Chapter 11 Intermolecular Forces IMF forces that exist























- Slides: 43

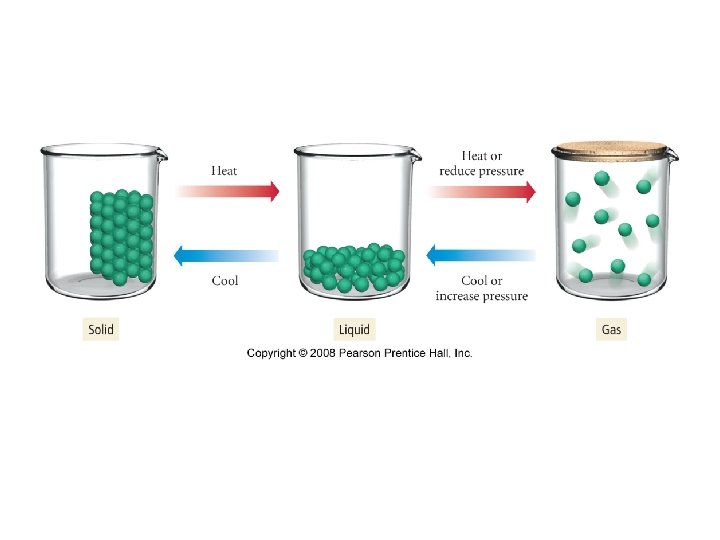
Chapter 11 Intermolecular Forces (IMF)- forces that exist between molecules Gases -IMF not strong enough to hold particles together -assumes shape and volume of container -expands to fill container -compressible -flow readily -diffusion within a gas occurs readily

Liquids -IMF strong enough to hold particles close together -assumes shape of container -does not expand to fill container -incompressible -flows readily -diffusion within a liquid occurs slowly -known as a condensed phase b/c particles are fairly close together

Solids -IMF very strong- virtually locks particles in place -has definite shape and volume -incompressible -does not flow -diffusion within a solid occurs slowly -known as a condensed phase

-states of matter can be changed by heating or cooling -changes the average KE EX-dec the temp of a gas will dec KE and form a liquid and then lock particles in place to form a solid -inc pressure of a gas can bring molecules closer together to form a liquid and then a solid


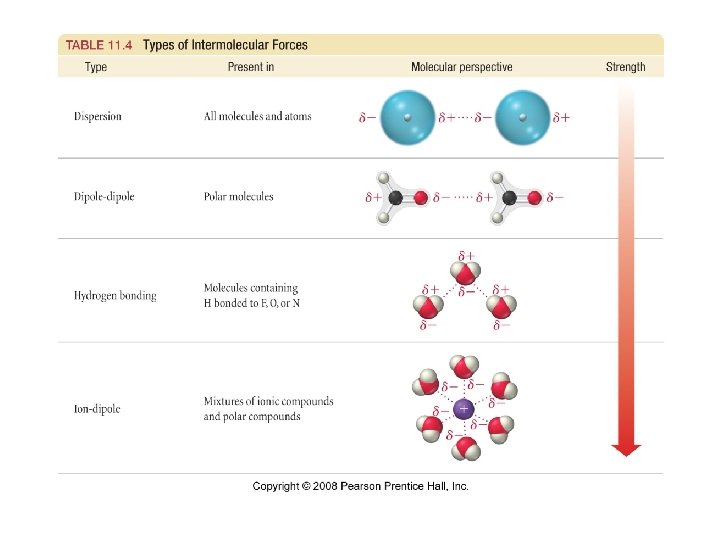
IMF -usually much weaker than intramolecular forces (ionic, metallic or covalent bonds) -less energy is required to change a state of matter than to break a bond -boiling/melting pt. inc with stronger IMF -page 428 Table 11. 2

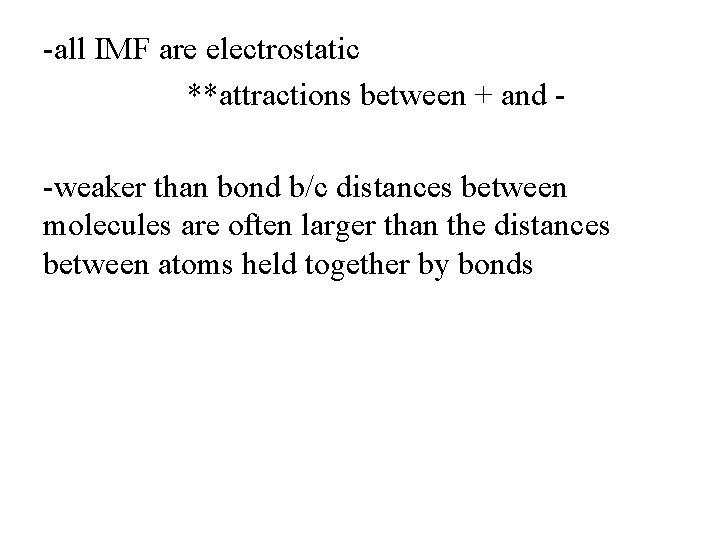
-all IMF are electrostatic **attractions between + and -weaker than bond b/c distances between molecules are often larger than the distances between atoms held together by bonds

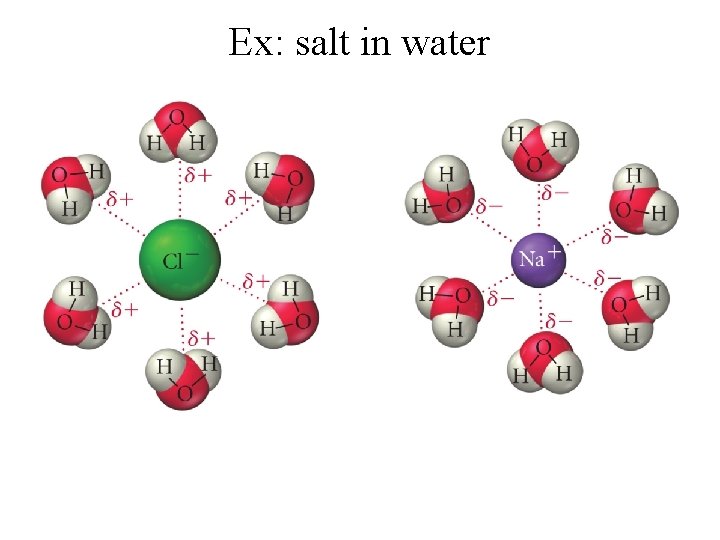
Ion-Dipole Forces -exists between an ion and a polar molecule -cations are attracted to - end of a dipole -anions are attracted to + end -magnitude of attraction inc as either the ionic charge or the magnitude of the dipole inc -strongest IMF *only found in ionic compounds dissolved in solution

Ex: salt in water

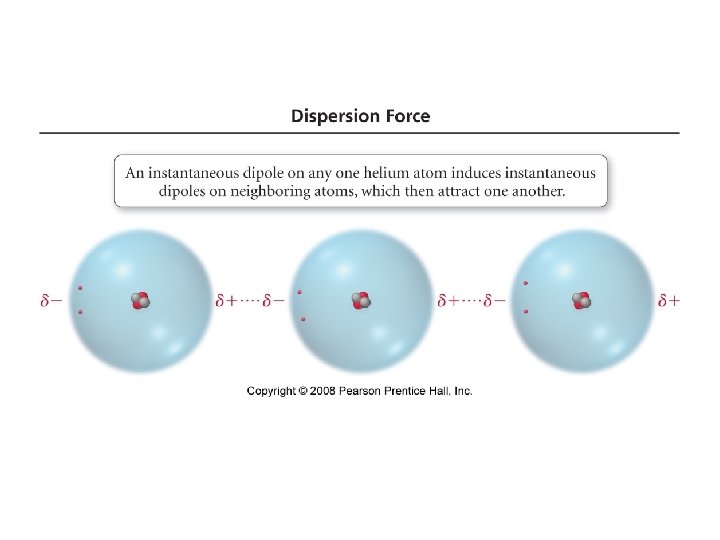
Dispersion Forces (London Dispersion) -present in all molecules and atoms -weakest IMF -caused by motion of e- and instantaneous dipole moment -can cause an instantaneous dipole moment on an adjacent atom, causing the atoms to be attracted -page 429 Fig 11. 4

-fluctuations in the electron distribution in atoms and molecules result in a temporary dipole – region with excess electron density has partial (─) charge – region with depleted electron density has partial (+) charge


-strength depends on ease with which the charge distribution can be distorted -called polarizability -the greater the polarizability the more easily an e- cloud can be distorted to give an instantaneous dipole -more polarizable molecules have larger dispersion forces -polarizability inc. as the # of e- in an atom or molecule inc. -strength inc. with inc. molecular weight

-shape also influences- longer straight chains are stronger b/c molecules can come into contact along entire molecule -more compact = less contact

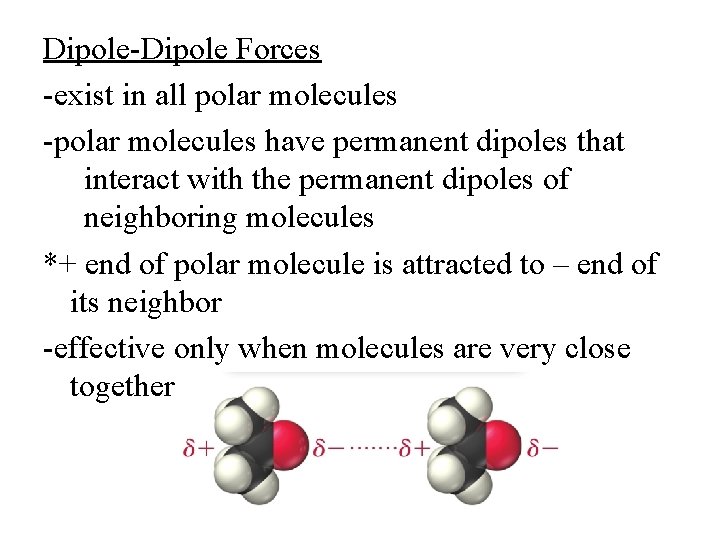
Dipole-Dipole Forces -exist in all polar molecules -polar molecules have permanent dipoles that interact with the permanent dipoles of neighboring molecules *+ end of polar molecule is attracted to – end of its neighbor -effective only when molecules are very close together

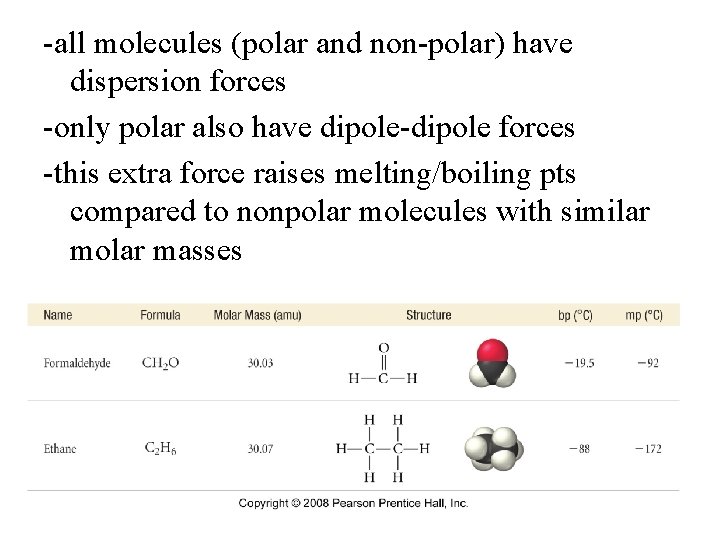
-all molecules (polar and non-polar) have dispersion forces -only polar also have dipole-dipole forces -this extra force raises melting/boiling pts compared to nonpolar molecules with similar molar masses

-for molecules of about equal mass and size, the strength of IMF inc. with inc. polarity Ex: CH 2 CN vs. CH 2 CH 3 Boiling pt. = 355 K and 231 K Mass= 40 amu and 43 amu *close masses so dispersion forces are similar *higher bp in CH 2 CN due to dipole-dipole forces -page 431 Figure 11. 8

Which of the following have dipole-dipole forces? 1) CO 2 -has polar bonds, but is nonpolar b/c it is linear no dipole-dipole forces 2) CH 2 Cℓ 2 -has polar bonds and net dipole has dipole-dipole forces 3) CH 4 -has nonpolar bonds no dipole-dipole forces

Hydrogen Bonding -occurs when polar molecules have H bonded to very electronegative atoms (N, O, and F) -H atom is attracted to nonbonding e- pair on another similar molecule -causes strong interaction between H and other atoms (N, O and F) in other molecules -second strongest IMF -substances with H-bonding have higher melting/boiling pts -water exhibits very strong H-bonding and this explains its behavior

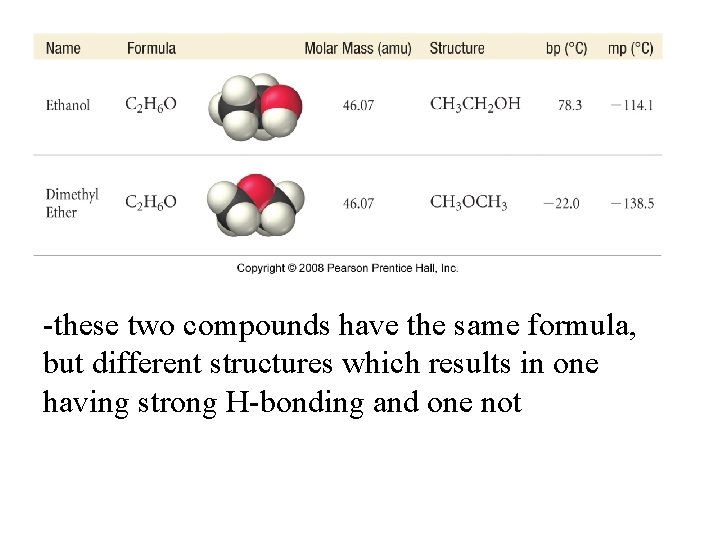
-these two compounds have the same formula, but different structures which results in one having strong H-bonding and one not

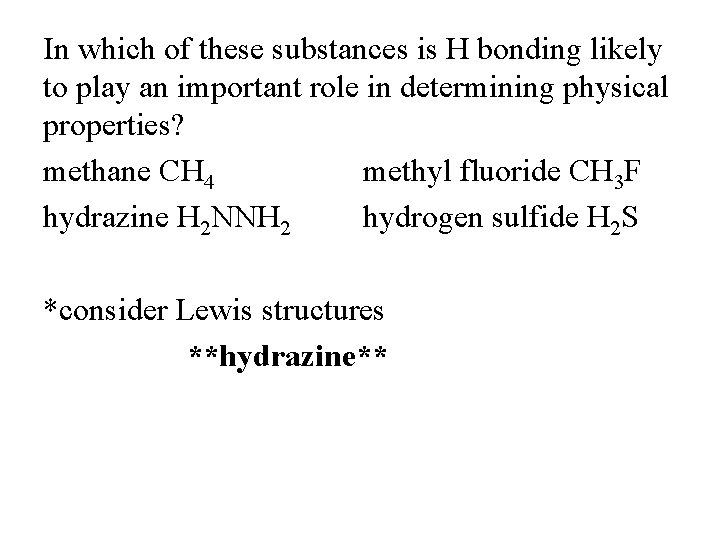
In which of these substances is H bonding likely to play an important role in determining physical properties? methane CH 4 methyl fluoride CH 3 F hydrazine H 2 NNH 2 hydrogen sulfide H 2 S *consider Lewis structures **hydrazine**

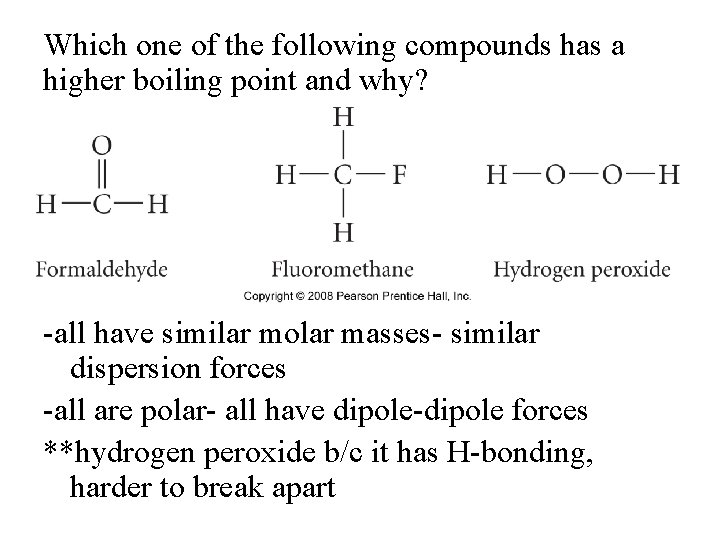
Which one of the following compounds has a higher boiling point and why? -all have similar molar masses- similar dispersion forces -all are polar- all have dipole-dipole forces **hydrogen peroxide b/c it has H-bonding, harder to break apart


List the substances Ba. Cℓ 2, H 2, CO, HF, and Ne in order of inc boiling point. * Ba. Cℓ 2 is ionic *all others have dispersion forces *MW= 2, 28, 19. 9, 20. 2 *CO and HF have dipole-dipole b/c polar *HF has H-bonding H 2 < Ne < CO < HF < Ba. Cℓ 2

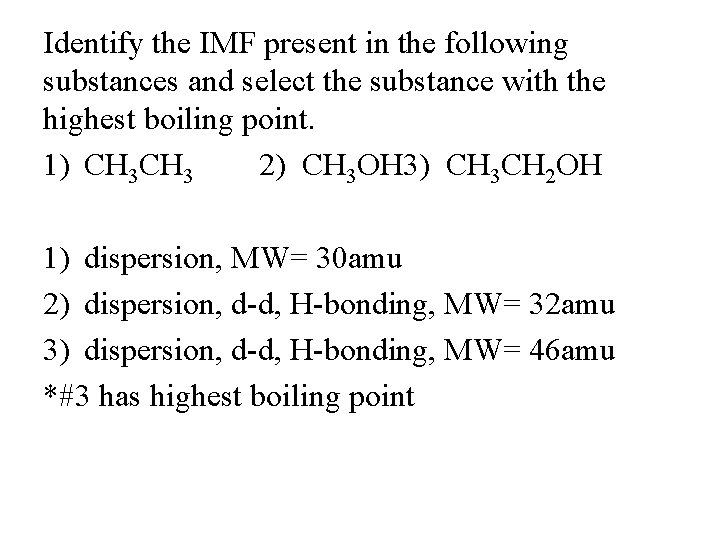
Identify the IMF present in the following substances and select the substance with the highest boiling point. 1) CH 3 2) CH 3 OH 3) CH 3 CH 2 OH 1) dispersion, MW= 30 amu 2) dispersion, d-d, H-bonding, MW= 32 amu 3) dispersion, d-d, H-bonding, MW= 46 amu *#3 has highest boiling point

Results of IMF 1) viscosity- the resistance of a liquid to flow -the higher the viscosity, the more slowly it flows -increases with greater IMF (or molar mass) b/c molecules cannot flow as easily -also depends on molecular shape- higher in longer molecules b/c they can become entangled -temp plays a part- the higher the temp, the lower the viscosity- higher KE can overcome the IMF

2) surface tension- inward force or pull that tends to minimize the surface area of a liquid -energy required to inc the surface area of a liquid by a unit amount -molecules with increased IMF have increased surface tension -water has an extremely high surface tension b/c of the strong H-bonding ex- forming spherical droplets, things being able to float on water -it is difficult to break the H-bonds -will dec by adding a surfactant (soap)

3) capillary action- ability of liquid to flow against gravity up a narrow tube cohesive forces- bind similar molecules to one another adhesive forces- bind a substance to a surface -if adhesive are greater than cohesive then the liquid will be drawn up -if cohesive are greater than adhesive than liquid does not rise -shape of meniscus determined by cohesion and adhesion

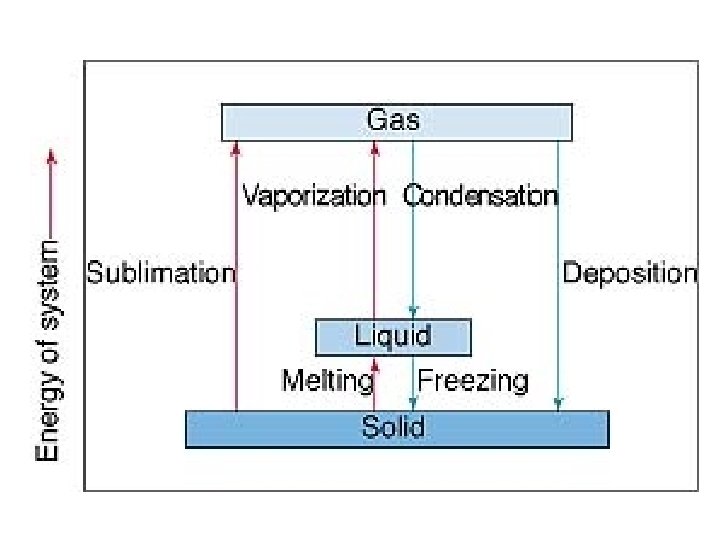
Phase Changes heat of fusion (∆Hfus) -amount of heat required to melt one mole of a solid - endothermic= + heat of solidification (∆Hsolid) -amount of heat required to solidify one mole of a liquid -exothermic= -same magnitude as ∆Hfus, but opposite sign b/c energy is given off

heat of vaporization (∆Hvap) -amount of heat required to vaporize one mole of a liquid - endothermic heat of condensation (∆Hcond) -amount of heat required to condense one mole of a gas to a liquid -exothermic -same magnitude as ∆Hvap, but opposite sign b/c energy is given off

heat of sublimation (∆Hsub) -goes from solid to gas without passing through liquid phase ∆Hsub= ∆Hfus + ∆Hvap -endothermic ex- frozen foods heat of deposition (∆Hdep) -gas to solid skipping liquid phase -exothermic -same magnitude as ∆Hsub, but opposite sign b/c energy is given off


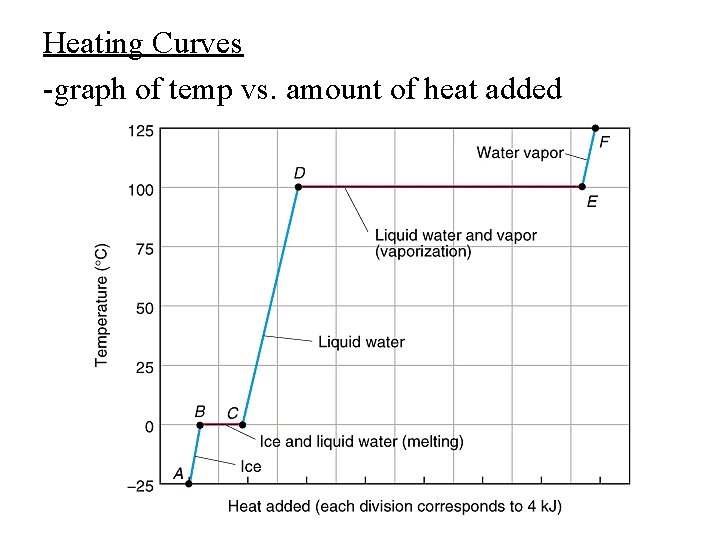
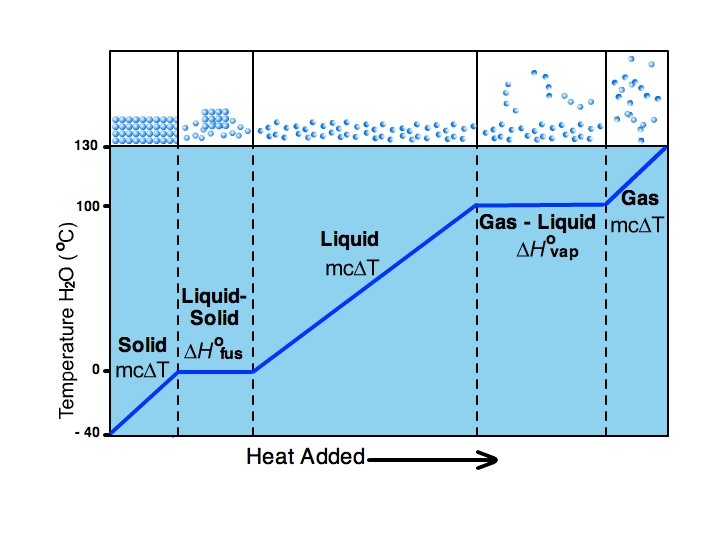
Heating Curves -graph of temp vs. amount of heat added

-can calculate enthalpy change of a system for each segment of the heating curve -in AB, CD, and EF a single phase is heated from one phase to another use ∆H = m. C∆T -for BC use ∆Hfus -for DE use ∆Hvap *energy used up to increase distance between particles


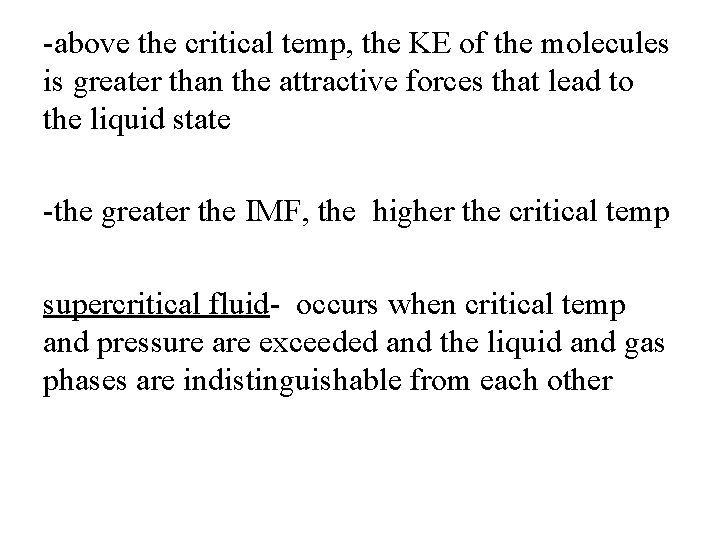
-a gas normally liquefies at some point when pressure is applied -if temp inc, pressure must inc even more -if temp reaches a certain point no amount of pressure can cause a liquid to form -at that point, as pressure inc and the gas becomes more steadily compressed critical temp- the highest temp at which a distinct liquid phase can form critical pressure- pressure required to bring about liquefaction at this critical temp

-above the critical temp, the KE of the molecules is greater than the attractive forces that lead to the liquid state -the greater the IMF, the higher the critical temp supercritical fluid- occurs when critical temp and pressure are exceeded and the liquid and gas phases are indistinguishable from each other

vapor pressure- pressure above the surface of the liquid, causes evaporation -will be lower with stronger IMF -if a container is sealed, the liquid will still vaporize and condense, just not into the atmosphere -when rate of condensation equals rate of vaporization the liquid has reached dynamic equilibrium -vapor pressure of a liquid is the pressure exerted by its vapor when liquid and vapor are in dynamic equilibrium

-equilibrium never occurs when vaporization happens in an open container volatile- liquids that evaporate easily -have high vapor pressure ex- nail polish remover nonvolatile- liquids that do not vaporize easily -have low vapor pressure ex- motor oil -vapor pressure inc with inc temp- particles move more and can escape to gas phase

-a liquid boils when vapor pressure equals the external pressure acting on the liquid surface normal boiling point- boiling point of a liquid at 1 atm *the higher the pressure the higher the boiling pt *food takes longer to cook at higher elevations WHY? -pressure is lower, lowering boiling point of water below 100°C, taking longer to cook

phase diagram- summarizes conditions under which equilibria exists between the different states of matter -can be used to predict which phase of a substance is present at any given temp and pressure -page 445 Fig 11. 27

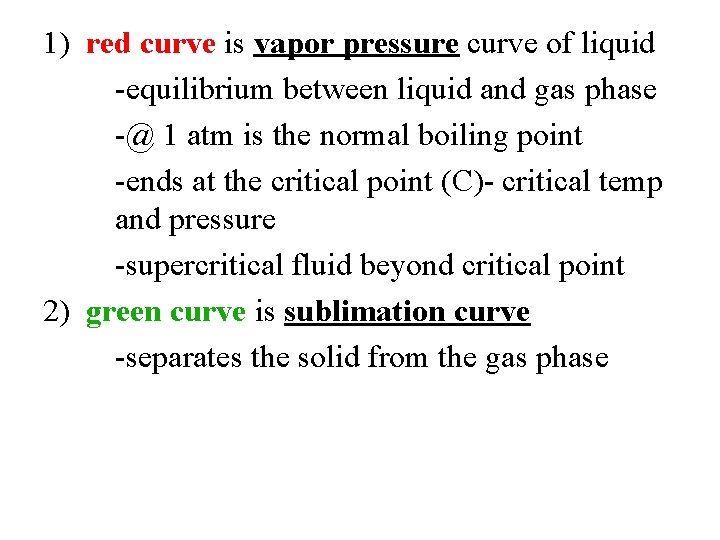
1) red curve is vapor pressure curve of liquid -equilibrium between liquid and gas phase -@ 1 atm is the normal boiling point -ends at the critical point (C)- critical temp and pressure -supercritical fluid beyond critical point 2) green curve is sublimation curve -separates the solid from the gas phase

3) blue curve is melting curve -separates solid phase from liquid phase -usually slopes to the right as pressure inc b/c most solids are denser than their liquids -@ 1 atm is the normal melting point triple point -point T, where three curves intersect -all three phases exist in equilibrium