CHAPTER 11 Gases Properties of Gases were the













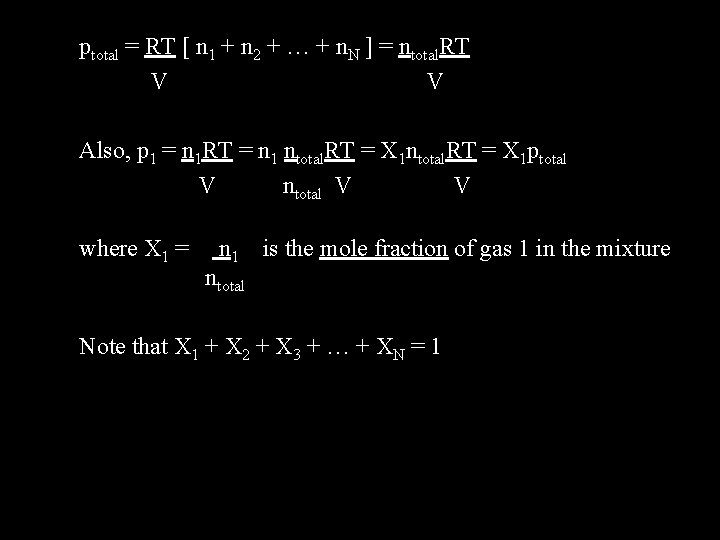










- Slides: 38

CHAPTER 11 Gases

Properties of Gases were the first state of matter extensively studied. This is because small changes in temperature and pressure lead to measurable changes in volume, and because gases, to a good first approximation, behave in a simple manner.

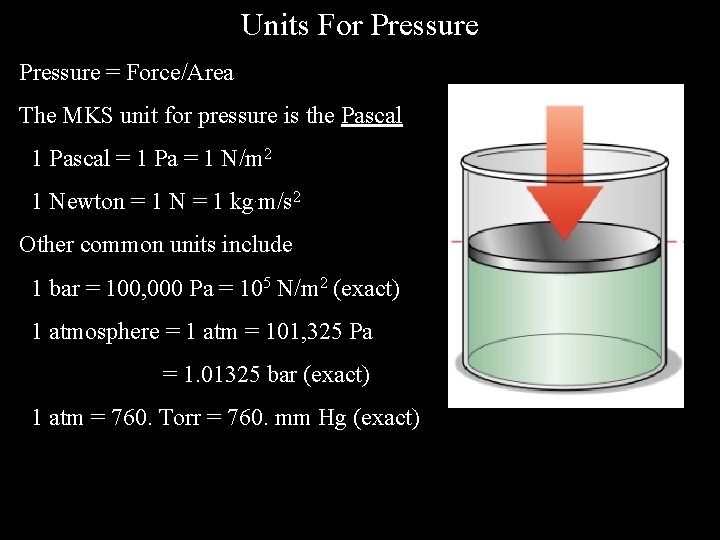
Units For Pressure = Force/Area The MKS unit for pressure is the Pascal 1 Pascal = 1 Pa = 1 N/m 2 1 Newton = 1 N = 1 kg. m/s 2 Other common units include 1 bar = 100, 000 Pa = 105 N/m 2 (exact) 1 atmosphere = 1 atm = 101, 325 Pa = 1. 01325 bar (exact) 1 atm = 760. Torr = 760. mm Hg (exact)

Barometer A barometer is a device for measuring atmospheric pressure. In a barometer pressure = force/area = hdg h = height (m) d = density (kg/m 3) g = gravitational constant (9. 807 m/s 2) The pressure unit atmosphere (atm) represent the approximate value for the pressure of the Earth’s atmosphere at sea level. The unit torr (mm Hg) comes from the most commonly used barometer for experimentally measuring pressure, the mercury barometer. 1 torr = 1 mm Hg (exact) d(Hg) = 13. 53 g/cm 3

Manometer A manometer is a device for measuring differences in pressure. In a mercury manometer h (in mm Hg) = pgas - patm

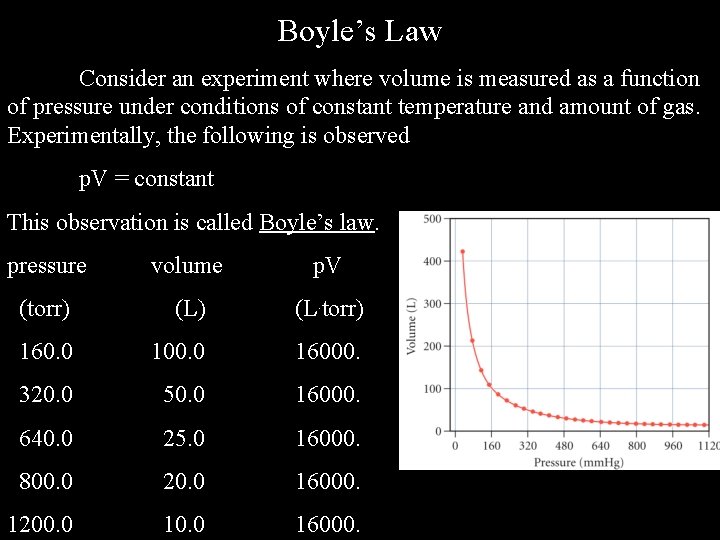
Boyle’s Law Consider an experiment where volume is measured as a function of pressure under conditions of constant temperature and amount of gas. Experimentally, the following is observed p. V = constant This observation is called Boyle’s law. pressure volume p. V (torr) (L. torr) 160. 0 100. 0 16000. 320. 0 50. 0 16000. 640. 0 25. 0 16000. 800. 0 20. 0 16000. 1200. 0 16000.

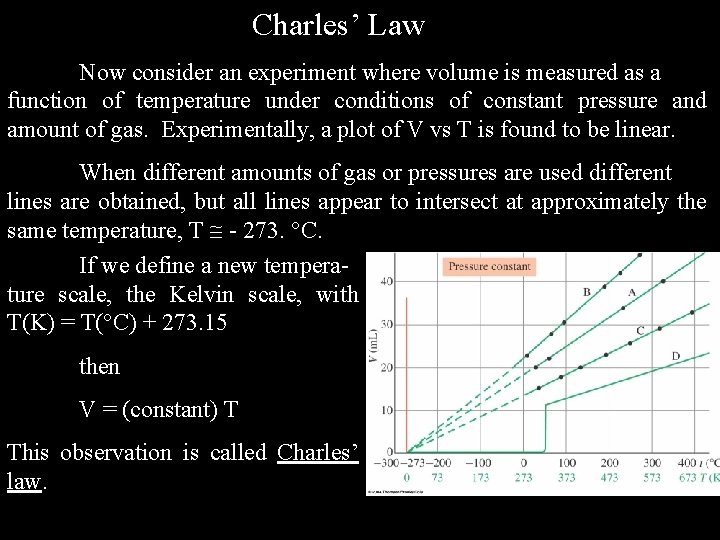
Charles’ Law Now consider an experiment where volume is measured as a function of temperature under conditions of constant pressure and amount of gas. Experimentally, a plot of V vs T is found to be linear. When different amounts of gas or pressures are used different lines are obtained, but all lines appear to intersect at approximately the same temperature, T - 273. C. If we define a new temperature scale, the Kelvin scale, with T(K) = T( C) + 273. 15 then V = (constant) T This observation is called Charles’ law.

Relationships From Boyle’s Law and Charles’ Law Useful relationships can be derived from Boyle’s law and Charles’ law. Boyle’s law. If n, T = constant, then p. V = constant. Therefore pi. Vi = pf. Vf Charles’ law. If n, p = constant, then V ~ T (or V = c. T, where c is a constant). From this, it follows that V/T = constant, or Vi/Ti = Vf/Tf. Example: A sample of gas at T = 300. K and p = 1. 00 atm occupies a volume V = 586. m. L. What volume will the gas occupy if the pressure is changed to 5. 00 atm while keeping temperature constant?

Example: A sample of gas at T = 300. K and p = 1. 00 atm occupies a volume V = 586. m. L. What volume will the gas occupy if the pressure is changed to 5. 00 atm while keeping temperature constant? Since n, T are constant, it follows that pi. Vi = pf. Vf Vf = Vi (pi/pf) = (586. m. L) (1. 00 atm/5. 00 atm) = 117. m. L

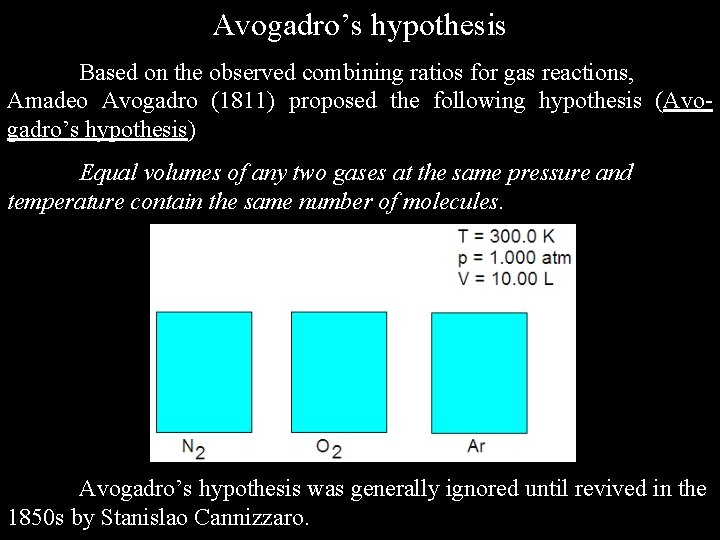
Avogadro’s hypothesis Based on the observed combining ratios for gas reactions, Amadeo Avogadro (1811) proposed the following hypothesis (Avogadro’s hypothesis) Equal volumes of any two gases at the same pressure and temperature contain the same number of molecules. Avogadro’s hypothesis was generally ignored until revived in the 1850 s by Stanislao Cannizzaro.

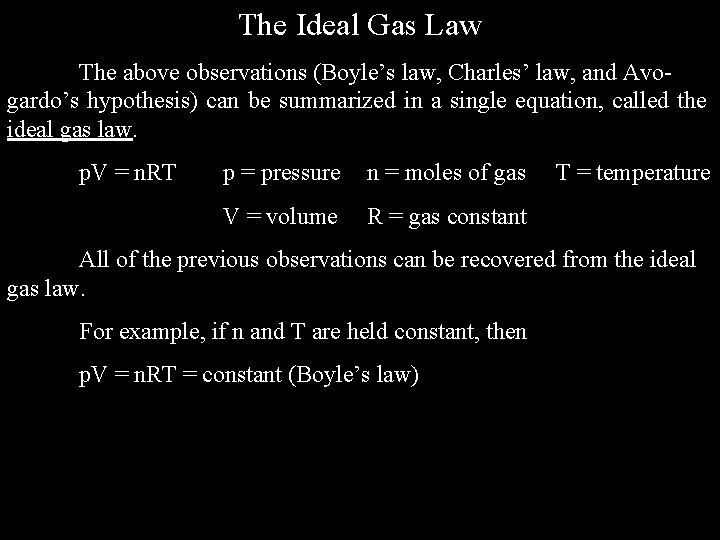
The Ideal Gas Law The above observations (Boyle’s law, Charles’ law, and Avogardo’s hypothesis) can be summarized in a single equation, called the ideal gas law. p. V = n. RT p = pressure n = moles of gas V = volume R = gas constant T = temperature All of the previous observations can be recovered from the ideal gas law. For example, if n and T are held constant, then p. V = n. RT = constant (Boyle’s law)

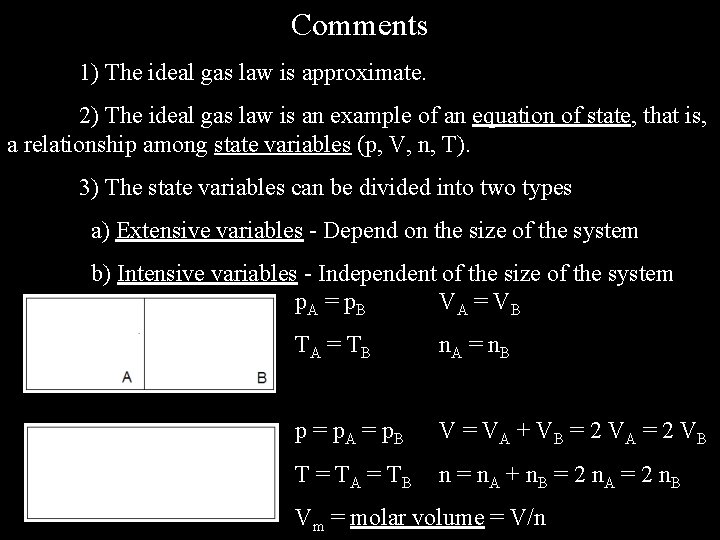
Comments 1) The ideal gas law is approximate. 2) The ideal gas law is an example of an equation of state, that is, a relationship among state variables (p, V, n, T). 3) The state variables can be divided into two types a) Extensive variables - Depend on the size of the system b) Intensive variables - Independent of the size of the system p. A = p. B VA = V B TA = T B n. A = n. B p = p. A = p. B V = VA + VB = 2 VA = 2 VB T = T A = TB n = n. A + n. B = 2 n. A = 2 n. B Vm = molar volume = V/n

4) The ideal gas law is based on two underlying assumptions a) The volume occupied by the gas molecules is small b) Attractive forces between gas molecules are small These assumptions become true in the following limits V (T, n held constant) p 0 (T, n held constant) T (p, n held constant) 5) The units for the gas constant, R, depend on the units used for p, V, and n. R = p. V/n. T can be used to determine the units. R = 8. 314 J/mol. K = (MKS unit) R = 0. 08314 L. bar/mol. K R = 0. 08206 L. atm/mol. K (1 J = 1 kg. m 2/s 2)

Sample Problems 1) A sample of N 2 gas with mass m = 8. 238 g is confined in a container of volume V = 2. 000 L at a temperature T = 30. 0 C. What is the pressure exerted by the gas? Give your answer in atm and torr. 2) A glass bulb has a mass m = 49. 618 g when empty and m = 51. 203 g when filled with an unknown pure gas. The volume of the bulb is V = 1. 283 L. The measurements are made at p = 0. 978 atm and T = 21. 5 C. What is the molecular mass of the gas?

1) A sample of N 2 gas with mass m = 8. 238 g is confined in a container of volume V = 2. 000 L at a temperature T = 30. 0 C. What is the pressure exerted by the gas? Give your answer in atm and torr. p. V = n. RT ; so p = n. RT M(N 2) = 28. 01 g/mol V n = 8. 238 g 1 mol = 0. 2941 mol T = 30. 0 C = 303. 2 K 28. 01 g p = (0. 2941 mol) (0. 08206 L. atm/mol. K) (303. 2 K) = 3. 659 atm 2. 000 L In units of torr p = 3. 659 atm 760 torr = 2781 torr 1 atm

2) A glass bulb has a mass m = 49. 618 g when empty and m = 51. 203 g when filled with an unknown pure gas. The volume of the bulb is V = 1. 283 L. The measurements are made at p = 0. 978 atm and T = 21. 5 C. What is the molecular mass of the gas? M = m/n mgas = mfilled - mempty = 51. 203 g - 49. 618 g = 1. 585 g p. V = n. RT ; so n = p. V RT n= T = 21. 5 C = 294. 6 K (0. 978 atm) (1. 283 L) = 0. 05190 mol (0. 08206 L. atm/mol. K) (294. 6 K) So M = m = 1. 585 g = 30. 5 g/mol n 0. 05190 mol

Density (d) is defined as d = m/V For solids and liquids density is approximately independent of temperature. For gases, density depends strongly on the conditions for which it is measured. Gas density is often reported at STP, standard temperature and pressure, which is T = 0 C = 273. 15 K p = 1. 000 atm Note that the molar volume for an ideal gas at STP is V = RT = (0. 08206 L. atm/mol. K) (273. 15 K) = 22. 41 L/mol n p (1. 000 atm)

Density and Molecular Mass The density of a gas can be related to its molecular mass. Since p. V = n. RT m = n. M n = m/M So p. V = m. RT M M = m. RT = m RT = d. RT p. V V p p So by measuring the density of a gas for known conditions of pressure and temperature, the molecular mass of the gas can be found. We can also rearrange the above equation to solve for other variables such as density, temperature, or pressure.

Reactions Involving Gases Since p. V = n. RT, then V = n. RT/p For gases at a particular pressure and temperature, n ~ V. We can therefore interpret a balanced chemical equation involving gases either in terms of moles of gas or volume of gas. For example 2 CO(g) + O 2(g) 2 CO 2(g) can be interpreted in two ways. CO 2. 1) 2 moles of CO will react with 1 mole of O 2 to form 2 moles of 2) 2 liters of CO will react with 1 liter of O 2 to form 2 liters of CO 2 (assuming the gases obey the ideal gas law, and are at the same temperature and pressure).

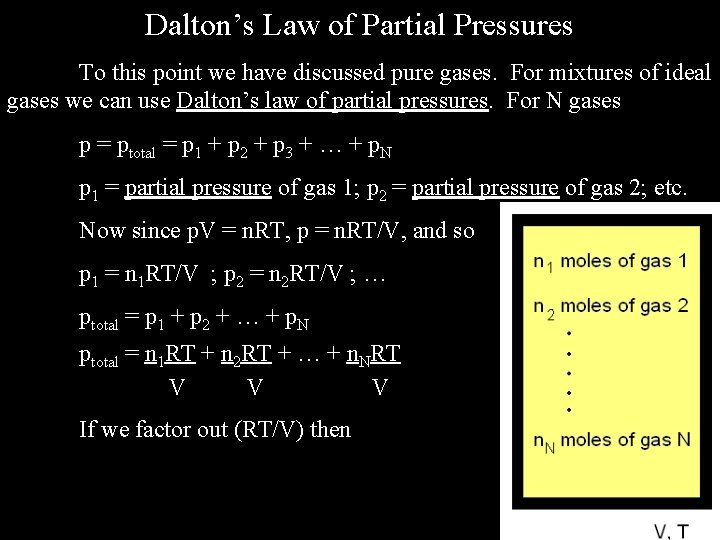
Dalton’s Law of Partial Pressures To this point we have discussed pure gases. For mixtures of ideal gases we can use Dalton’s law of partial pressures. For N gases p = ptotal = p 1 + p 2 + p 3 + … + p. N p 1 = partial pressure of gas 1; p 2 = partial pressure of gas 2; etc. Now since p. V = n. RT, p = n. RT/V, and so p 1 = n 1 RT/V ; p 2 = n 2 RT/V ; … ptotal = p 1 + p 2 + … + p. N ptotal = n 1 RT + n 2 RT + … + n. NRT V V V If we factor out (RT/V) then
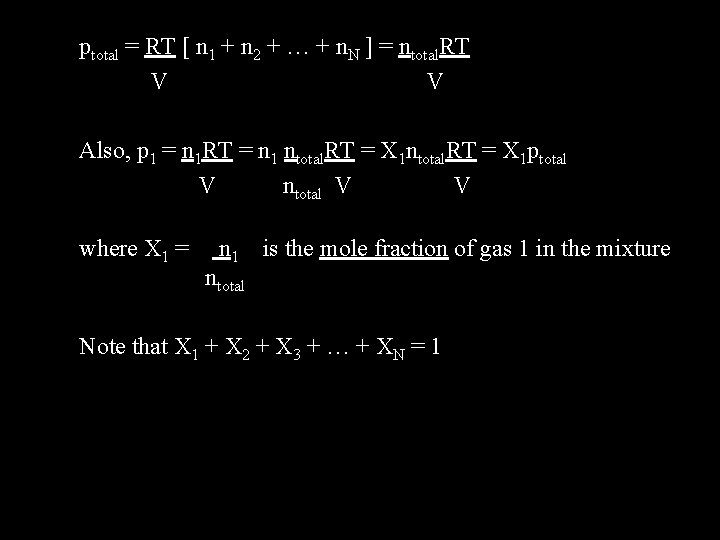
ptotal = RT [ n 1 + n 2 + … + n. N ] = ntotal. RT V V Also, p 1 = n 1 RT = n 1 ntotal. RT = X 1 ptotal V ntotal V V where X 1 = n 1 is the mole fraction of gas 1 in the mixture ntotal Note that X 1 + X 2 + X 3 + … + XN = 1

Sample Problem A gas mixture is prepared by mixing together 10. 00 g of nitrogen (N 2, M = 28. 01 g/mol) and 10. 00 g of argon (Ar, M = 39. 95 g/mol). The gas is confined in a container with volume V = 20. 00 L. The total pressure of the gas mixture is ptotal = 0. 8488 atm. Find the following: 1) Partial pressure of N 2 and Ar in the gas mixture. 2) Temperature of the gas mixture.

A gas mixture is prepared by mixing together 10. 00 g of nitrogen (N 2, M = 28. 01 g/mol) and 10. 00 g of argon (Ar, M = 39. 95 g/mol). The gas is confined in a container with volume V = 20. 00 L. The total pressure of the gas mixture is ptotal = 0. 8488 atm. Find the following: 1) Partial pressure of N 2 and Ar in the gas mixture. 2) Temperature of the gas mixture. moles N 2 = 10. 00 g N 2 1 mol N 2 = 0. 3570 mol N 2 28. 01 g N 2 moles Ar = 10. 00 g Ar 1 mol Ar = 0. 2503 mol Ar 39. 95 g Ar Total number of moles of gas = 0. 3570 mol + 0. 2503 mol = 0. 6073 mol X(N 2) = 0. 3570 mol = 0. 5878 0. 6073 mol X(Ar) = 0. 2503 mol = 0. 4122 0. 6073 mol

From Dalton’s law, pi = Xi ptotal, so p(N 2)= (0. 5878)(0. 8488 atm) = 0. 4989 atm p(Ar) = (0. 4122)(0. 8488 atm) = 0. 3499 atm Finally, since p. V = n. RT, it follows that T = p. V/n. R so T = (0. 8488 atm)(20. 00 L) (0. 6073 mol)(0. 08206 L. atm/mol. K) = 340. 6 K

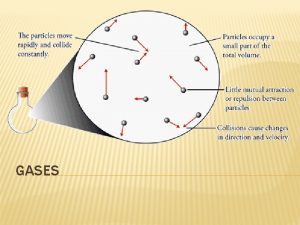
Kinetic Theory The above description of gas behavior is based on experimental observation. However, by use of kinetic theory, a description of the behavior of gases on a molecular level, the above equations can be derived. Assumptions: 1) Gases are composed of molecules in random motion. 2) The volume occupied by the molecules is small. 3) The interaction forces between molecules are weak. 4) Collisions between molecules, or between a molecule and the walls of the container, are elastic (total kinetic energy remains constant).

Average Speed and Kinetic Energy Based on the above assumptions the following equations can be derived for urms, the root mean square average speed and (KE)ave, the average kinetic energy of a gas molecule urms = [3 RT/M]1/2 (KE)ave = (3/2)RT (per mole of gas) Note the following: 1) For a particular value of temperature all gases have the same value for average kinetic energy. 2) urms ~ T 1/2 urms ~ (1/M)1/2 So gas speeds increase as temperature increases, and at a particular temperature light gases move faster (on average) than heavy gases. So, at 300. K, one mole of helium (M = 4. 0 g/mol) and one mole of methane (M = 16. 0 g/mol) have the same average amount of kinetic energy, but the helium atoms, on average, move twice as fast as the methane molecules.

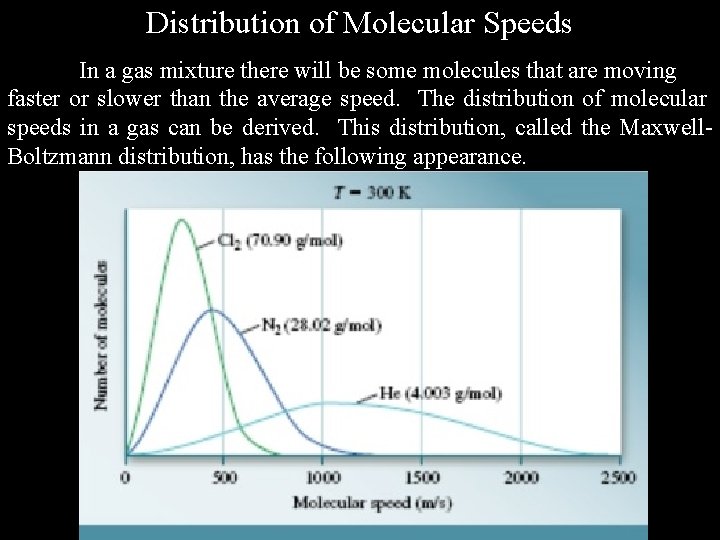
Distribution of Molecular Speeds In a gas mixture there will be some molecules that are moving faster or slower than the average speed. The distribution of molecular speeds in a gas can be derived. This distribution, called the Maxwell. Boltzmann distribution, has the following appearance.

Temperature Dependence of the Maxwell-Boltzmann Distribution As temperature increases the peak in the Maxwell-Boltzmann distribution shifts to higher speeds, and the height of the distribution decreases.

Example: What is the average speed of an N 2 (MW = 28. 01 g/mol) molecule at T = 300. K and at T = 1000. K?

Example: What is the average speed of an N 2 (MW = 28. 01 g/mol) molecule at T = 300. K and at T = 1000. K? urms = [3 RT/M]1/2 At T = 300. K urms = [3 (8. 314 J/mol. K) (300. K)/ (28. 01 x 10 -3 kg/mol)]1/2 = 517. m/s (1160 mph) At T = 1000. K urms = [3 (8. 314 J/mol. K) (1000. K)/ (28. 01 x 10 -3 kg/mol)]1/2 = 944. m/s Notice we use R in MKS units (J/mol. K), and M in units of kg/mol.

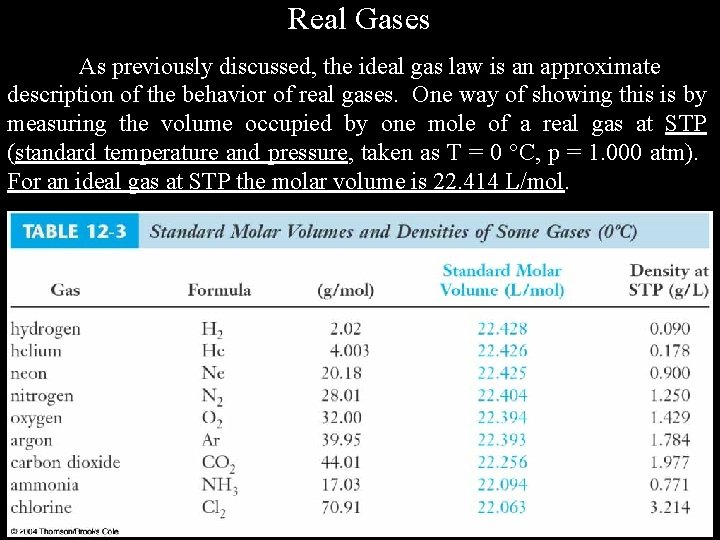
Real Gases As previously discussed, the ideal gas law is an approximate description of the behavior of real gases. One way of showing this is by measuring the volume occupied by one mole of a real gas at STP (standard temperature and pressure, taken as T = 0 C, p = 1. 000 atm). For an ideal gas at STP the molar volume is 22. 414 L/mol.

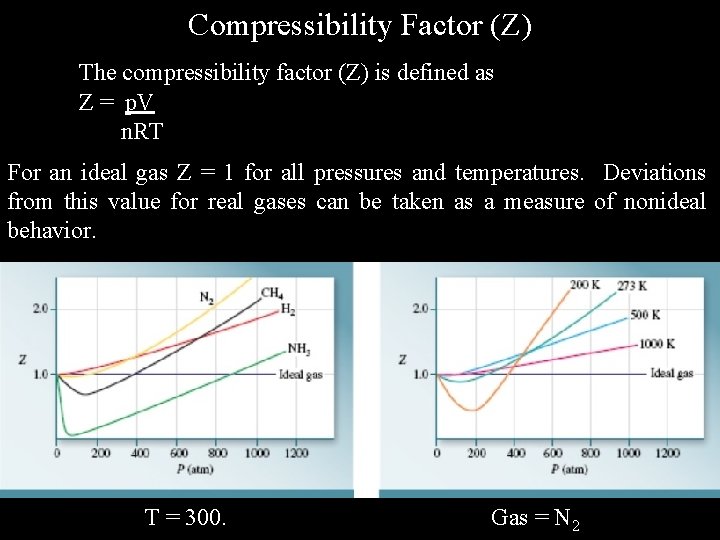
Compressibility Factor (Z) The compressibility factor (Z) is defined as Z = p. V n. RT For an ideal gas Z = 1 for all pressures and temperatures. Deviations from this value for real gases can be taken as a measure of nonideal behavior. T = 300. Gas = N 2

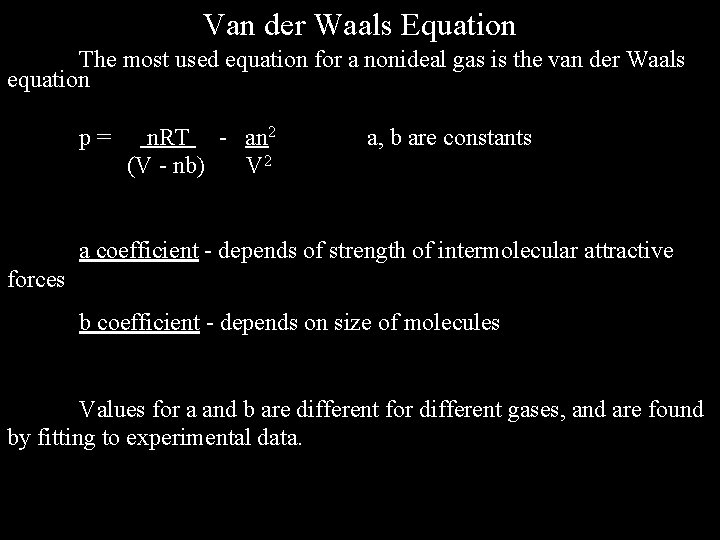
Van der Waals Equation The most used equation for a nonideal gas is the van der Waals equation p= n. RT - an 2 (V - nb) V 2 a, b are constants a coefficient - depends of strength of intermolecular attractive forces b coefficient - depends on size of molecules Values for a and b are different for different gases, and are found by fitting to experimental data.

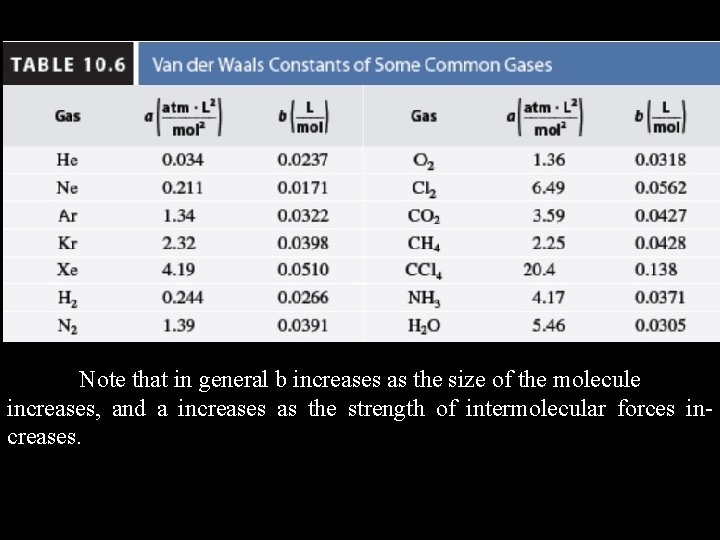
Note that in general b increases as the size of the molecule increases, and a increases as the strength of intermolecular forces increases.

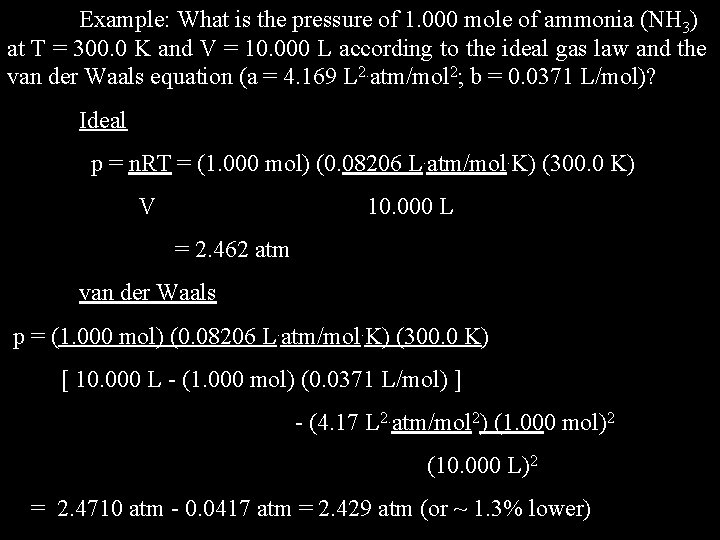
Example: What is the pressure of 1. 000 mole of ammonia (NH 3) at T = 300. 0 K and V = 10. 000 L according to the ideal gas law and according to the van der Waals equation (a = 4. 17 L 2. atm/mol 2; b = 0. 0371 L/mol)? Ideal gas law p = n. RT V van der Waals equation p = n. RT - an 2 (V - nb) V 2

Example: What is the pressure of 1. 000 mole of ammonia (NH 3) at T = 300. 0 K and V = 10. 000 L according to the ideal gas law and the van der Waals equation (a = 4. 169 L 2. atm/mol 2; b = 0. 0371 L/mol)? Ideal p = n. RT = (1. 000 mol) (0. 08206 L. atm/mol. K) (300. 0 K) V 10. 000 L = 2. 462 atm van der Waals p = (1. 000 mol) (0. 08206 L. atm/mol. K) (300. 0 K) [ 10. 000 L - (1. 000 mol) (0. 0371 L/mol) ] - (4. 17 L 2. atm/mol 2) (1. 000 mol)2 (10. 000 L)2 = 2. 4710 atm - 0. 0417 atm = 2. 429 atm (or ~ 1. 3% lower)

So V(ideal) = 2. 462 atm V(van der Waals) = 2. 429 atm Which volume is correct? The result from the van der Waals equation is likely to be closer to the correct (experimental) value because the van der Waals equation usually does a better job of predicting pressure, volume, etc. than the ideal gas law. However, since all gas laws are approximate, it is incorrect to say that one result is wrong and the other result is right. It is better to say that the van der Waals equation does a better job of predicting these quantities than the ideal gas law.

End of Chapter 11 “. . . equal volumes of gases, at the same temperature and pressure, contain the same number of molecules. ” - Amadeo Avogadro “ Two attacks on Boyle’s work were immediately published, one by Thomas Hobbes…and the other by…Franciscus Linus. Hobbs based his criticism on the physical impossibility of a vacuum (“A vacuum is nothing, and what is nothing cannot exist”). Linus claimed that the mercury column (in the barometer) was held up by an invisible thread, which fastened itself to the upper end of the tube. The theory seemed quite reasonable, he said, for anyone could easily feel the pull of the thread by covering the end of the barometer tube with his finger. ” - John Moore, Physical Chemistry
 Buoyancyability
Buoyancyability Solid
Solid General properties of gases
General properties of gases Four properties of gases
Four properties of gases 5 properties of gases
5 properties of gases Properties of gases
Properties of gases Noble gases general properties
Noble gases general properties What are the different properties of gas?
What are the different properties of gas? Gases have low densities
Gases have low densities What is noble gas
What is noble gas Liquid information
Liquid information Charles's law
Charles's law Properties of gases
Properties of gases List 2 of the important properties of gases
List 2 of the important properties of gases Was/were going to and was/were supposed to
Was/were going to and was/were supposed to Extensive vs intensive quantity
Extensive vs intensive quantity Physical properties and chemical properties
Physical properties and chemical properties Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worm breton
Tư thế worm breton Hát lên người ơi alleluia
Hát lên người ơi alleluia Các môn thể thao bắt đầu bằng tiếng nhảy
Các môn thể thao bắt đầu bằng tiếng nhảy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng xinh xinh thế chỉ nói điều hay thôi
Cái miệng xinh xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ