Chapter 11 Properties of Gases Gases have a

















- Slides: 49

Chapter 11: Properties of Gases • Gases have a number of properties that are very different from liquids and solids: 1) Gases are compressible 2) Gases exert a pressure 3) Gas pressure depends on the amount of confined gas 4) Gases fill their container 5) Gases mix freely with each other 6) Gas pressure increases with temperature

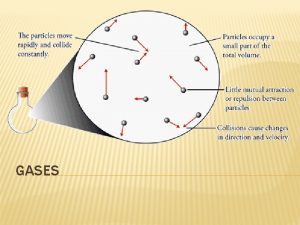
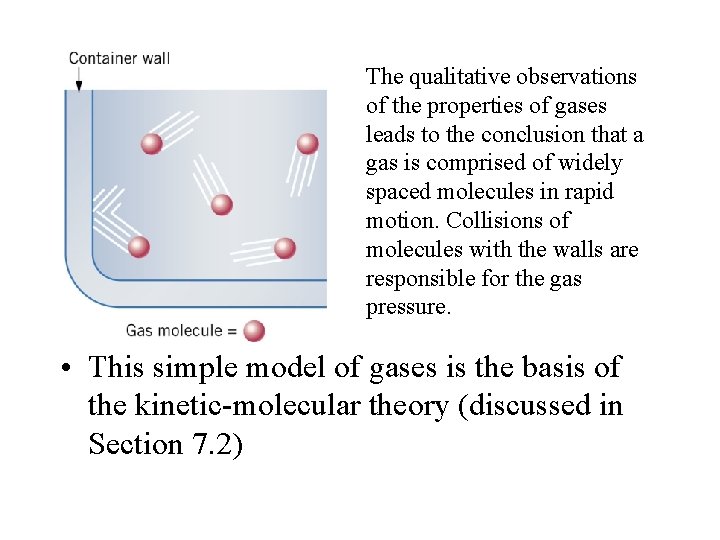
The qualitative observations of the properties of gases leads to the conclusion that a gas is comprised of widely spaced molecules in rapid motion. Collisions of molecules with the walls are responsible for the gas pressure. • This simple model of gases is the basis of the kinetic-molecular theory (discussed in Section 7. 2)

• Recall the pressure is a force per unit area • The earth exerts a gravitational force on everything with mass near it • What we call weight is the gravitational force acting on an object • The pressure due to air molecules colliding with an object is called the atmospheric pressure

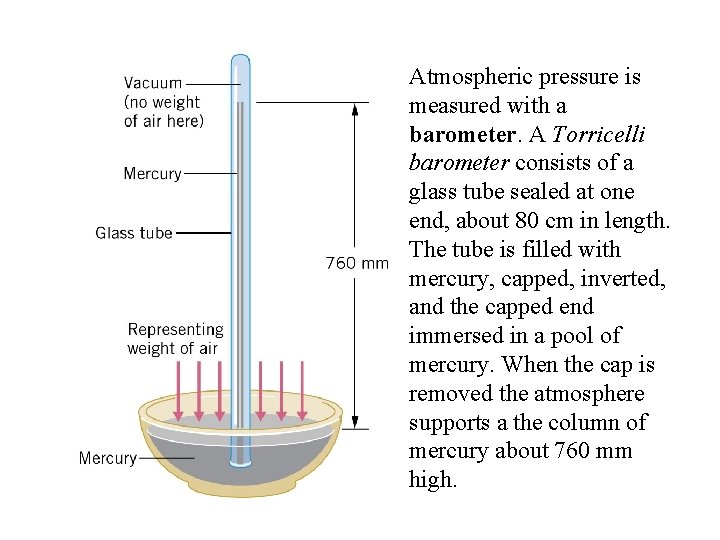
Atmospheric pressure is measured with a barometer. A Torricelli barometer consists of a glass tube sealed at one end, about 80 cm in length. The tube is filled with mercury, capped, inverted, and the capped end immersed in a pool of mercury. When the cap is removed the atmosphere supports a the column of mercury about 760 mm high.

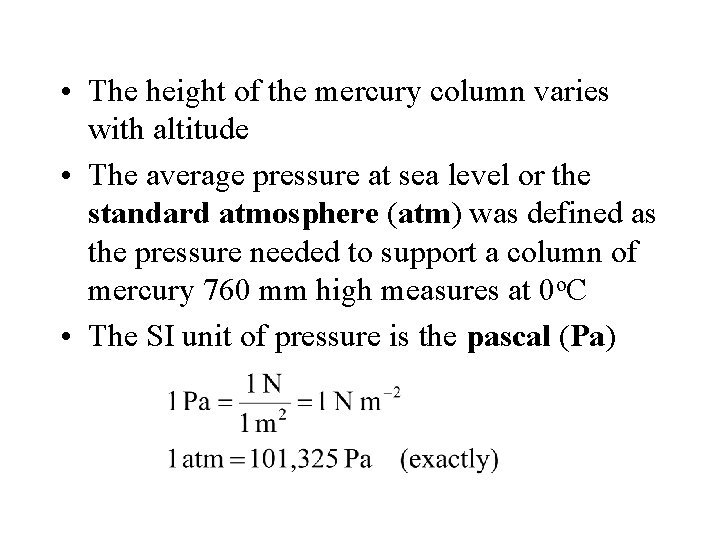
• The height of the mercury column varies with altitude • The average pressure at sea level or the standard atmosphere (atm) was defined as the pressure needed to support a column of mercury 760 mm high measures at 0 o. C • The SI unit of pressure is the pascal (Pa)

• You may encounter a number of pressure units • The standard atmosphere is • Chemical reactions often involve gases

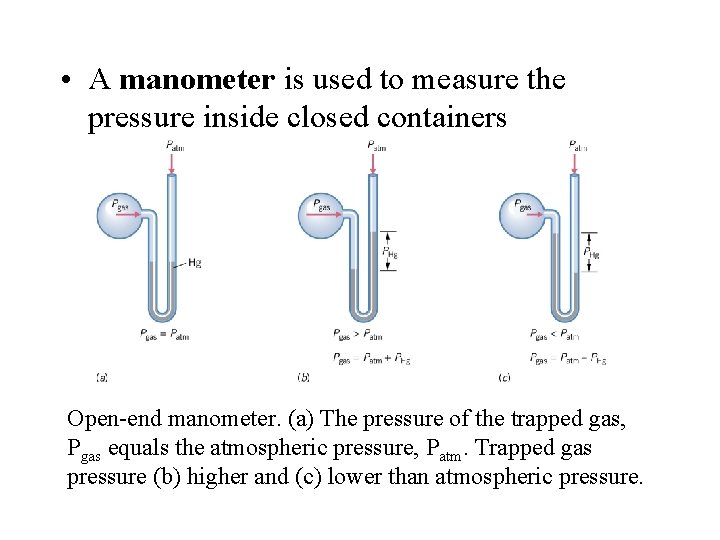
• A manometer is used to measure the pressure inside closed containers Open-end manometer. (a) The pressure of the trapped gas, Pgas equals the atmospheric pressure, Patm. Trapped gas pressure (b) higher and (c) lower than atmospheric pressure.

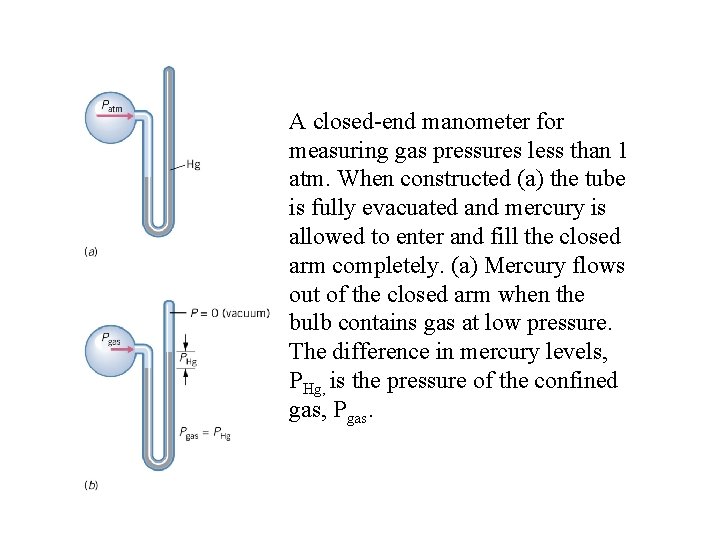
A closed-end manometer for measuring gas pressures less than 1 atm. When constructed (a) the tube is fully evacuated and mercury is allowed to enter and fill the closed arm completely. (a) Mercury flows out of the closed arm when the bulb contains gas at low pressure. The difference in mercury levels, PHg, is the pressure of the confined gas, Pgas.

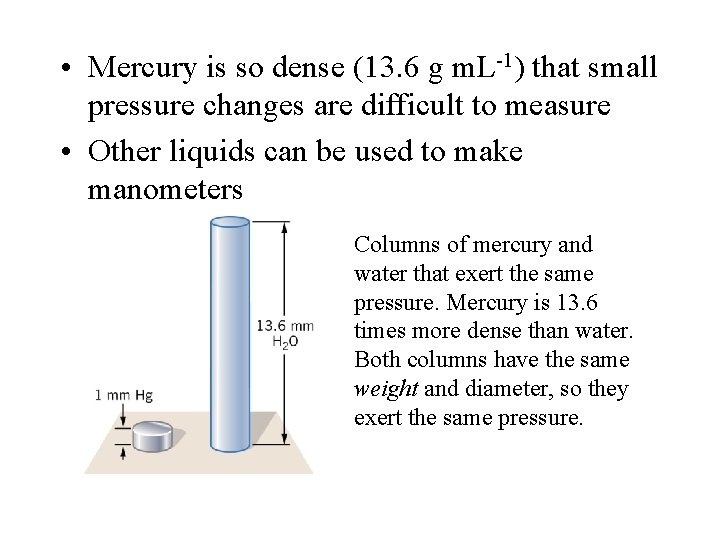
• Mercury is so dense (13. 6 g m. L-1) that small pressure changes are difficult to measure • Other liquids can be used to make manometers Columns of mercury and water that exert the same pressure. Mercury is 13. 6 times more dense than water. Both columns have the same weight and diameter, so they exert the same pressure.

• Thus for a given difference in pressure, the difference in heights between the two levels is inversely proportional to the density of the liquid used in the manometer • There are four variables that affect the properties of a gas: pressure, volume, temperature, and the amount of the gas • Simple experiments can be conducted that relate how these variables change • The gas laws summarize these experiments

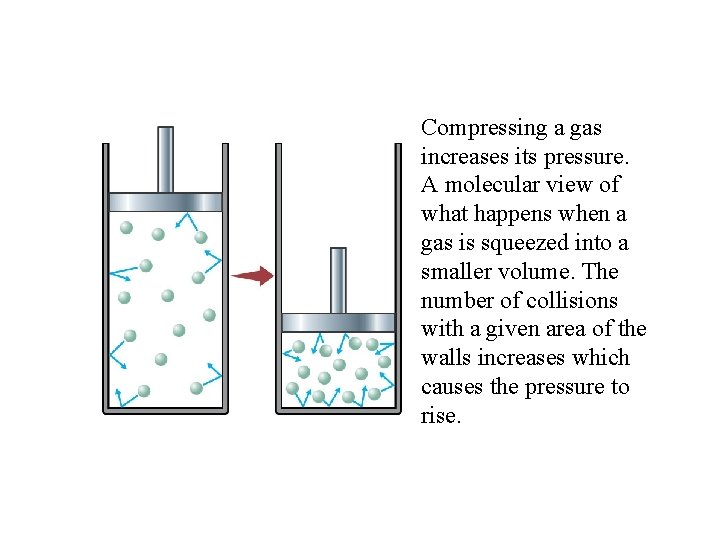
Compressing a gas increases its pressure. A molecular view of what happens when a gas is squeezed into a smaller volume. The number of collisions with a given area of the walls increases which causes the pressure to rise.

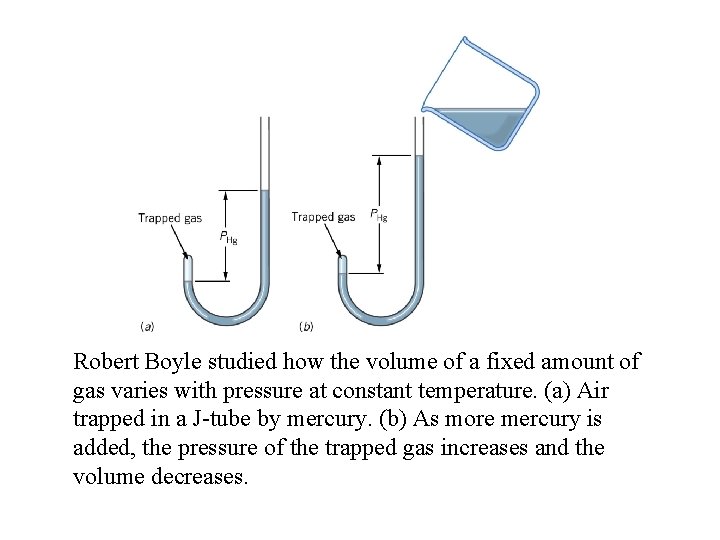
Robert Boyle studied how the volume of a fixed amount of gas varies with pressure at constant temperature. (a) Air trapped in a J-tube by mercury. (b) As more mercury is added, the pressure of the trapped gas increases and the volume decreases.

(a) A typical graph of volume versus pressure showing volume decreasing as pressure increases. (b) A straight line is obtained when volume is plotted against (1/P), which shows that

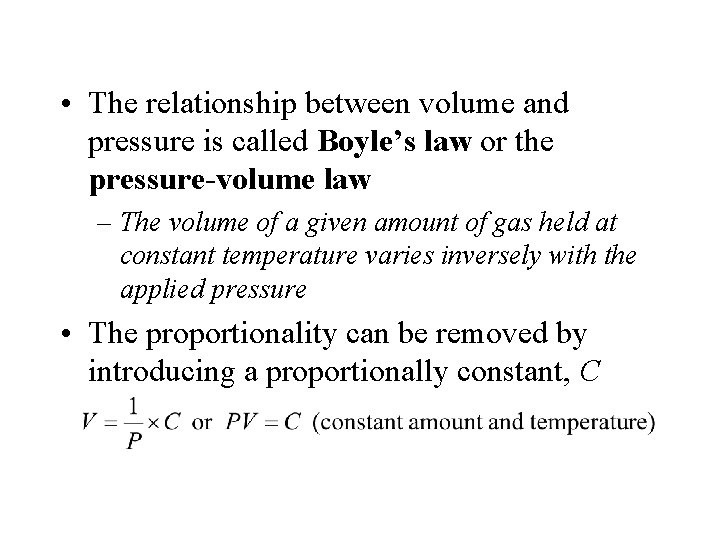
• The relationship between volume and pressure is called Boyle’s law or the pressure-volume law – The volume of a given amount of gas held at constant temperature varies inversely with the applied pressure • The proportionality can be removed by introducing a proportionally constant, C

• Boyle’s law is remarkably successful, especially for common laboratory conditions • However, no real gas obeys Boyle’s law exactly over a wide range of temperatures and pressures • The hypothetical gas that does exactly obey Boyle’s law is called an ideal gas • Real gases act more like ideal gases as their pressures decrease and temperatures increase

• Jacques Alexander Charles studied how the volume of a gas sample varied with temperature Charles’ law plots. Each line shows how the gas volume changes with temperature for different sized samples of the same gas.

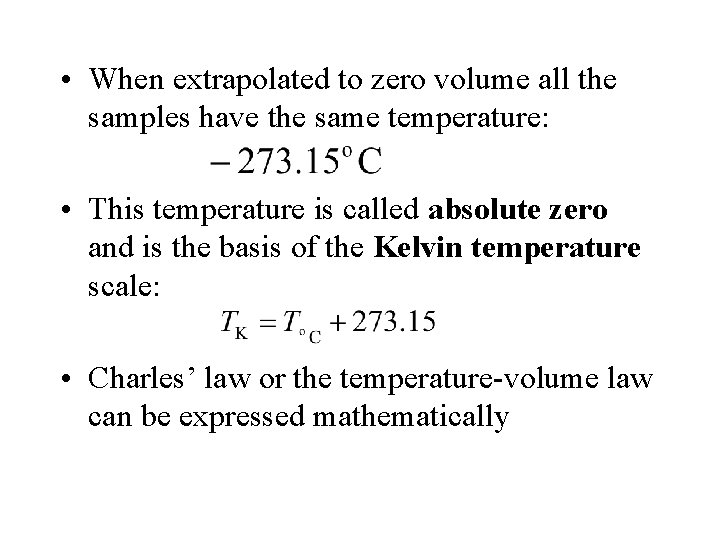
• When extrapolated to zero volume all the samples have the same temperature: • This temperature is called absolute zero and is the basis of the Kelvin temperature scale: • Charles’ law or the temperature-volume law can be expressed mathematically

• Joseph Louis Gay-Lussac studied how the pressure and temperature of a fixed amount of gas at constant volume are related • Gay-Lussacs’ law or the pressuretemperature law states: – The pressure of a fixed amount of gas held at constant volume is directly proportional to the Kelvin temperature • Mathematically this is

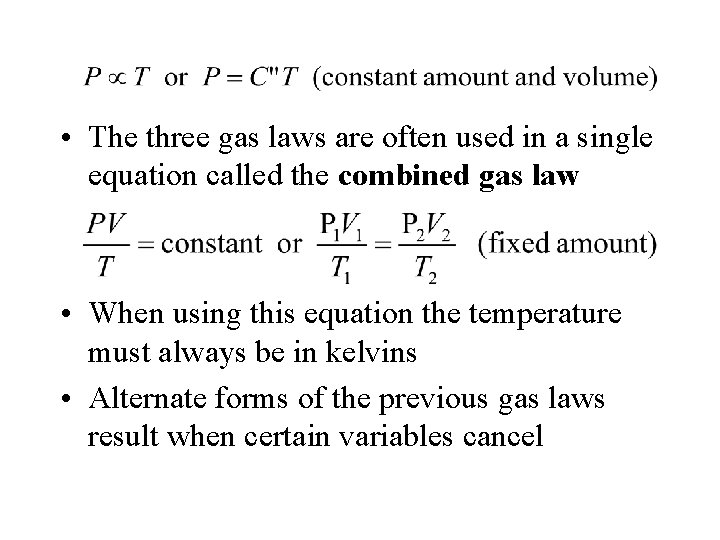
• The three gas laws are often used in a single equation called the combined gas law • When using this equation the temperature must always be in kelvins • Alternate forms of the previous gas laws result when certain variables cancel

• Problems involving the gas laws are important

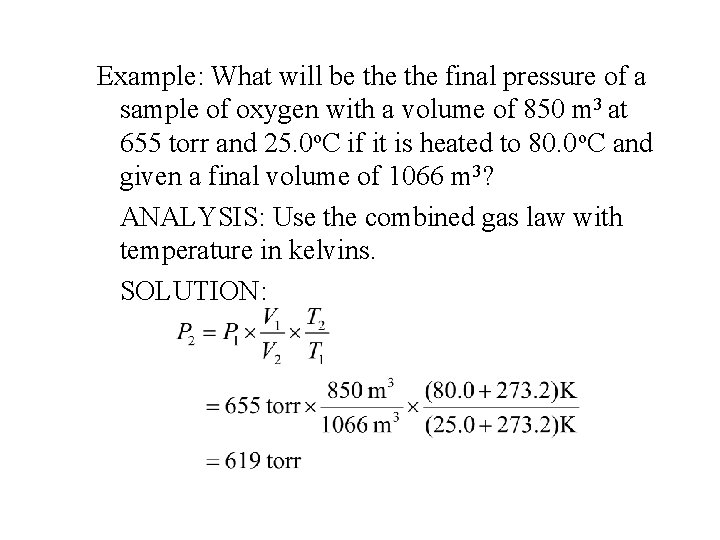
Example: What will be the final pressure of a sample of oxygen with a volume of 850 m 3 at 655 torr and 25. 0 o. C if it is heated to 80. 0 o. C and given a final volume of 1066 m 3? ANALYSIS: Use the combined gas law with temperature in kelvins. SOLUTION:

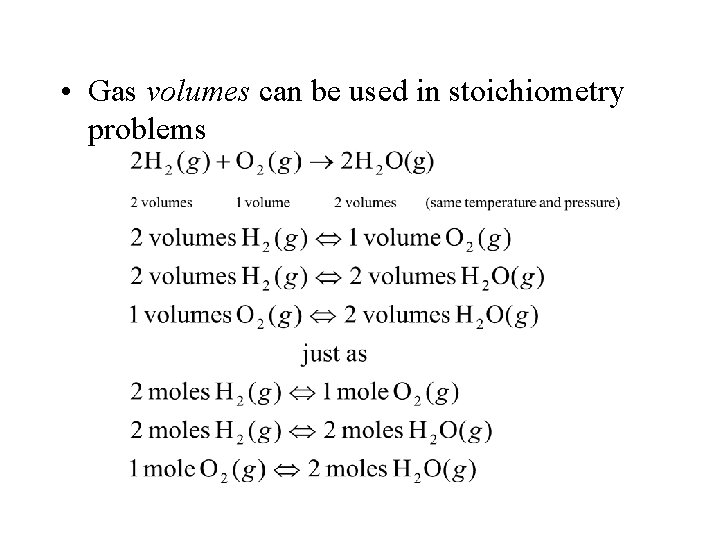
• The law of combining volume states: – When gases react at the same temperature and pressure, their combining volumes are in ratios of simple, whole numbers • Example: • Amedeo Avogadro studied this and devised Avogadro’s principle: – When measured at the same temperature and pressure, equals volumes of gases contain equal number of moles

• A corollary to Avogadro’s principle is: – The volume of a gas is directly proportional to its number of moles, n • Thus, the volume of one mole of any gas at standard temperature and pressure (STP) or 0 o. C and 1 atm is 22. 4 L (a constant for all ideal gases) • This is called the standard molar volume of a gas

• The combined gas law can be generalized to include changes in the number of moles of sample • The ideal gas law is

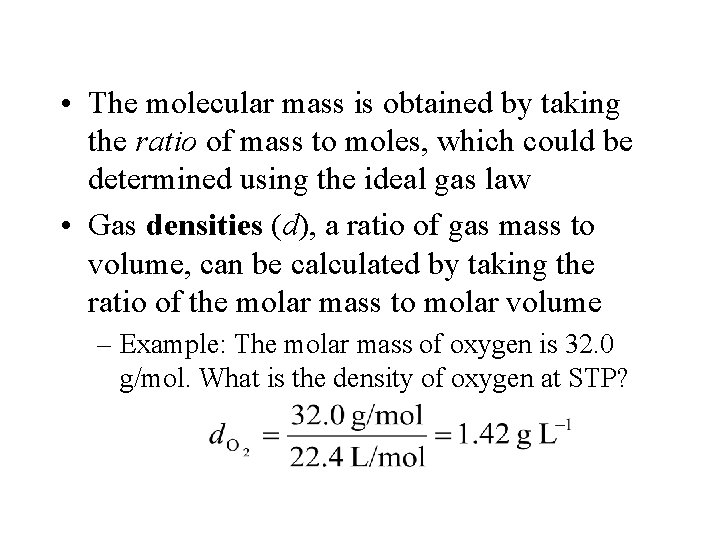
• The molecular mass is obtained by taking the ratio of mass to moles, which could be determined using the ideal gas law • Gas densities (d), a ratio of gas mass to volume, can be calculated by taking the ratio of the molar mass to molar volume – Example: The molar mass of oxygen is 32. 0 g/mol. What is the density of oxygen at STP?

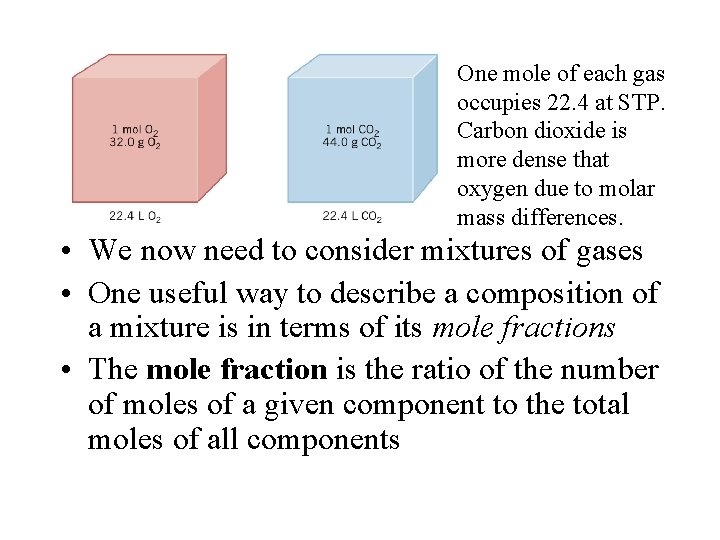
One mole of each gas occupies 22. 4 at STP. Carbon dioxide is more dense that oxygen due to molar mass differences. • We now need to consider mixtures of gases • One useful way to describe a composition of a mixture is in terms of its mole fractions • The mole fraction is the ratio of the number of moles of a given component to the total moles of all components

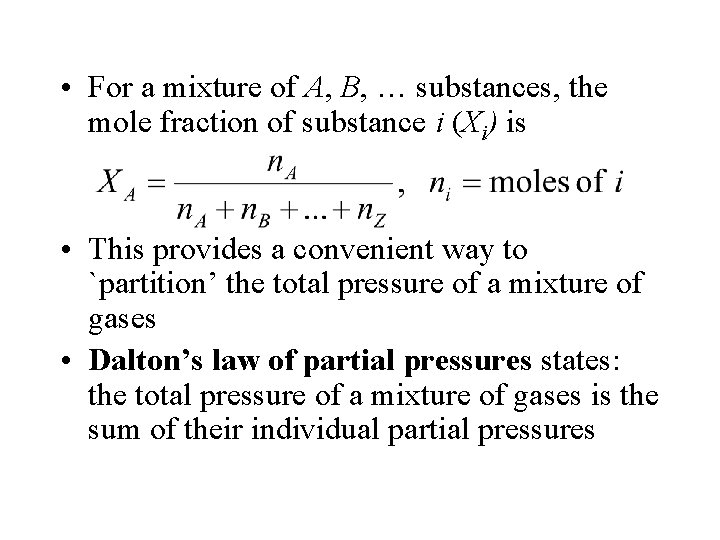
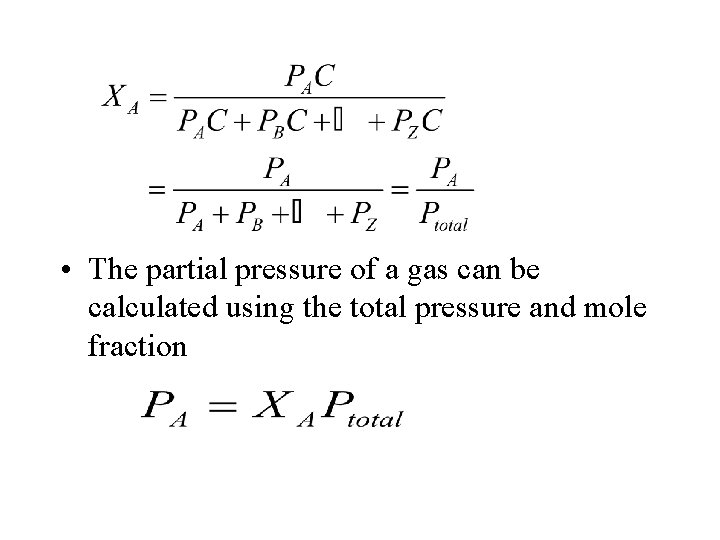
• For a mixture of A, B, … substances, the mole fraction of substance i (Xi) is • This provides a convenient way to `partition’ the total pressure of a mixture of gases • Dalton’s law of partial pressures states: the total pressure of a mixture of gases is the sum of their individual partial pressures

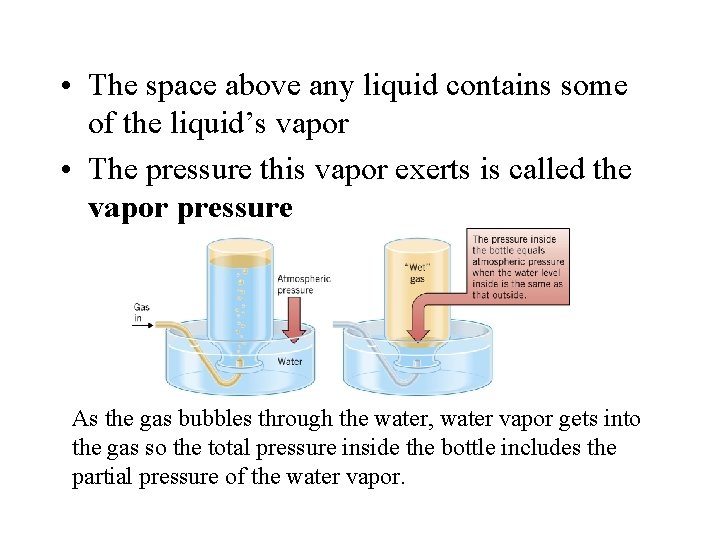
• For a system of only gases, mole fractions and partial pressure partition the total pressure in the same fashion • Gases are often collected over water in the laboratory • These (collected) gases are saturated with water

• The space above any liquid contains some of the liquid’s vapor • The pressure this vapor exerts is called the vapor pressure As the gas bubbles through the water, water vapor gets into the gas so the total pressure inside the bottle includes the partial pressure of the water vapor.

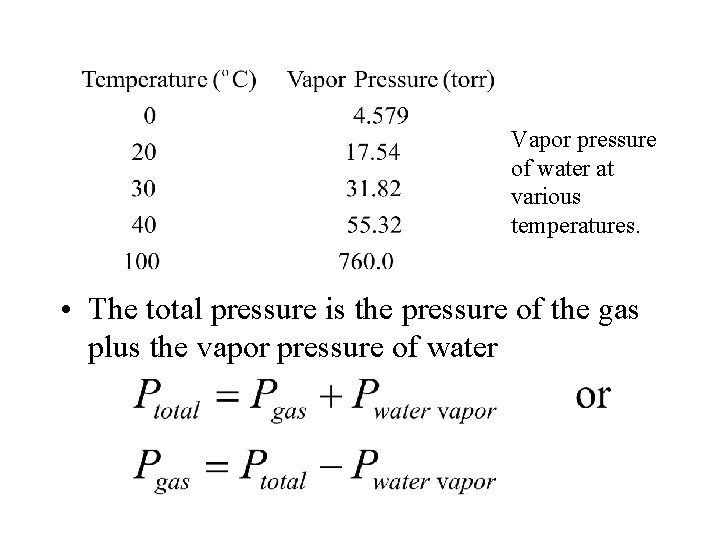
Vapor pressure of water at various temperatures. • The total pressure is the pressure of the gas plus the vapor pressure of water

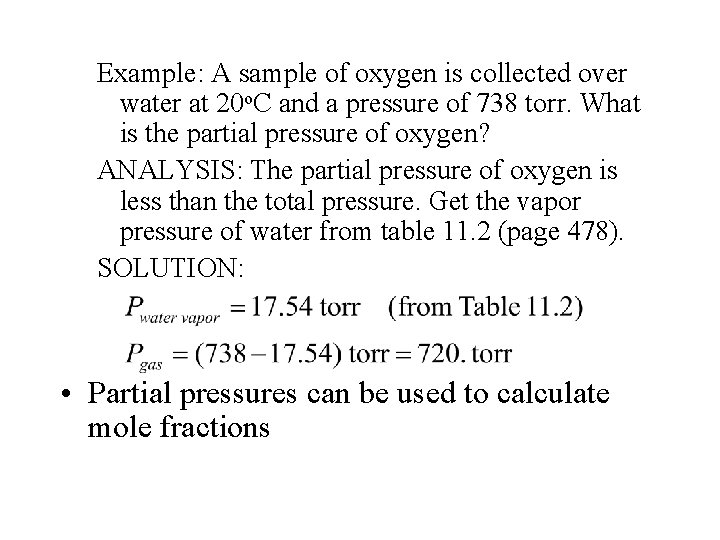
Example: A sample of oxygen is collected over water at 20 o. C and a pressure of 738 torr. What is the partial pressure of oxygen? ANALYSIS: The partial pressure of oxygen is less than the total pressure. Get the vapor pressure of water from table 11. 2 (page 478). SOLUTION: • Partial pressures can be used to calculate mole fractions

• This is possible because the number of moles of each gas is directly proportional to its partial pressure • Using the ideal gas equation for each gas • For a given mixture of gases, the volume and temperature is the same for all gases • Using C=V/RT gives

• The partial pressure of a gas can be calculated using the total pressure and mole fraction

• Gas volumes can be used in stoichiometry problems

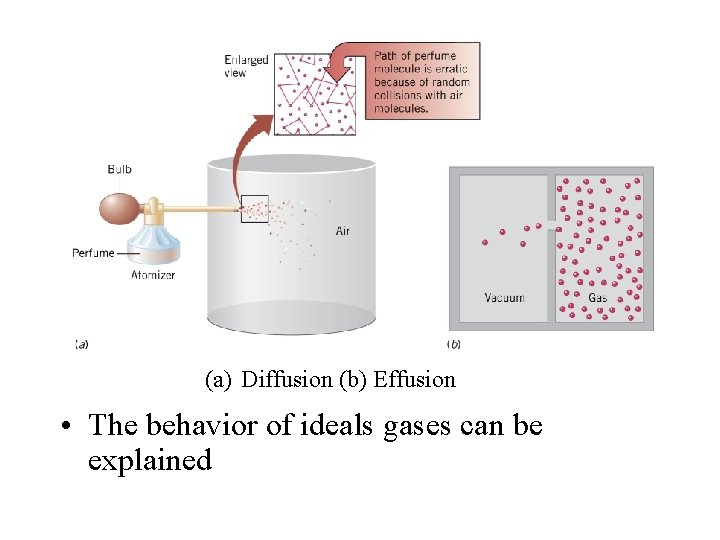
• Diffusion is the spontaneous intermingling of the molecules of one gas with another • Effusion is the movement of gas molecules through a tiny hole into a vacuum • The rates of both diffusion and effusion depend on the speed of the gas molecules • The faster the molecules, the faster diffusion and effusion occur • Thomas Graham studied effusion

• He found that the effusion rate of a gas was inversely proportional to the square root of the density (d) • This is known as Graham’s law • Where Mi is the molar mass of species i

(a) Diffusion (b) Effusion • The behavior of ideals gases can be explained

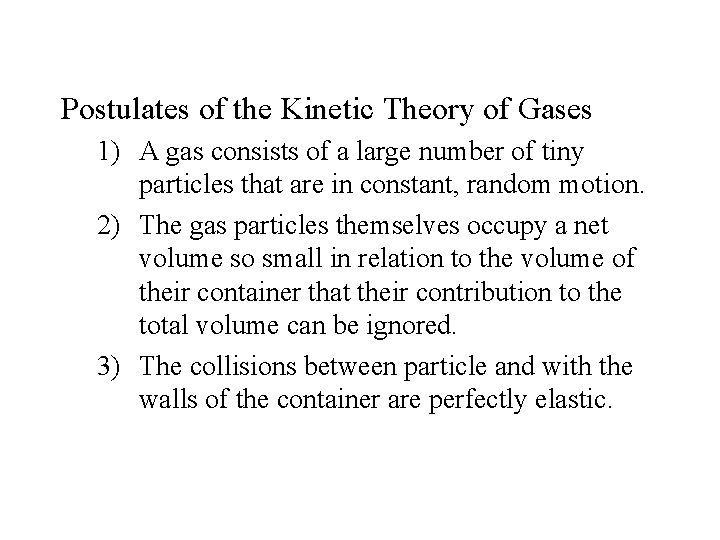
Postulates of the Kinetic Theory of Gases 1) A gas consists of a large number of tiny particles that are in constant, random motion. 2) The gas particles themselves occupy a net volume so small in relation to the volume of their container that their contribution to the total volume can be ignored. 3) The collisions between particle and with the walls of the container are perfectly elastic.

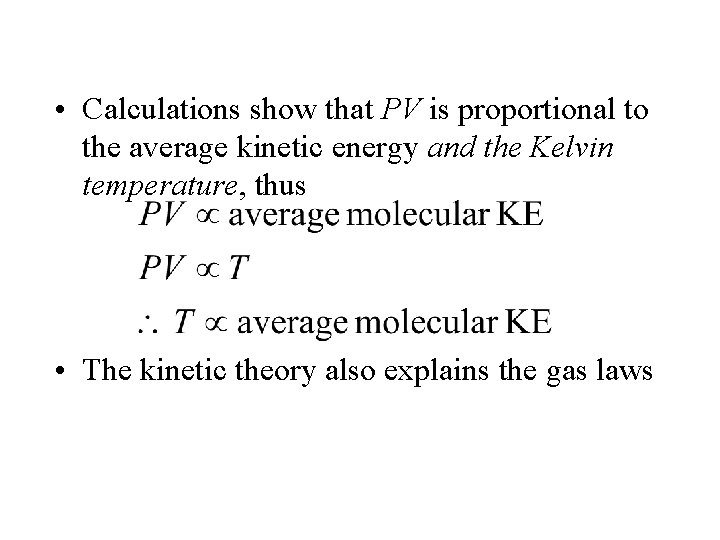
• Calculations show that PV is proportional to the average kinetic energy and the Kelvin temperature, thus • The kinetic theory also explains the gas laws

The kinetic theory and the pressure volume law (Boyle’s law). When the gas volume is made smaller going from (a) to (b), the frequency of collisions per unit area of the containers’ wall increases. Thus the pressure increases.

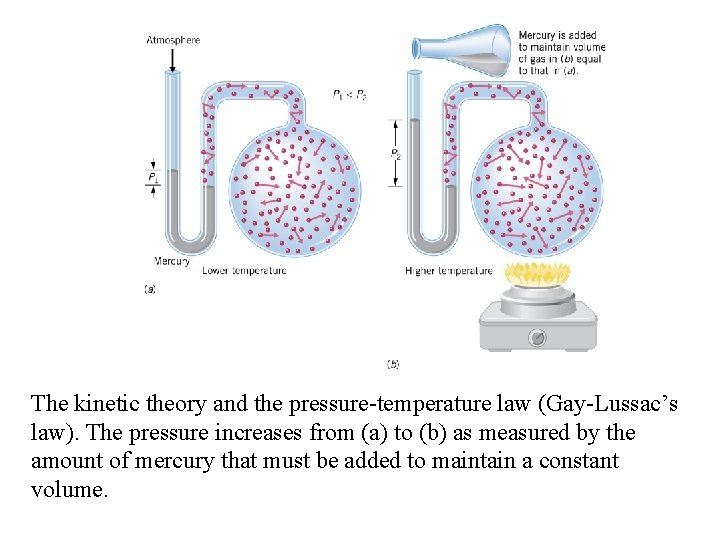
The kinetic theory and the pressure-temperature law (Gay-Lussac’s law). The pressure increases from (a) to (b) as measured by the amount of mercury that must be added to maintain a constant volume.

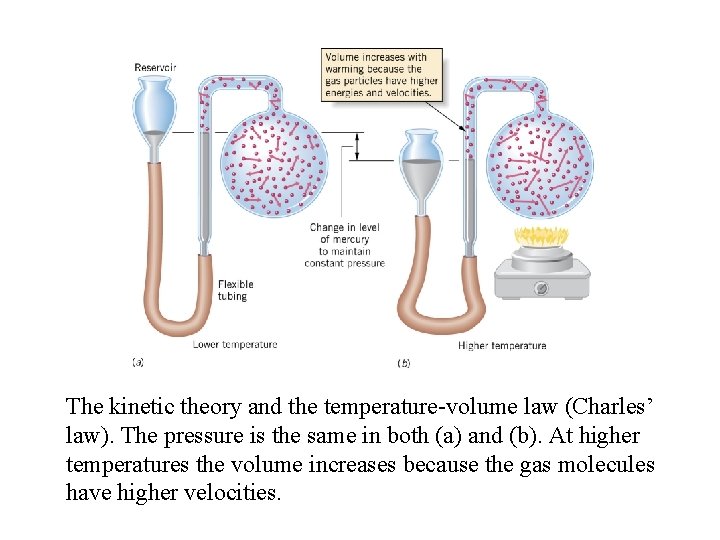
The kinetic theory and the temperature-volume law (Charles’ law). The pressure is the same in both (a) and (b). At higher temperatures the volume increases because the gas molecules have higher velocities.

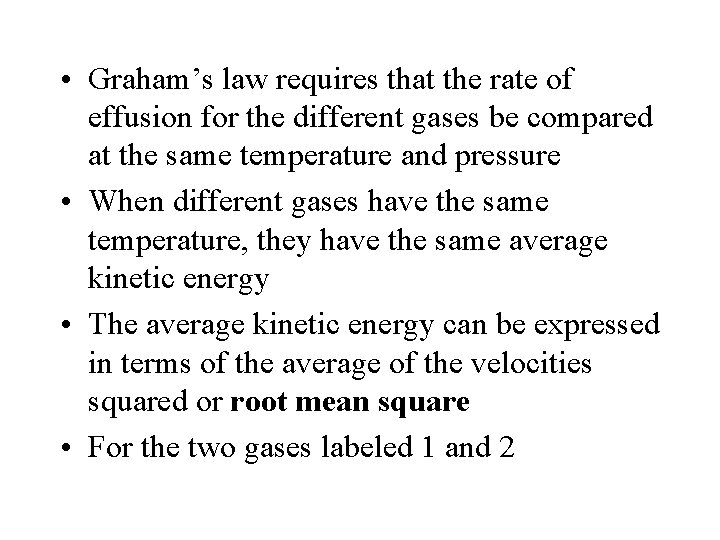
• Graham’s law requires that the rate of effusion for the different gases be compared at the same temperature and pressure • When different gases have the same temperature, they have the same average kinetic energy • The average kinetic energy can be expressed in terms of the average of the velocities squared or root mean square • For the two gases labeled 1 and 2

• Note that heavier gases move slower than lighter gases

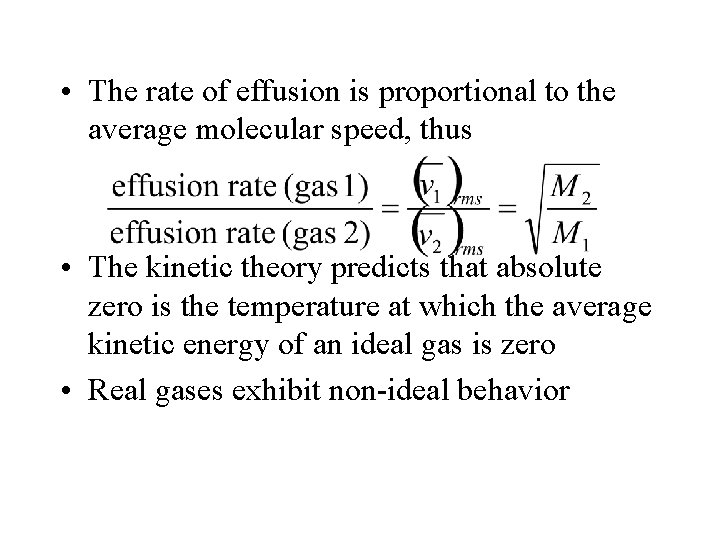
• The rate of effusion is proportional to the average molecular speed, thus • The kinetic theory predicts that absolute zero is the temperature at which the average kinetic energy of an ideal gas is zero • Real gases exhibit non-ideal behavior

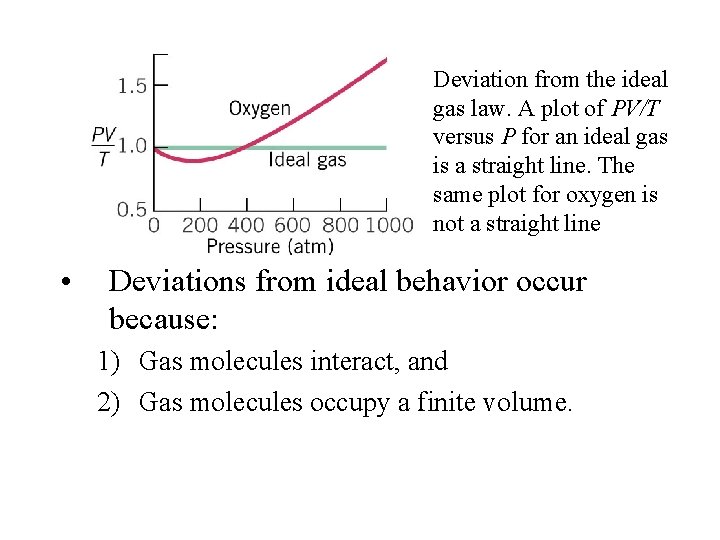
Deviation from the ideal gas law. A plot of PV/T versus P for an ideal gas is a straight line. The same plot for oxygen is not a straight line • Deviations from ideal behavior occur because: 1) Gas molecules interact, and 2) Gas molecules occupy a finite volume.

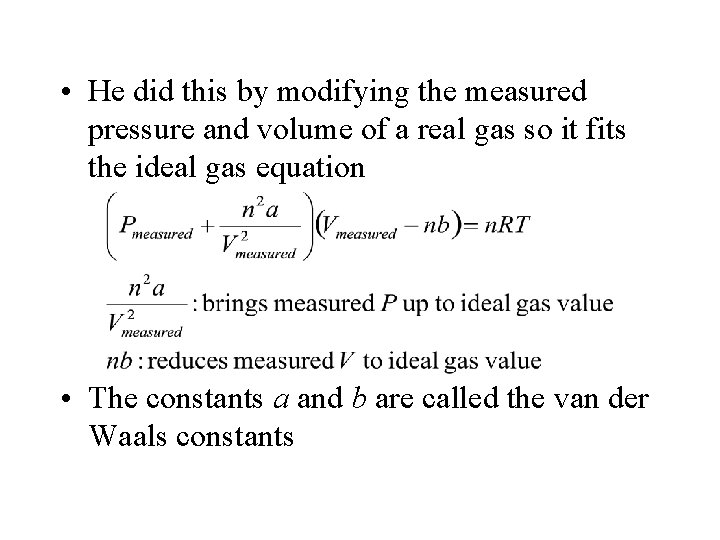
(a) In an ideal gas, molecules would travel in straight lines. (b) In a real gas, the paths would curve due to the attractions between molecules. • J. D. van der Waals corrected the ideal gas equation in a simple, but useful, way

• He did this by modifying the measured pressure and volume of a real gas so it fits the ideal gas equation • The constants a and b are called the van der Waals constants

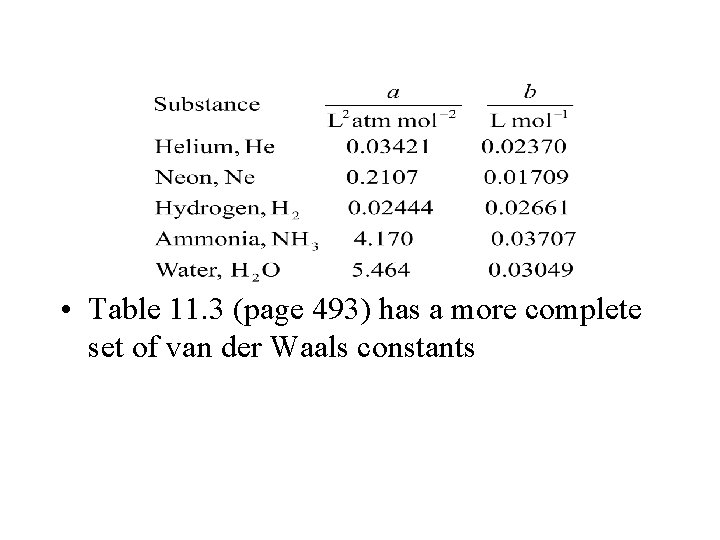
• Table 11. 3 (page 493) has a more complete set of van der Waals constants
 Has six faces 8 vertices and 12 edges
Has six faces 8 vertices and 12 edges Solids liquids and gases section 2 properties of fluids
Solids liquids and gases section 2 properties of fluids The properties of solids liquids and gases
The properties of solids liquids and gases Properties of gas
Properties of gas Four properties of gas
Four properties of gas 5 properties of gases
5 properties of gases Properties of gases
Properties of gases Noble gases general properties
Noble gases general properties Characteristics of gases
Characteristics of gases Gases have low densities
Gases have low densities What is noble gas
What is noble gas Properties of solid liquid and gas
Properties of solid liquid and gas Properties of gases
Properties of gases Properties of gas
Properties of gas List 2 of the important properties of gases
List 2 of the important properties of gases Extensive and intensive examples
Extensive and intensive examples Chemical property of matter
Chemical property of matter Avogadro's law relationship
Avogadro's law relationship Chapter 11 review gases section 1
Chapter 11 review gases section 1 Ideal gas law examples
Ideal gas law examples Chapter 14 the behavior of gases
Chapter 14 the behavior of gases 13-4 practice problems chemistry
13-4 practice problems chemistry Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Timestamps must have following properties namely
Timestamps must have following properties namely Why do different polymers have different properties?
Why do different polymers have different properties? Emergent properties
Emergent properties Words have meaning and names have power
Words have meaning and names have power Does congress have the power to make no mail on saturdays
Does congress have the power to make no mail on saturdays Modals in the past
Modals in the past It is not you they have rejected but me
It is not you they have rejected but me I have decided i have resolved
I have decided i have resolved Ideas have consequences bad ideas have victims
Ideas have consequences bad ideas have victims Have been to or have gone to
Have been to or have gone to Some animals are dangerous *
Some animals are dangerous * Past tense modal
Past tense modal Endoskeleton and usually a spiny skin
Endoskeleton and usually a spiny skin Properties and attributes of triangles
Properties and attributes of triangles Chapter 11 properties of the hair and scalp answers
Chapter 11 properties of the hair and scalp answers Chapter 9 properties of transformations answer key
Chapter 9 properties of transformations answer key Group of carbon
Group of carbon Properties of circles
Properties of circles Limits and their properties
Limits and their properties 2 properties of matter
2 properties of matter Tinea favosa milady
Tinea favosa milady Ap chemistry chapter 7 periodic properties of the elements
Ap chemistry chapter 7 periodic properties of the elements List the properties of x radiation chapter 38
List the properties of x radiation chapter 38 Properties of pure substances
Properties of pure substances Chapter 2 properties of matter answer key
Chapter 2 properties of matter answer key Chapter 1 limits and their properties
Chapter 1 limits and their properties