CHAPTER 5 GASES 5 1 PRESSURE Properties Gases





- Slides: 13

CHAPTER 5 - GASES

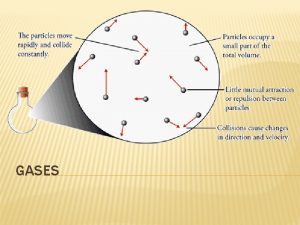
5. 1 PRESSURE � Properties � Gases of Gases uniformly fill any container � Gases are easily compressed � Gases mix completely with any other gas � Gases exert pressure on their surroundings

5. 1 PRESSURE � Measuring � The barometric pressure barometer � Invented by Evangelista Torricelli in 1643 � Units � mm Hg (torr) � newtons/m 2 (pascal (Pa)) � Atmospheres 760 torr = 1 atm 101, 325 Pa = 101. 3 k. Pa 1 atm = standard pressure

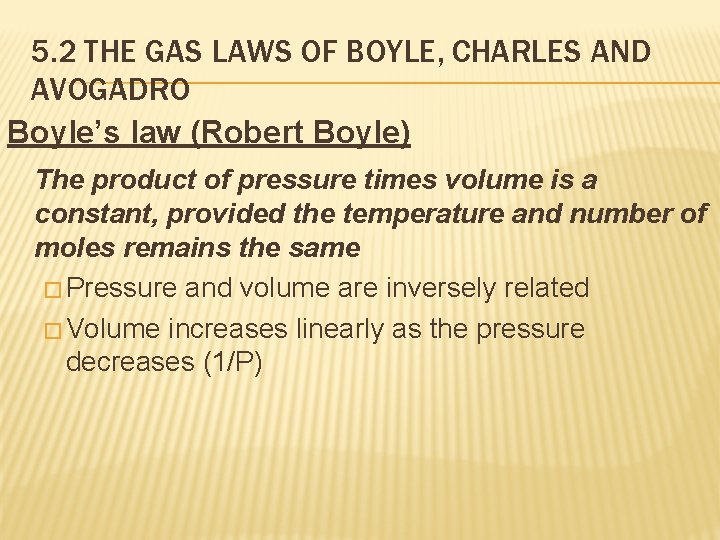
5. 2 THE GAS LAWS OF BOYLE, CHARLES AND AVOGADRO Boyle’s law (Robert Boyle) The product of pressure times volume is a constant, provided the temperature and number of moles remains the same � Pressure and volume are inversely related � Volume increases linearly as the pressure decreases (1/P)

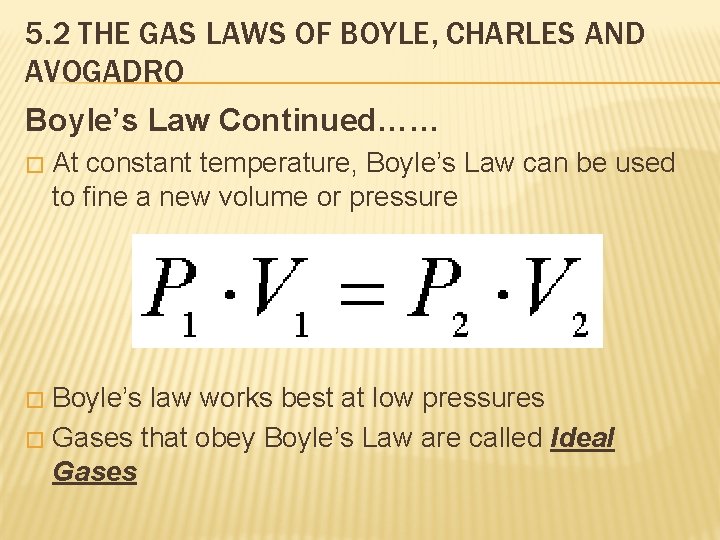
5. 2 THE GAS LAWS OF BOYLE, CHARLES AND AVOGADRO Boyle’s Law Continued…… � At constant temperature, Boyle’s Law can be used to fine a new volume or pressure Boyle’s law works best at low pressures � Gases that obey Boyle’s Law are called Ideal Gases �

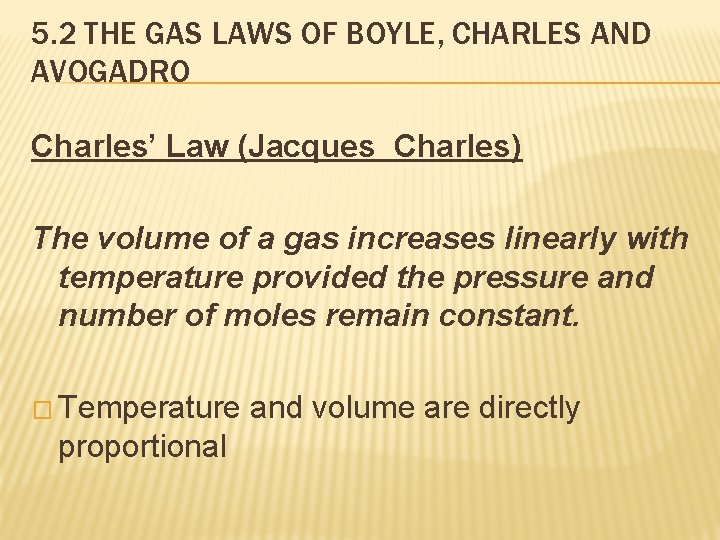
5. 2 THE GAS LAWS OF BOYLE, CHARLES AND AVOGADRO Charles’ Law (Jacques Charles) The volume of a gas increases linearly with temperature provided the pressure and number of moles remain constant. � Temperature proportional and volume are directly

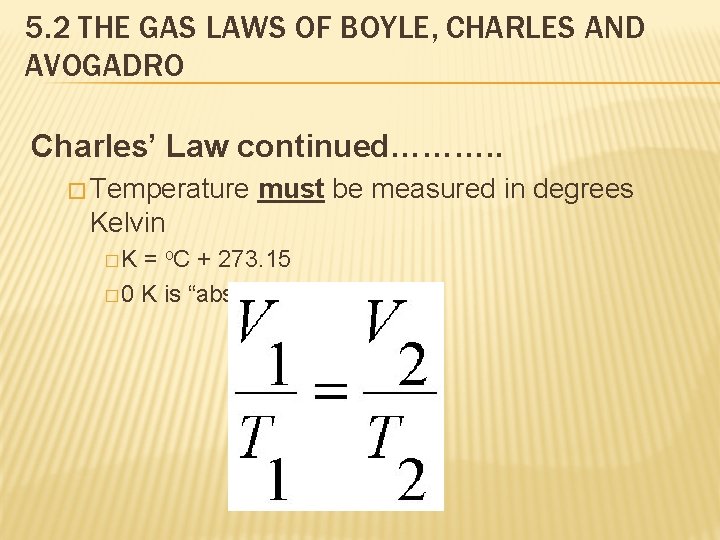
5. 2 THE GAS LAWS OF BOYLE, CHARLES AND AVOGADRO Charles’ Law continued………. . � Temperature must be measured in degrees Kelvin �K = o. C + 273. 15 � 0 K is “absolute zero”

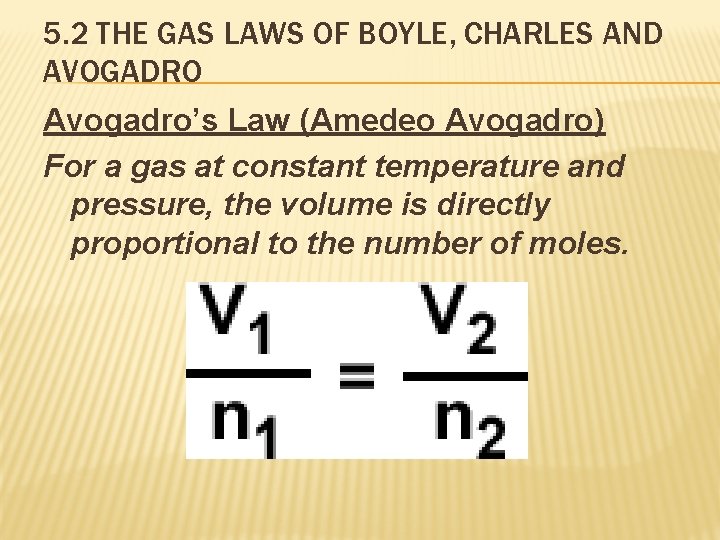
5. 2 THE GAS LAWS OF BOYLE, CHARLES AND AVOGADRO Avogadro’s Law (Amedeo Avogadro) For a gas at constant temperature and pressure, the volume is directly proportional to the number of moles.

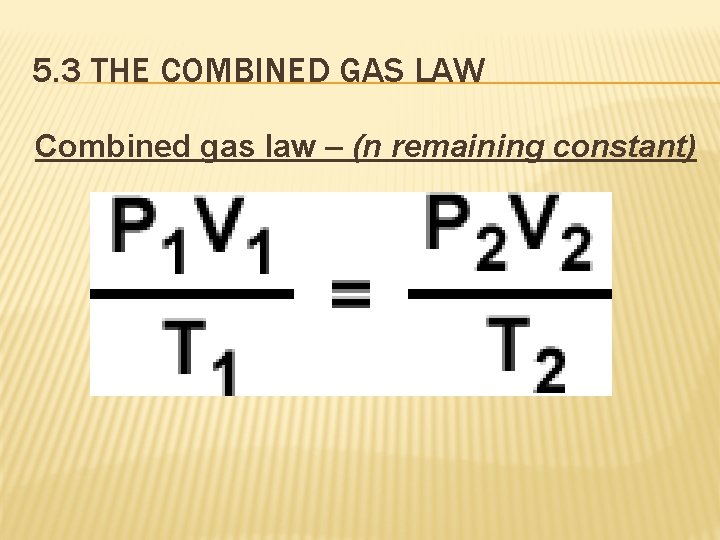
5. 3 THE COMBINED GAS LAW Combined gas law – (n remaining constant)

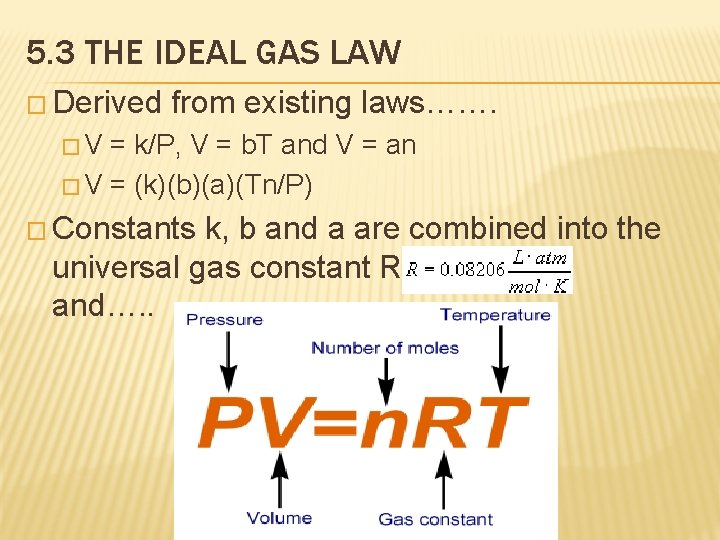
5. 3 THE IDEAL GAS LAW � Derived from existing laws……. �V = k/P, V = b. T and V = an � V = (k)(b)(a)(Tn/P) � Constants k, b and a are combined into the universal gas constant R and…. .

5. 3 THE IDEAL GAS LAW � Limitations � Works of the Ideal Gas Law well at low pressure and high temperatures � Most gases do not behave ideally above 1 atm � Does not work well near the condensation conditions of a gas

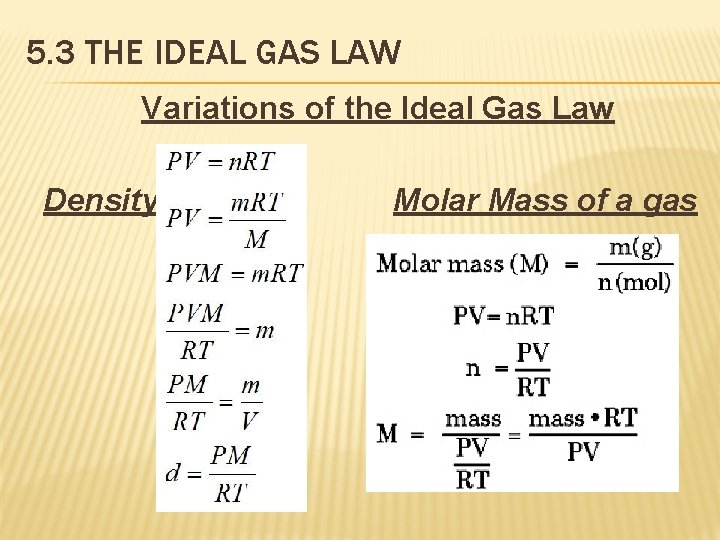
5. 3 THE IDEAL GAS LAW Variations of the Ideal Gas Law Density Molar Mass of a gas

5. 4 GAS STOICHIOMETRY At Standard Temperature and Pressure (STP) T = 273. 15 K (0 o. C) P = 1 atm (760 torr or 101. 3 k. Pa) 1 mole of an ideal gas occupies 22. 4 L of volume Remember……. . Density = mass/volume and
 How does a gas exert pressure
How does a gas exert pressure Do gases exert pressure on whatever surrounds them
Do gases exert pressure on whatever surrounds them Buoyancyability
Buoyancyability Properties of solids and liquids
Properties of solids and liquids Properties of gas
Properties of gas Four properties of gases
Four properties of gases 5 properties of gases
5 properties of gases Properties of gases
Properties of gases Properties of noble gas
Properties of noble gas What are the different properties of gas?
What are the different properties of gas? Why do gases have low densities
Why do gases have low densities What is noble gas
What is noble gas Liquid information
Liquid information Properties of gasses
Properties of gasses