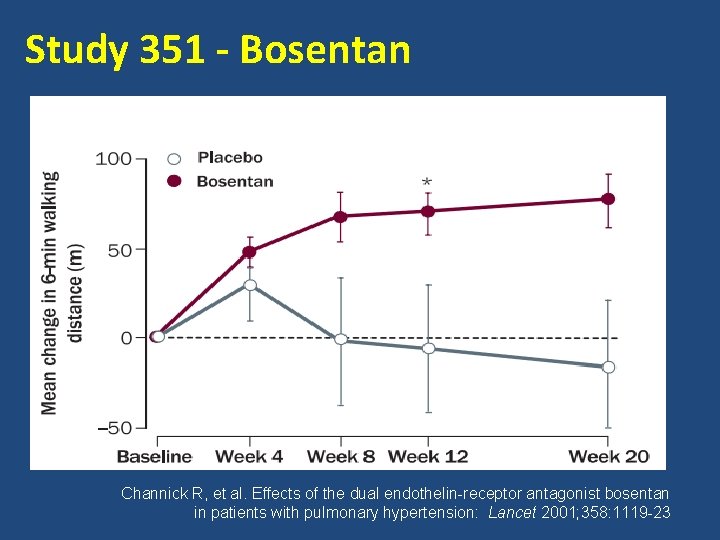
Study 351 - Bosentan Channick R, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: Lancet 2001; 358: 1119 -23

Study 351 - Bosentan Channick R, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: Lancet 2001; 358: 1119 -23

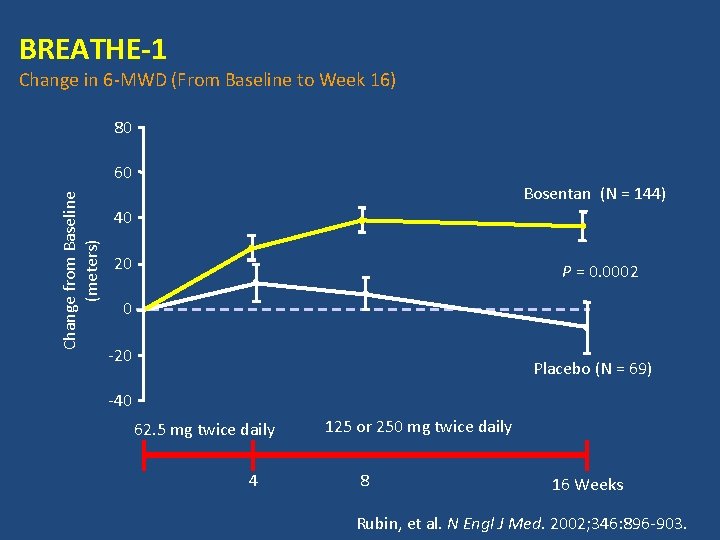
BREATHE-1

BREATHE-1 Change in 6 -MWD (From Baseline to Week 16) 80 Change from Baseline (meters) 60 Bosentan (N = 144) 40 20 P = 0. 0002 0 -20 Placebo (N = 69) -40 62. 5 mg twice daily 4 125 or 250 mg twice daily 8 16 Weeks Rubin, et al. N Engl J Med. 2002; 346: 896 -903.

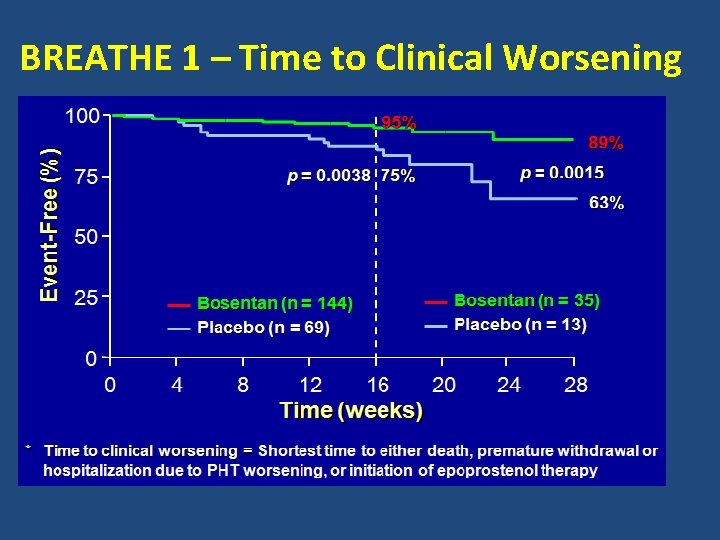
BREATHE 1 – Time to Clinical Worsening

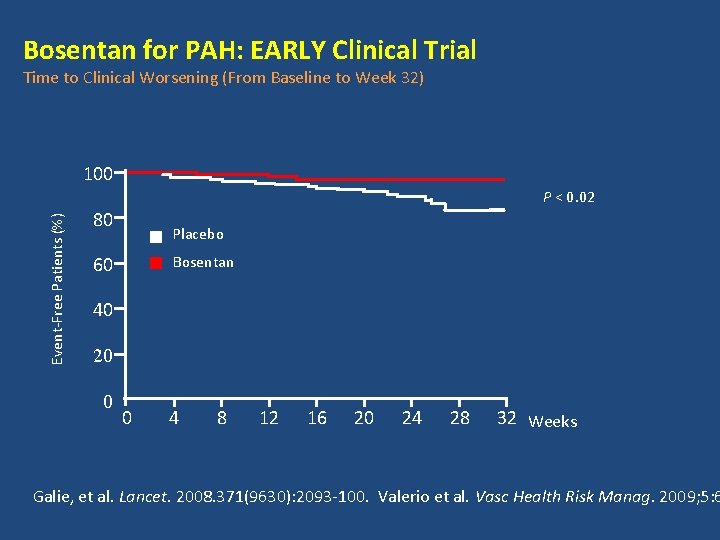
Bosentan for PAH: EARLY Clinical Trial Time to Clinical Worsening (From Baseline to Week 32) 100 Event-Free Patients (%) P < 0. 02 80 Placebo Bosentan 60 40 20 0 0 4 8 12 16 20 24 28 32 Weeks Galie, et al. Lancet. 2008. 371(9630): 2093 -100. Valerio et al. Vasc Health Risk Manag. 2009; 5: 6

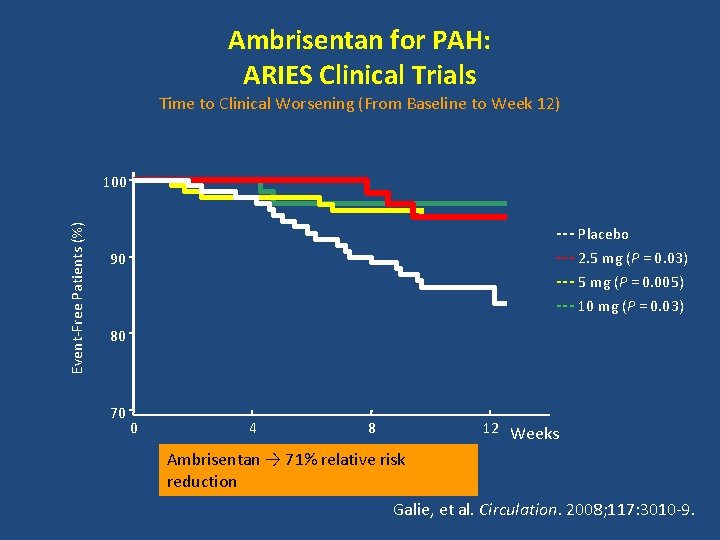
Ambrisentan for PAH: ARIES Clinical Trials Time to Clinical Worsening (From Baseline to Week 12) Event-Free Patients (%) 100 --- Placebo --- 2. 5 mg (P = 0. 03) --- 5 mg (P = 0. 005) --- 10 mg (P = 0. 03) 90 80 70 0 4 8 12 Weeks Ambrisentan → 71% relative risk reduction Galie, et al. Circulation. 2008; 117: 3010 -9.

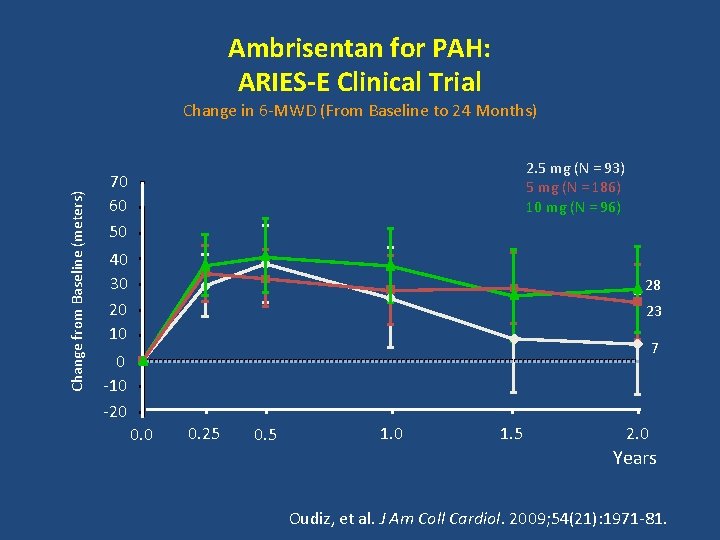
Ambrisentan for PAH: ARIES-E Clinical Trial Change from Baseline (meters) Change in 6 -MWD (From Baseline to 24 Months) 2. 5 mg (N = 93) 5 mg (N = 186) 10 mg (N = 96) 70 60 50 40 30 20 10 28 23 7 0 -10 -20 0. 25 0. 5 1. 0 1. 5 2. 0 Years Oudiz, et al. J Am Coll Cardiol. 2009; 54(21): 1971 -81.

Macitentan- SERAPHIN STUDY

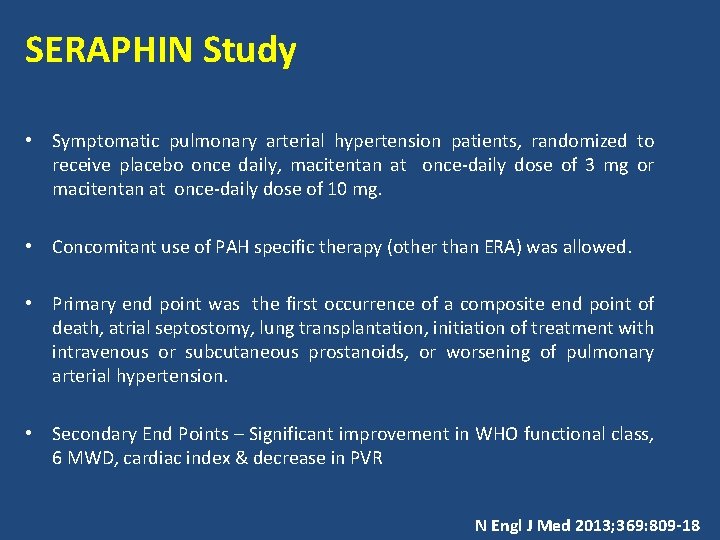
SERAPHIN Study • Symptomatic pulmonary arterial hypertension patients, randomized to receive placebo once daily, macitentan at once-daily dose of 3 mg or macitentan at once-daily dose of 10 mg. • Concomitant use of PAH specific therapy (other than ERA) was allowed. • Primary end point was the first occurrence of a composite end point of death, atrial septostomy, lung transplantation, initiation of treatment with intravenous or subcutaneous prostanoids, or worsening of pulmonary arterial hypertension. • Secondary End Points – Significant improvement in WHO functional class, 6 MWD, cardiac index & decrease in PVR N Engl J Med 2013; 369: 809 -18

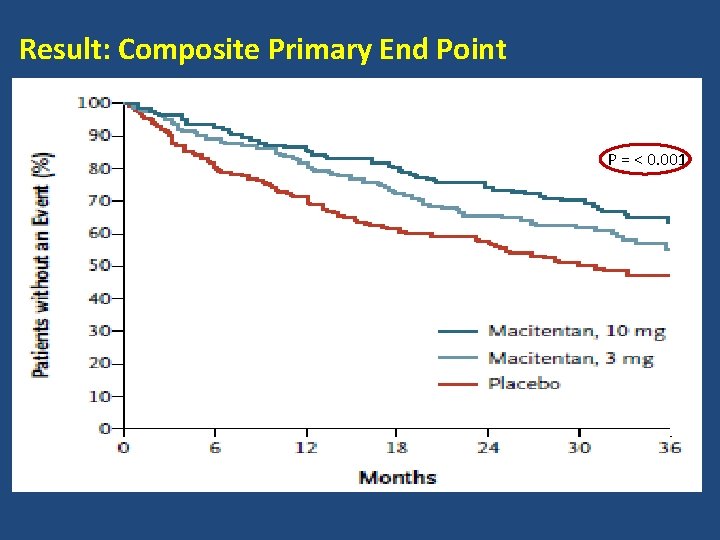
Result: Composite Primary End Point P = < 0. 001

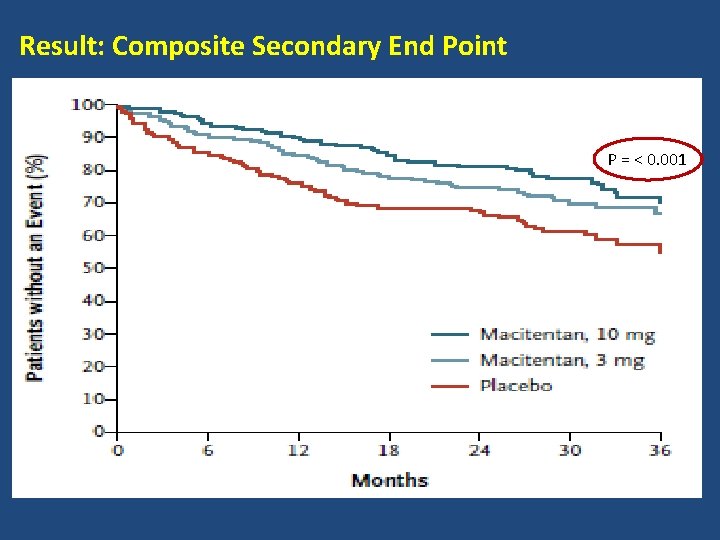
Result: Composite Secondary End Point P = < 0. 001

• Conclusion - Macitentan significantly reduced morbidity and mortality among patients with pulmonary arterial hypertension in this event-driven study. • Received US Food and Drug Administration approval in Oct 2013 for the treatment of pulmonary arterial hypertension (PAH, WHO Group I) to delay disease progression.

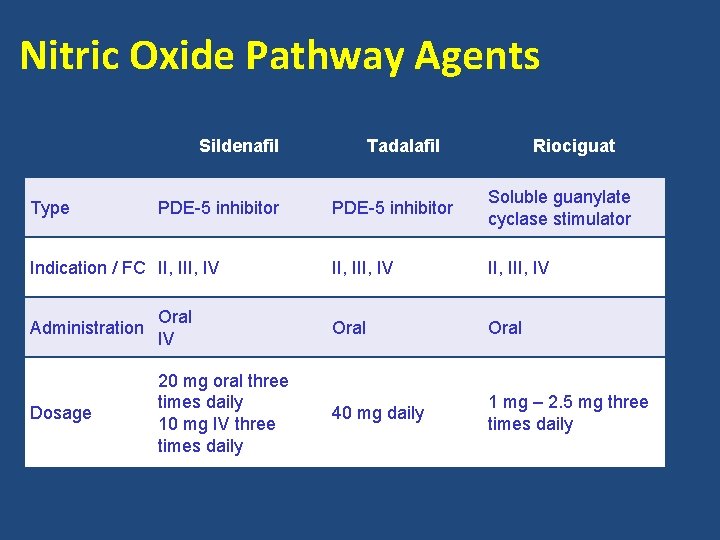
Nitric Oxide Pathway Agents Sildenafil Type PDE-5 inhibitor Indication / FC II, IV Tadalafil Riociguat PDE-5 inhibitor Soluble guanylate cyclase stimulator II, III, IV Administration Oral IV Oral Dosage 20 mg oral three times daily 10 mg IV three times daily 40 mg daily 1 mg – 2. 5 mg three times daily

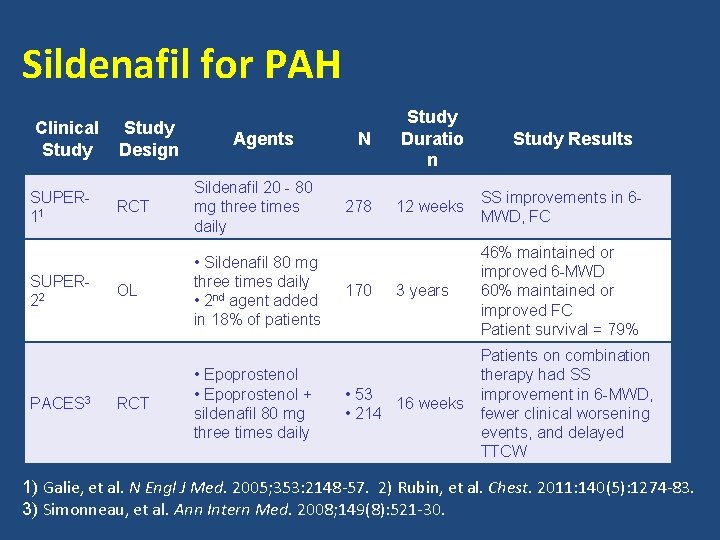
Sildenafil for PAH Clinical Study SUPER 11 SUPER 22 PACES 3 Study Design Agents RCT Sildenafil 20 - 80 mg three times daily OL • Sildenafil 80 mg three times daily • 2 nd agent added in 18% of patients RCT • Epoprostenol + sildenafil 80 mg three times daily N Study Duratio n 278 12 weeks SS improvements in 6 MWD, FC 3 years 46% maintained or improved 6 -MWD 60% maintained or improved FC Patient survival = 79% 170 • 53 16 weeks • 214 Study Results Patients on combination therapy had SS improvement in 6 -MWD, fewer clinical worsening events, and delayed TTCW 1) Galie, et al. N Engl J Med. 2005; 353: 2148 -57. 2) Rubin, et al. Chest. 2011: 140(5): 1274 -83. 3) Simonneau, et al. Ann Intern Med. 2008; 149(8): 521 -30.

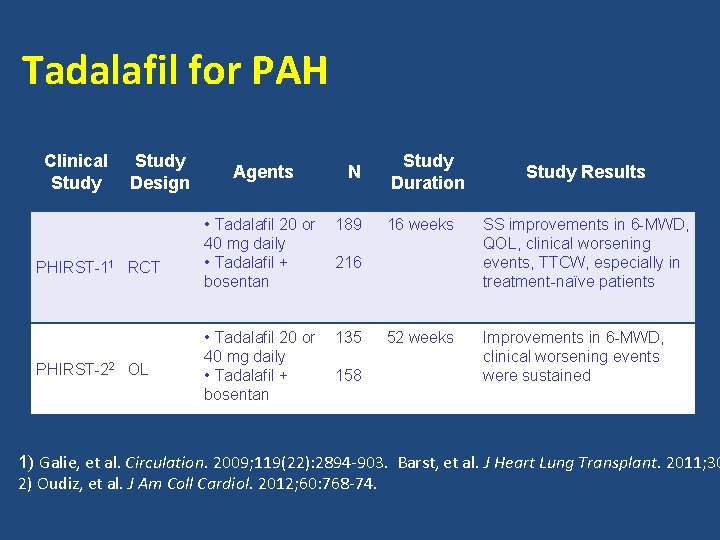
Tadalafil for PAH Clinical Study Design PHIRST-11 RCT PHIRST-22 OL Agents N • Tadalafil 20 or 40 mg daily • Tadalafil + bosentan 189 • Tadalafil 20 or 40 mg daily • Tadalafil + bosentan 135 Study Duration 16 weeks SS improvements in 6 -MWD, QOL, clinical worsening events, TTCW, especially in treatment-naïve patients 52 weeks Improvements in 6 -MWD, clinical worsening events were sustained 216 158 Study Results 1) Galie, et al. Circulation. 2009; 119(22): 2894 -903. Barst, et al. J Heart Lung Transplant. 2011; 30 2) Oudiz, et al. J Am Coll Cardiol. 2012; 60: 768 -74.

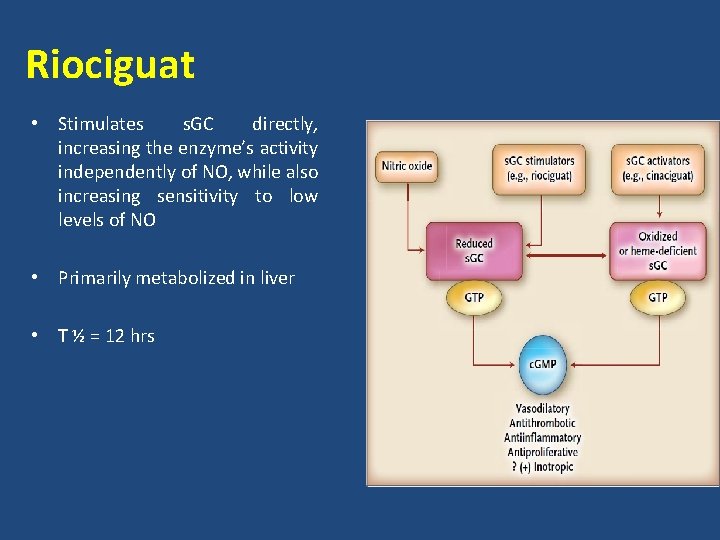
Riociguat • Stimulates s. GC directly, increasing the enzyme’s activity independently of NO, while also increasing sensitivity to low levels of NO • Primarily metabolized in liver • T ½ = 12 hrs

Riociguat • Contraindications – Pregnancy – Use with nitrates, PDEIs • Precaution – PVOD – Bleeding – Hypotension • Received US FDA approval for PAH and CTEPH in Oct 2013

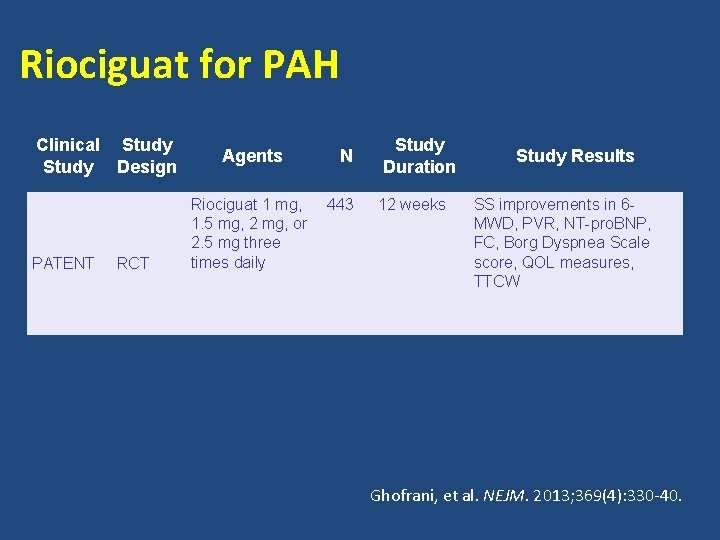
Riociguat for PAH Clinical Study Design PATENT RCT Agents Riociguat 1 mg, 1. 5 mg, 2 mg, or 2. 5 mg three times daily N 443 Study Duration 12 weeks Study Results SS improvements in 6 MWD, PVR, NT-pro. BNP, FC, Borg Dyspnea Scale score, QOL measures, TTCW Ghofrani, et al. NEJM. 2013; 369(4): 330 -40.

PATENT 1 STUDY

PATENT -1 Study • PAH (Group 1) pts randomized to – Placebo (126) – Riociguat 2. 5 mg max dose (254) – Riociguat 1. 5 mg max dose (63) • Primary end point – 6 MWD • Secondary end points – Changes in PVR, NT pro BNP, WHO functional class, Time to clinical worsening, Borg Dyspnoea Score, QOL scores • Follow up – 12 wks • Concomitant use of ERAs & prostanoids (non I/V) allowed N Engl J Med 2013; 369: 330 -40.

Results & Conclusion • Primary end point (placebo vs 2. 5 mg group) – 6 MWD had increased from baseline by a mean of 30 m in the 2. 5 mg– maximum group and had decreased by a mean of 6 m in the placebo group (P<0. 001) • Secondary end points – Significant improvement in NT pro BNP, WHO functional class, Borg Dyspnoea Score, clinical worsening events – Significant improvement in PVR, m. PAP, CI – No significant difference seen in Euro Qo. L 5 D • Conclusion : Riociguat significantly improved exercise capacity and secondary efficacy end points in patients with pulmonary arterial hypertension.

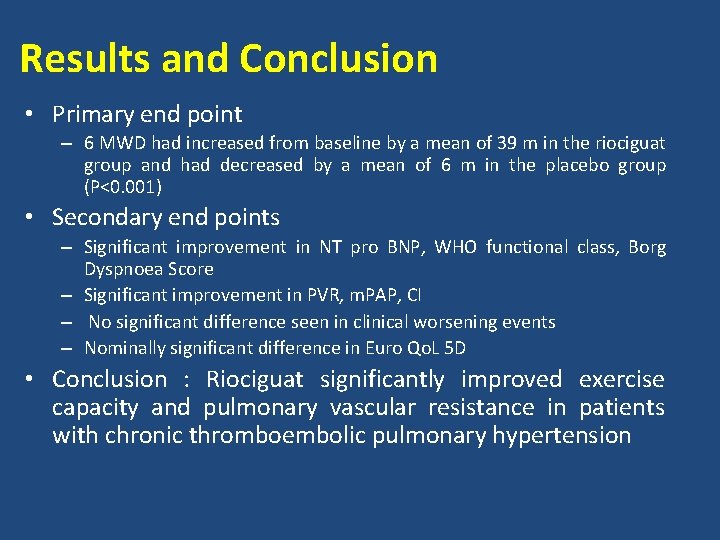
CHEST-1 • Double blind randomized 16 wk trial of Riociguat (2. 5 mg) vs Placebo recruiting 261 pts of CTEPH – Considered inoperable ( 70%) – Persistent PAH after TEA (30%) • Primary end point – 6 MWD • Secondary end points – Changes in PVR, NT pro BNP, WHO functional class, Time to clinical worsening, Borg Dyspnea Score, QOL scores N Engl J Med 2013; 369: 319 -29

Results and Conclusion • Primary end point – 6 MWD had increased from baseline by a mean of 39 m in the riociguat group and had decreased by a mean of 6 m in the placebo group (P<0. 001) • Secondary end points – Significant improvement in NT pro BNP, WHO functional class, Borg Dyspnoea Score – Significant improvement in PVR, m. PAP, CI – No significant difference seen in clinical worsening events – Nominally significant difference in Euro Qo. L 5 D • Conclusion : Riociguat significantly improved exercise capacity and pulmonary vascular resistance in patients with chronic thromboembolic pulmonary hypertension

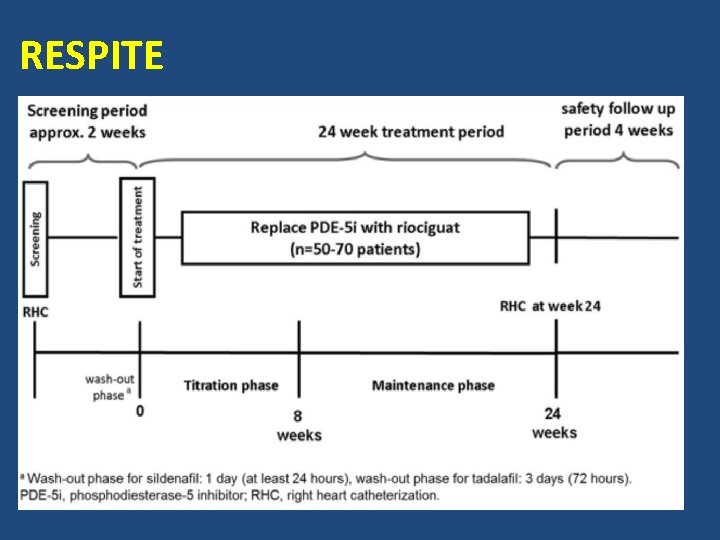
Riociguat for PAH: RESPITE Clinical Trial Study design • • Open-label N = 60 patients with poor response to a PDE-5 i Study duration = 24 weeks Study endpoints – 6 -MWD, cardiac index, NT-pro. BNP, functional class, quality of life, TTCW • Study is ongoing www. clinicaltrials. gov/ct 2/show/NCT 02007629

RESPITE

Selexipag for PAH • Latest drug to be approved. • Mechanism of action – Prostacyclin IP receptor agonist – Targets the prostacyclin pathway • Administration – oral Image: www. chemspider. com/Chemical-Structure. 8089417. html

Selexipag • Selective agonist of prostacyclin IP receptor • A phase II study, involving 43 PAH patients showed significant reductions in PVR (− 30. 3% versus placebo) compared with placebo ERJ October 1, 2012 vol. 40 no. 4 874 -880

GRIPHON

Combination Therapy for PAH Background • Rationale – to target multiple disease pathways • REVEAL: 34% of patients on 2 or more treatments Sequential Therapy • Starting in one drug class and adding an agent from another class • Used when therapy needs to be augmented because response to initial therapy is inadequate Upfront Therapy • Used in early PAH disease • May improve patient outcomes, slow disease progression, and reduce costs associated with managing clinical worsening Galie, et al. J Am Coll Cardiol. 2013; 62(25): S 60 -72. Badesch, et al. Chest. 2010; 137(2): 37



Upfront Combination Therapy: AMBITION Clinical Trial Study design • RCT • N = 500 treatment-naïve patients • Study groups – Ambrisentan – Tadalafil – Ambrisentan + tadalafil • Study duration = event driven • Primary endpoint = TTCW Study results *P < 0. 05 • Combination therapy reduced the risk of clinical failure events by 50%* • SS* improvements in: – 6 -MWD – NT-pro. BNP – % Patients with a satisfactory clinical response Galie, et al. Eur Respir J. 2014; 44(58): ABSTRACT.

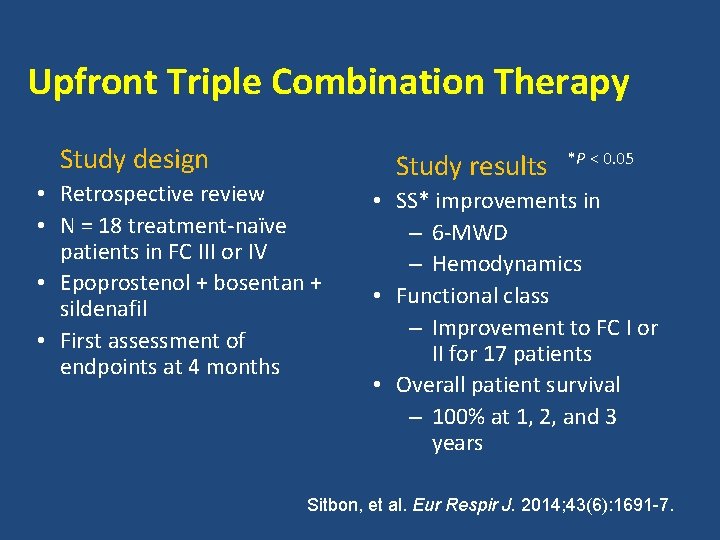
Upfront Triple Combination Therapy Study design • Retrospective review • N = 18 treatment-naïve patients in FC III or IV • Epoprostenol + bosentan + sildenafil • First assessment of endpoints at 4 months Study results *P < 0. 05 • SS* improvements in – 6 -MWD – Hemodynamics • Functional class – Improvement to FC I or II for 17 patients • Overall patient survival – 100% at 1, 2, and 3 years Sitbon, et al. Eur Respir J. 2014; 43(6): 1691 -7.

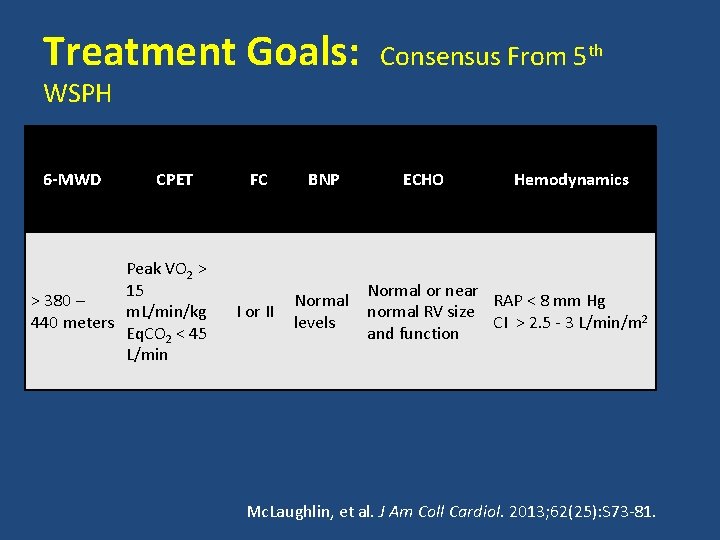
Treatment Goals: Consensus From 5 th WSPH 6 -MWD CPET Peak VO 2 > 15 > 380 – m. L/min/kg 440 meters Eq. CO 2 < 45 L/min FC BNP I or II Normal levels ECHO Hemodynamics Normal or near RAP < 8 mm Hg normal RV size CI > 2. 5 - 3 L/min/m 2 and function Mc. Laughlin, et al. J Am Coll Cardiol. 2013; 62(25): S 73 -81.

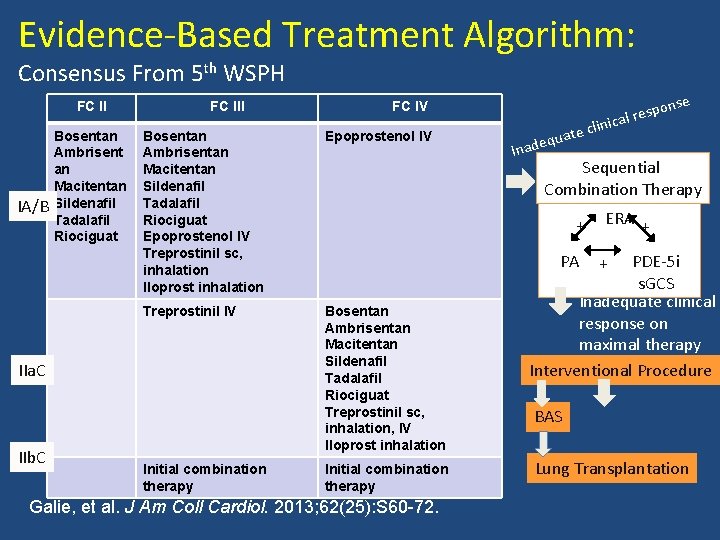
Evidence-Based Treatment Algorithm: Consensus From 5 th WSPH FC II IA/B Bosentan Ambrisent an Macitentan Sildenafil Tadalafil Riociguat FC III Bosentan Ambrisentan Macitentan Sildenafil Tadalafil Riociguat Epoprostenol IV Treprostinil sc, inhalation Iloprost inhalation Epoprostenol IV Treprostinil IV Bosentan Ambrisentan Macitentan Sildenafil Tadalafil Riociguat Treprostinil sc, inhalation, IV Iloprost inhalation IIa. C IIb. C FC IV Initial combination therapy ate dequ Ina PA Galie, et al. J Am Coll Cardiol. 2013; 62(25): S 60 -72. clini Sequential Combination Therapy + Initial combination therapy nse spo cal re ERA + PDE-5 i s. GCS Inadequate clinical response on maximal therapy Interventional Procedure + BAS Lung Transplantation

Thank you
 Dr kailash kumar goyal
Dr kailash kumar goyal Pah clearance
Pah clearance Clearance equation renal
Clearance equation renal Med
Med Pah in echo
Pah in echo Bmpr gene
Bmpr gene Ww2 military alphabet
Ww2 military alphabet Tagore international school east of kailash
Tagore international school east of kailash Conclusion of kothari commission
Conclusion of kothari commission Kailash faces towards north
Kailash faces towards north Tagore international school east of kailash parent portal
Tagore international school east of kailash parent portal Nilay goyal
Nilay goyal Vikram goyal architect
Vikram goyal architect Summary response essay introduction
Summary response essay introduction Devyani goyal
Devyani goyal Gourav goyal
Gourav goyal How to convert paper br to electronic batch record
How to convert paper br to electronic batch record Ashok kumar pandey iit hyderabad
Ashok kumar pandey iit hyderabad Mohak kumar
Mohak kumar Deepak kumar bryn mawr
Deepak kumar bryn mawr Xshhz
Xshhz Kamal kumar ips
Kamal kumar ips G ravindra kumar
G ravindra kumar Kalyan kumar hcl
Kalyan kumar hcl Ravi kumar kopparapu
Ravi kumar kopparapu Uiuc cs 445
Uiuc cs 445 Ranjana kumar gavi
Ranjana kumar gavi Pavan kumar vijay
Pavan kumar vijay Kumar mangalam birla committee
Kumar mangalam birla committee Kumar mangalam birla committee
Kumar mangalam birla committee Dr anuj kumar tripathi neurosurgeon
Dr anuj kumar tripathi neurosurgeon Kumar venkitanarayanan
Kumar venkitanarayanan Kumar is producing the photoelectric effect by using
Kumar is producing the photoelectric effect by using Kumar raj kharel
Kumar raj kharel Radial palmar grasp
Radial palmar grasp Kumar n. sivarajan
Kumar n. sivarajan Emani kumar
Emani kumar Anil kumar polsani mainframe
Anil kumar polsani mainframe Akshay kumar assistant
Akshay kumar assistant Eprg framework
Eprg framework Medisep hospitals in kerala
Medisep hospitals in kerala Dr pulin kumar gupta
Dr pulin kumar gupta Rohtash kumar vs state of haryana
Rohtash kumar vs state of haryana Rakesh kumar ntnu
Rakesh kumar ntnu Tan steinbach kumar
Tan steinbach kumar Jay kumar kar
Jay kumar kar Senthil kumar palanisamy
Senthil kumar palanisamy Saturn
Saturn Ravi kumar sacramento
Ravi kumar sacramento Santosh kumar swain kiit
Santosh kumar swain kiit Ca sripriya kumar
Ca sripriya kumar Management assertions
Management assertions Kumar
Kumar Dr v kumar
Dr v kumar Dr. pradip kumar khastagir
Dr. pradip kumar khastagir Sunesh kumar aiims
Sunesh kumar aiims Kumar satish ravi
Kumar satish ravi Dr alok kumar pandey
Dr alok kumar pandey Akshay kumar
Akshay kumar Mahendra kumar fiji
Mahendra kumar fiji Dilip kumar
Dilip kumar Ibmqx
Ibmqx Bedford interview skills
Bedford interview skills Alok kumar jagadev
Alok kumar jagadev Alok kumar jagadev
Alok kumar jagadev Chapter 9 kumar steinbach tan
Chapter 9 kumar steinbach tan Kumar
Kumar Is life of pi a true story
Is life of pi a true story Prof. dr. pradeep kumar gupta
Prof. dr. pradeep kumar gupta Pyloric stenosis
Pyloric stenosis Manas kumar chaudhuri
Manas kumar chaudhuri Vikas kumar
Vikas kumar Hamiltonian formulation of general relativity
Hamiltonian formulation of general relativity Kumar samrudhi society
Kumar samrudhi society Manas kumar chaudhuri
Manas kumar chaudhuri Chapter 9 kumar steinbach tan
Chapter 9 kumar steinbach tan Rfm
Rfm Dr ashish kumar bhutani
Dr ashish kumar bhutani Krishnan kumar bajaj vs pepsico
Krishnan kumar bajaj vs pepsico Hari ram kumar
Hari ram kumar Rajesh kumar bhagat
Rajesh kumar bhagat Kumar
Kumar Vikas kumar
Vikas kumar Saha chapter 1
Saha chapter 1 Samir kumar khanal
Samir kumar khanal Nirupama prakash kumar
Nirupama prakash kumar Arwin kumar
Arwin kumar Srinidhi sampath kumar
Srinidhi sampath kumar