Atomic Structure I The Evolution of Atomic Theories










- Slides: 37

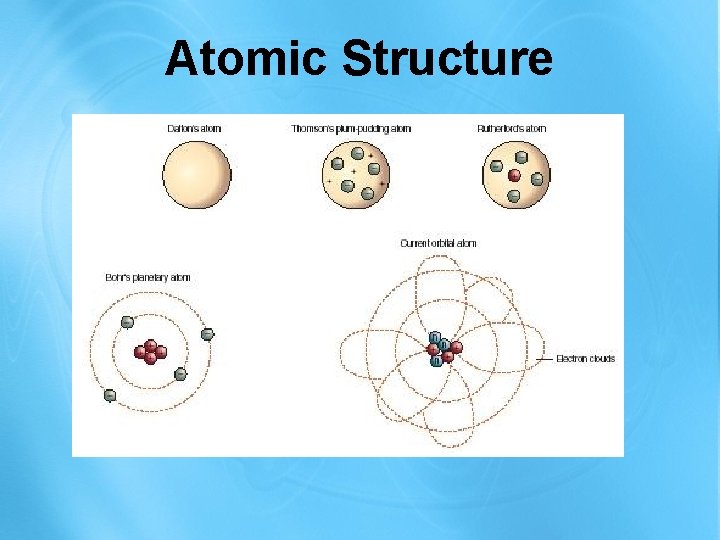
Atomic Structure

I. The Evolution of Atomic Theories Around 400 BC A. Democritus (Year ________) First to suggest the existence of fundamental particles – called them atoms • • Believed they were indivisible and indestructible • Smallest particle of matter

1803 -1805 B. John Dalton (Year _____) • Dalton studied the ratios in which elements combine in chemical reactions. Based on his experiments, he formulated the first theories about atoms. • Dalton’s Atomic Theory: 1. All matter is composed of ATOMS 2. All elements are composed of INDIVISIBLE ATOMS (SOLID/HARD SPHERE) 3. All atoms of a given element are IDENTICAL (SAME MASS AND PROPERTIES). Atoms of different elements have DIFFERENT MASSES/PROPERTIES

• Dalton’s Atomic Theory (Continued): 4. Law of Definite Proportions: Atoms can combine with each other in simple-whole number ratios to form compounds (Ex – H 20, Ag. NO 3) 5. Law of Multiple Proportions: Atoms can combine in 2 or more ways to form compounds (Ex – Fe 2 O 3 vs. Fe. O or H 2 O vs. H 2 O 2)

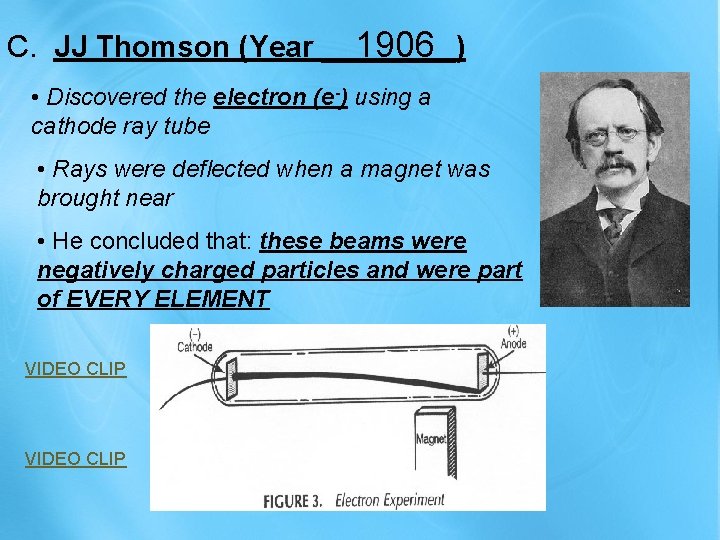
1906 C. JJ Thomson (Year ____) • Discovered the electron (e-) using a cathode ray tube • Rays were deflected when a magnet was brought near • He concluded that: these beams were negatively charged particles and were part of EVERY ELEMENT VIDEO CLIP

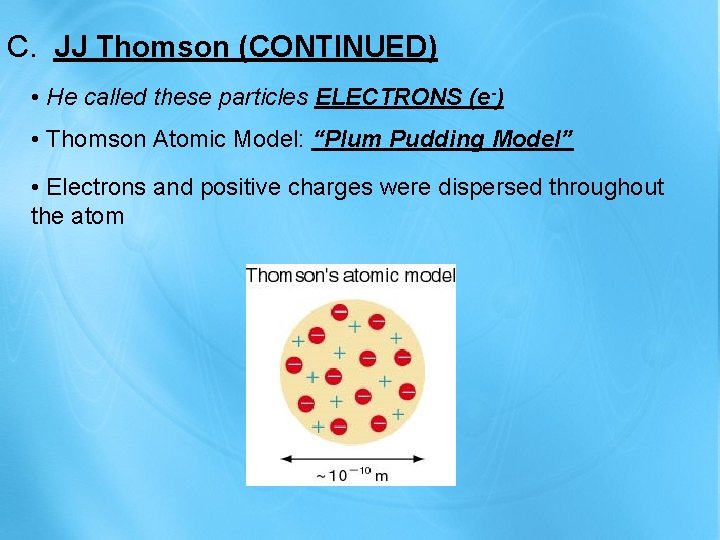
C. JJ Thomson (CONTINUED) • He called these particles ELECTRONS (e-) • Thomson Atomic Model: “Plum Pudding Model” • Electrons and positive charges were dispersed throughout the atom

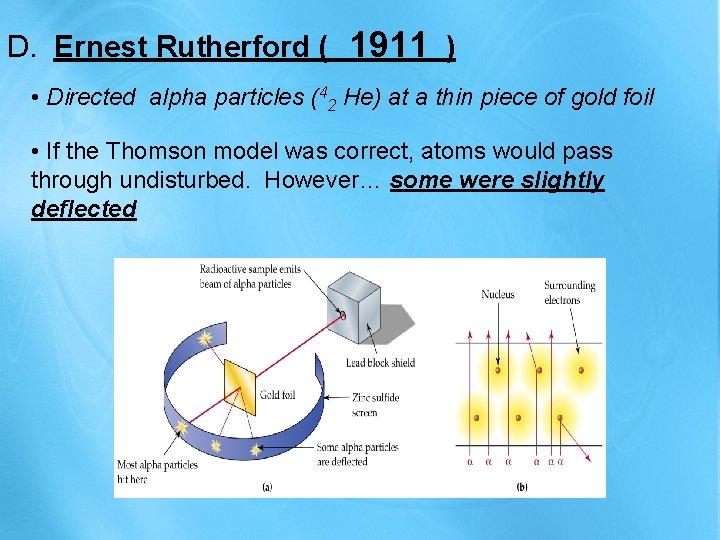
1911 D. Ernest Rutherford ( ) • Directed alpha particles (42 He) at a thin piece of gold foil • If the Thomson model was correct, atoms would pass through undisturbed. However… some were slightly deflected

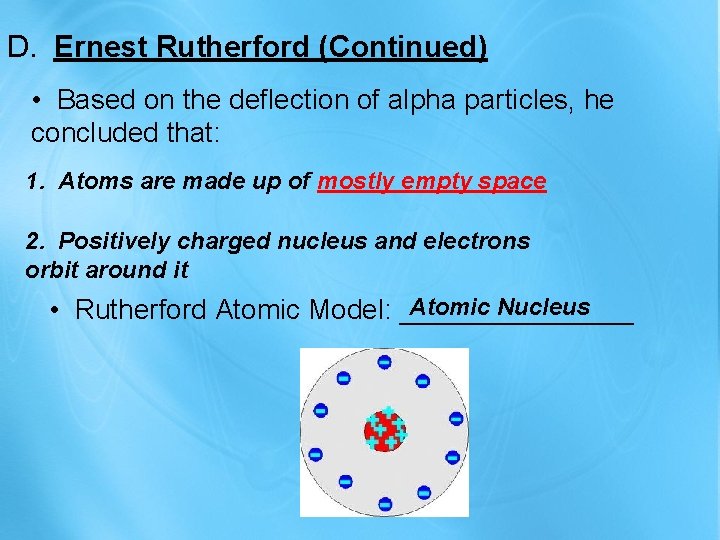
D. Ernest Rutherford (Continued) • Based on the deflection of alpha particles, he concluded that: 1. Atoms are made up of mostly empty space 2. Positively charged nucleus and electrons orbit around it Atomic Nucleus • Rutherford Atomic Model: ________

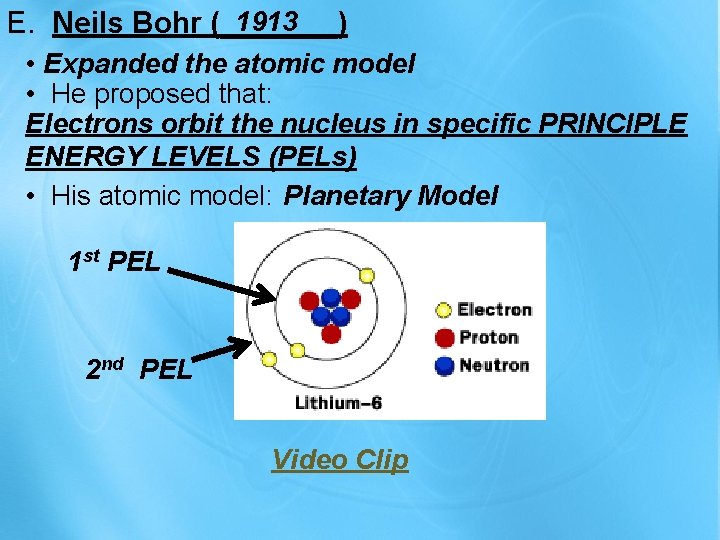
1913 E. Neils Bohr (_______) • Expanded the atomic model • He proposed that: Electrons orbit the nucleus in specific PRINCIPLE ENERGY LEVELS (PELs) • His atomic model: Planetary Model 1 st PEL 2 nd PEL Video Clip

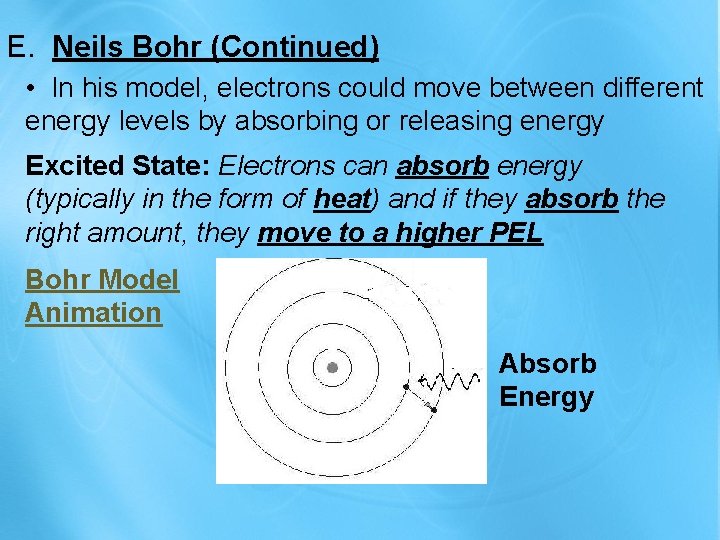
E. Neils Bohr (Continued) • In his model, electrons could move between different energy levels by absorbing or releasing energy Excited State: Electrons can absorb energy (typically in the form of heat) and if they absorb the right amount, they move to a higher PEL Bohr Model Animation Absorb Energy

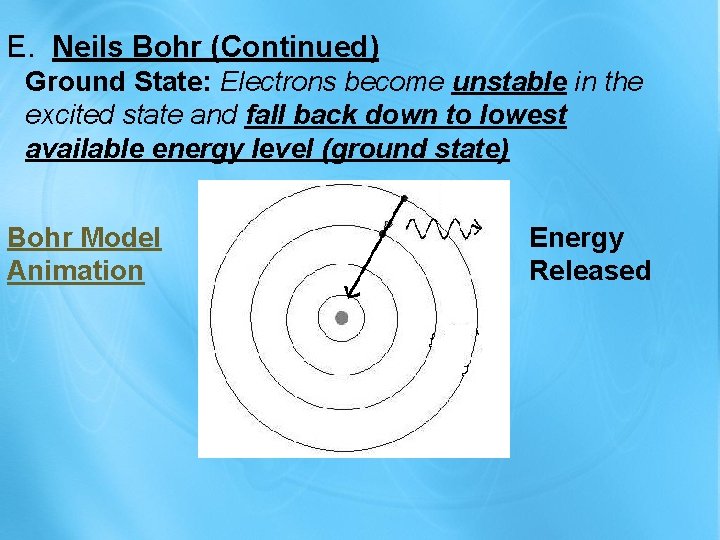
E. Neils Bohr (Continued) Ground State: Electrons become unstable in the excited state and fall back down to lowest available energy level (ground state) Bohr Model Animation Energy Released

E. Neils Bohr (Continued) Electrons want to be in the ground (stable) state Photon Release: when the electron returns to ground state it releases the energy it absorbed in the form of a PHOTON (light energy) to become stable This can be seen in Fireworks Energy (Photon) Released

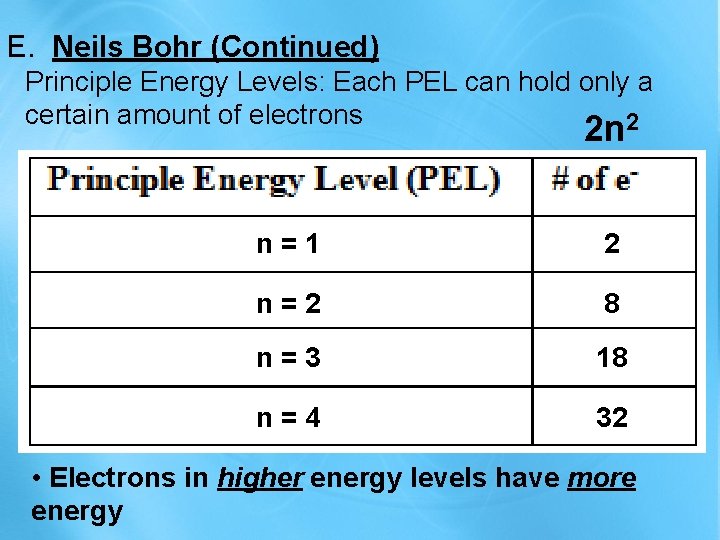
E. Neils Bohr (Continued) Principle Energy Levels: Each PEL can hold only a certain amount of electrons 2 2 n n = 1 2 n = 2 8 n = 3 18 n = 4 32 • Electrons in higher energy levels have more energy

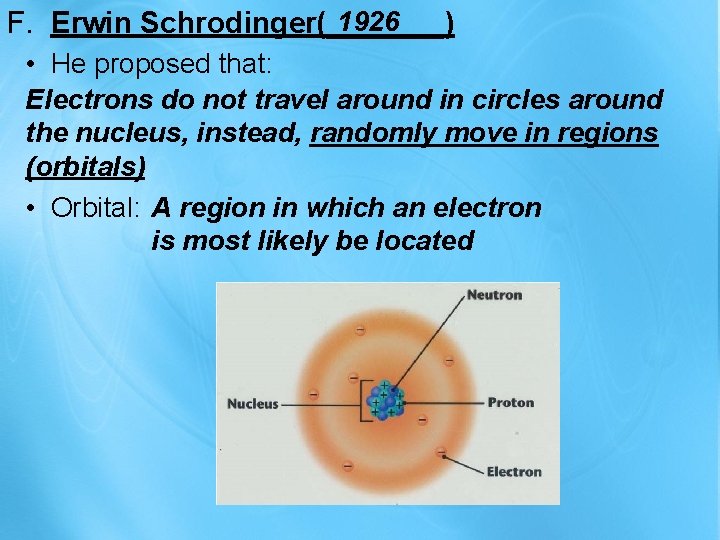
1926 F. Erwin Schrodinger(_______) • He proposed that: Electrons do not travel around in circles around the nucleus, instead, randomly move in regions (orbitals) • Orbital: A region in which an electron is most likely be located

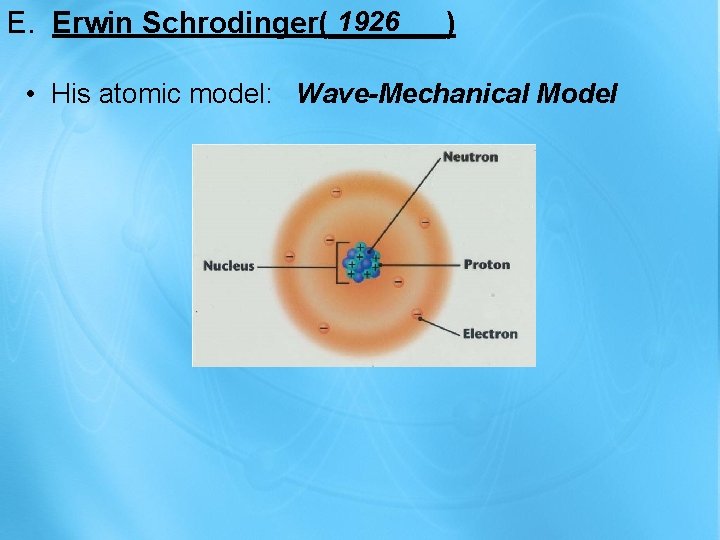
1926 E. Erwin Schrodinger(_______) • His atomic model: Wave-Mechanical Model

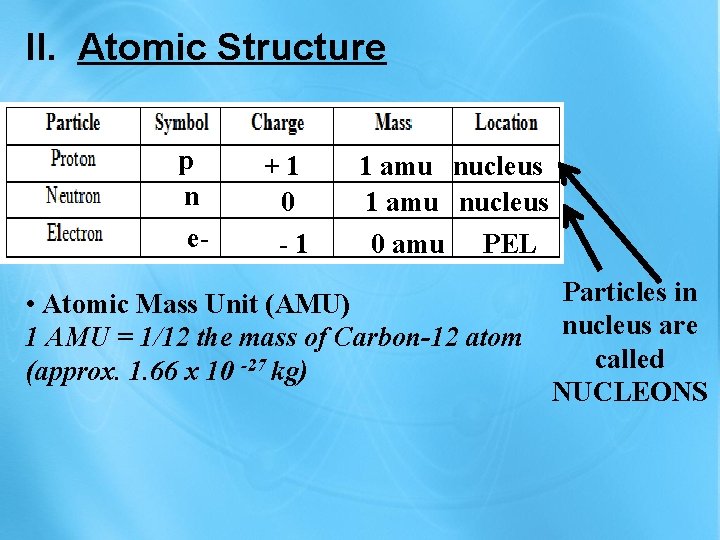
II. Atomic Structure p n e- +1 0 -1 1 amu nucleus 0 amu PEL • Atomic Mass Unit (AMU) 1 AMU = 1/12 the mass of Carbon-12 atom (approx. 1. 66 x 10 -27 kg) Particles in nucleus are called NUCLEONS

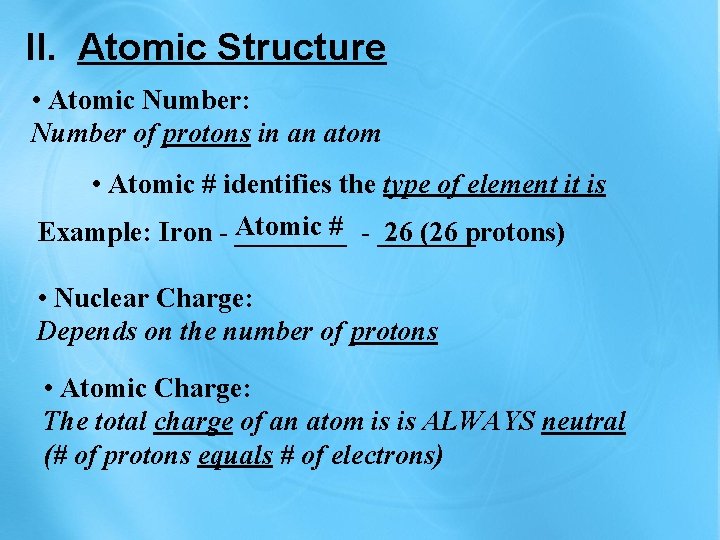
II. Atomic Structure • Atomic Number: Number of protons in an atom • Atomic # identifies the type of element it is # - _______ Example: Iron - Atomic ____ 26 (26 protons) • Nuclear Charge: Depends on the number of protons • Atomic Charge: The total charge of an atom is is ALWAYS neutral (# of protons equals # of electrons)

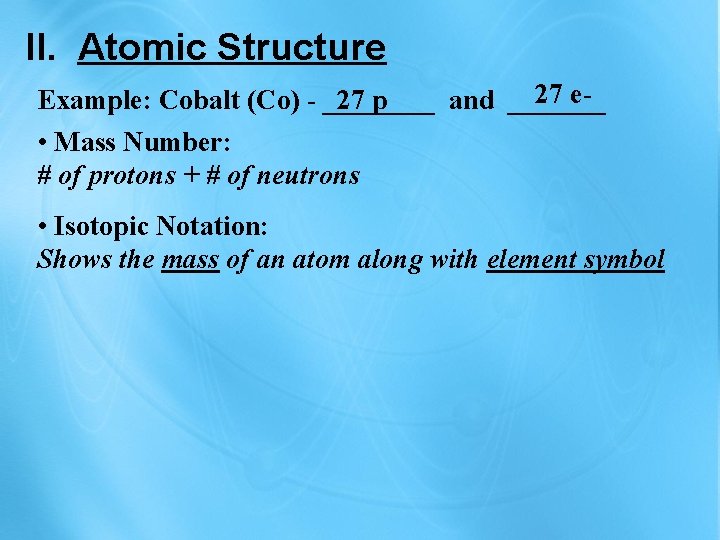
II. Atomic Structure 27 e. Example: Cobalt (Co) - ____ 27 p and _______ • Mass Number: # of protons + # of neutrons • Isotopic Notation: Shows the mass of an atom along with element symbol

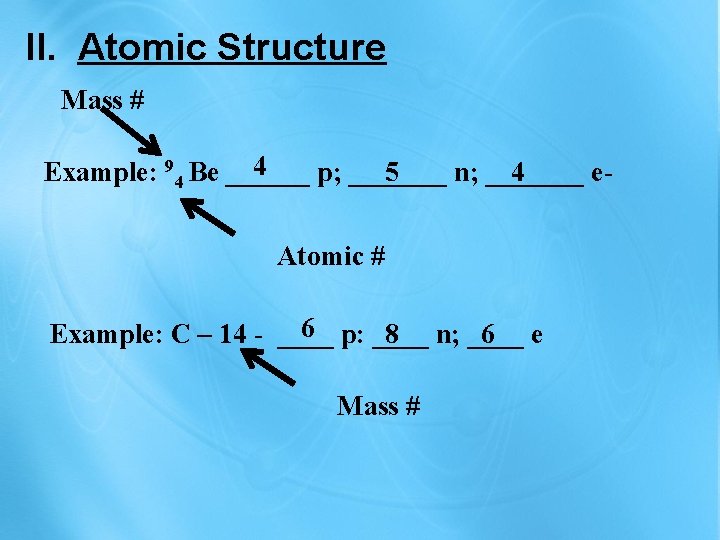
II. Atomic Structure Mass # 4 Example: 94 Be ______ p; _______ 5 n; _______ 4 e. Atomic # 6 p: ____ Example: C – 14 - ____ 8 n; ____ 6 e Mass #

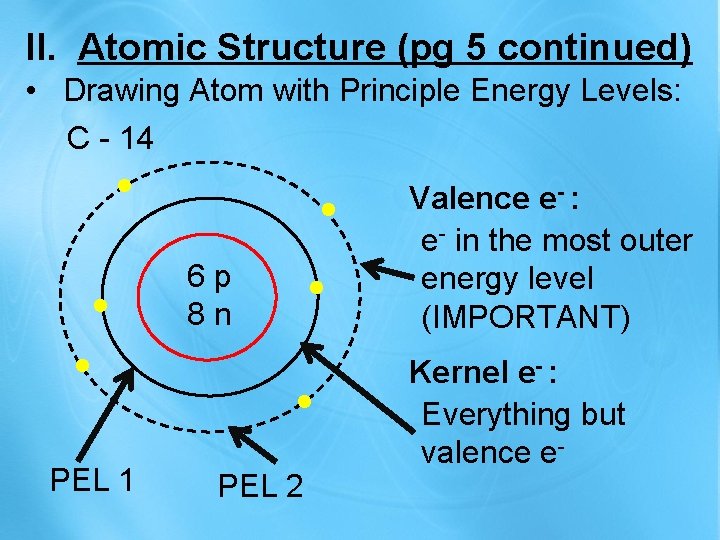
II. Atomic Structure (pg 5 continued) • Drawing Atom with Principle Energy Levels: C - 14 . . . PEL 1 6 p 8 n . . . PEL 2 Valence e- : e- in the most outer energy level (IMPORTANT) Kernel e- : Everything but valence e-

SKIPPING TO SECTION IV

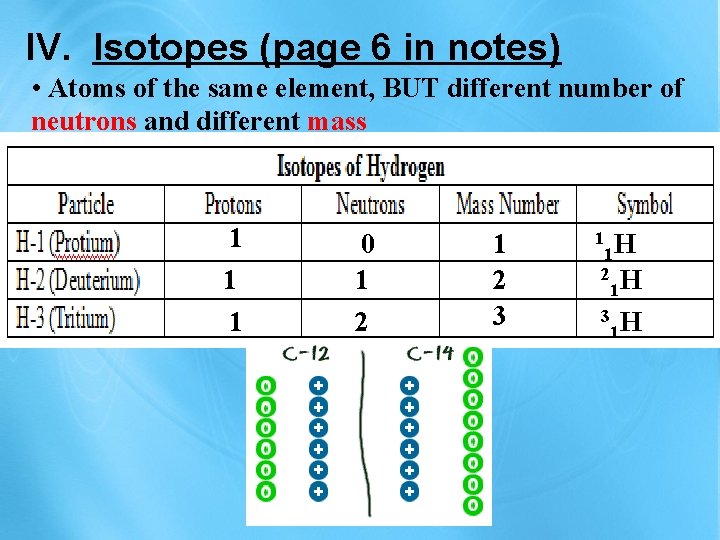
IV. Isotopes (page 6 in notes) • Atoms of the same element, BUT different number of neutrons and different mass 1 1 1 0 1 2 3 1 H 1 2 H 1 3 H 1

IV. Isotopes (page 6 in notes) • Example: Find the number of neutrons in an atom of Se-79 Atomic # = 34 Mass # = 79 79 Se 34 # of n = Mass # - Atomic # = 79 – 34 = 45 n

IV. Isotopes (page 6 in notes) • Example: Find the number of neutrons in an atom of 52 Cr 24 Atomic # = 24 Mass # = 52 # of n = Mass # - Atomic # = 52 – 24 = 28

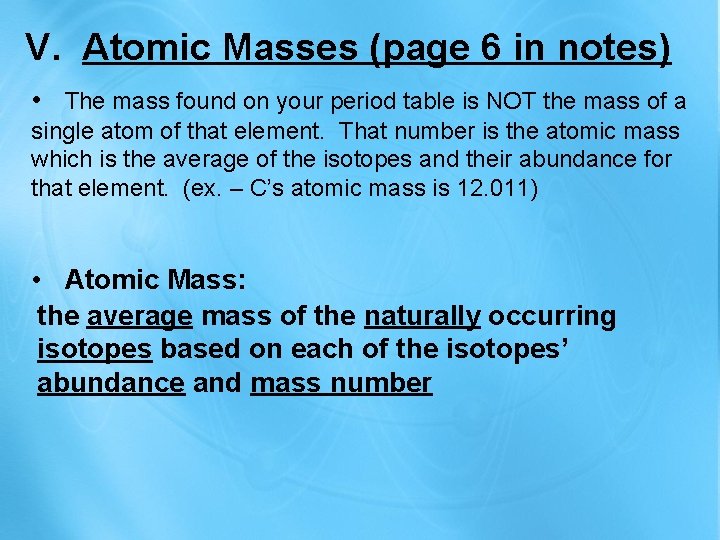
V. Atomic Masses (page 6 in notes) • The mass found on your period table is NOT the mass of a single atom of that element. That number is the atomic mass which is the average of the isotopes and their abundance for that element. (ex. – C’s atomic mass is 12. 011) • Atomic Mass: the average mass of the naturally occurring isotopes based on each of the isotopes’ abundance and mass number

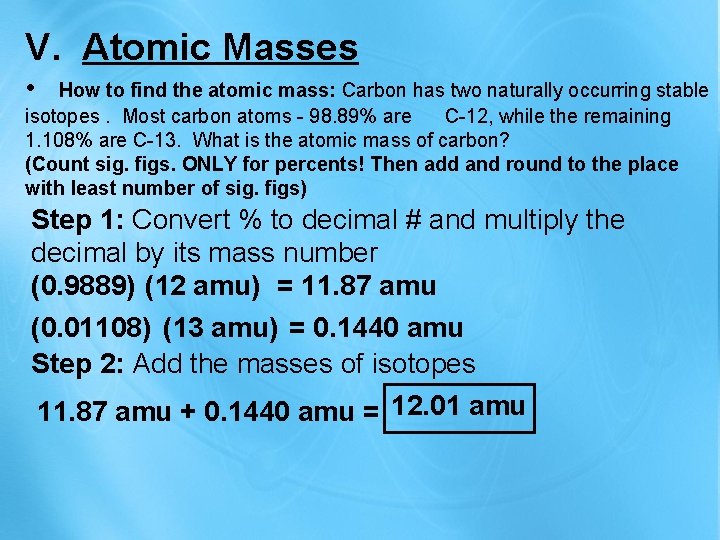
V. Atomic Masses • How to find the atomic mass: Carbon has two naturally occurring stable isotopes. Most carbon atoms - 98. 89% are C-12, while the remaining 1. 108% are C-13. What is the atomic mass of carbon? (Count sig. figs. ONLY for percents! Then add and round to the place with least number of sig. figs) Step 1: Convert % to decimal # and multiply the decimal by its mass number (0. 9889) (12 amu) = 11. 87 amu (0. 01108) (13 amu) = 0. 1440 amu Step 2: Add the masses of isotopes 11. 87 amu + 0. 1440 amu = 12. 01 amu

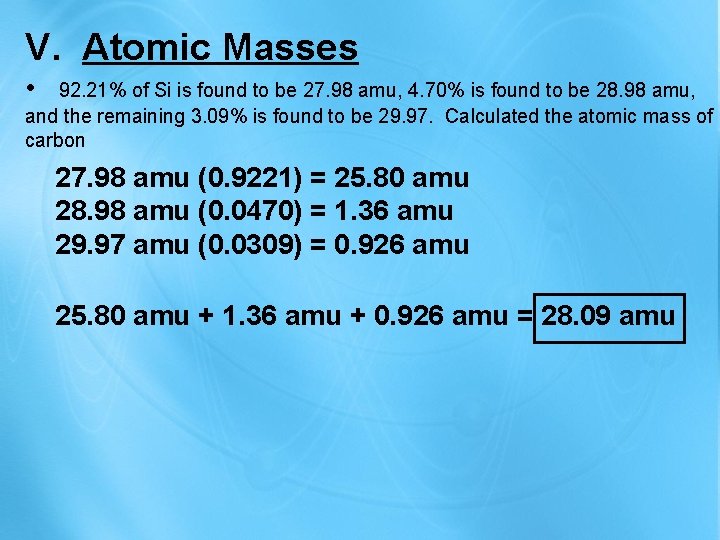
V. Atomic Masses • 92. 21% of Si is found to be 27. 98 amu, 4. 70% is found to be 28. 98 amu, and the remaining 3. 09% is found to be 29. 97. Calculated the atomic mass of carbon 27. 98 amu (0. 9221) = 25. 80 amu 28. 98 amu (0. 0470) = 1. 36 amu 29. 97 amu (0. 0309) = 0. 926 amu 25. 80 amu + 1. 36 amu + 0. 926 amu = 28. 09 amu

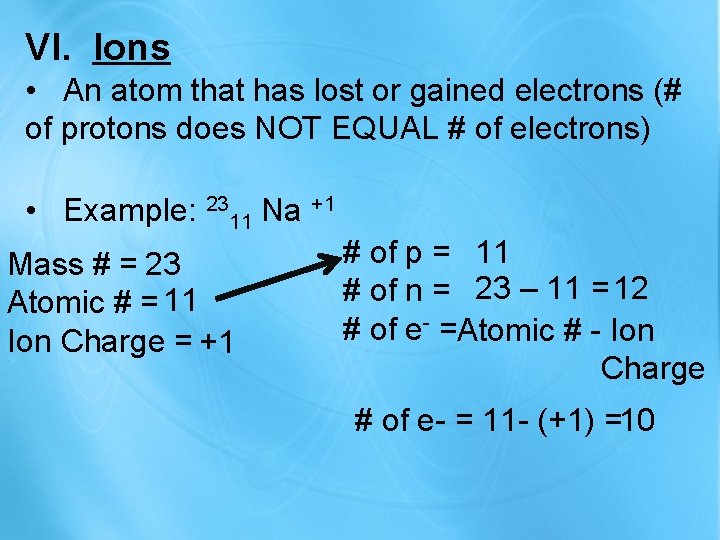
VI. Ions • An atom that has lost or gained electrons (# of protons does NOT EQUAL # of electrons) • Example: 2311 Na +1 Mass # = 23 Atomic # = 11 Ion Charge = +1 # of p = 11 # of n = 23 – 11 = 12 # of e- =Atomic # - Ion Charge # of e- = 11 - (+1) =10

VI. Ions • Anion: Negatively charged ion (atom GAINED e-) • Cation: Positively charged ion (atom LOST e-) Remember: a CATion is PAWsitive

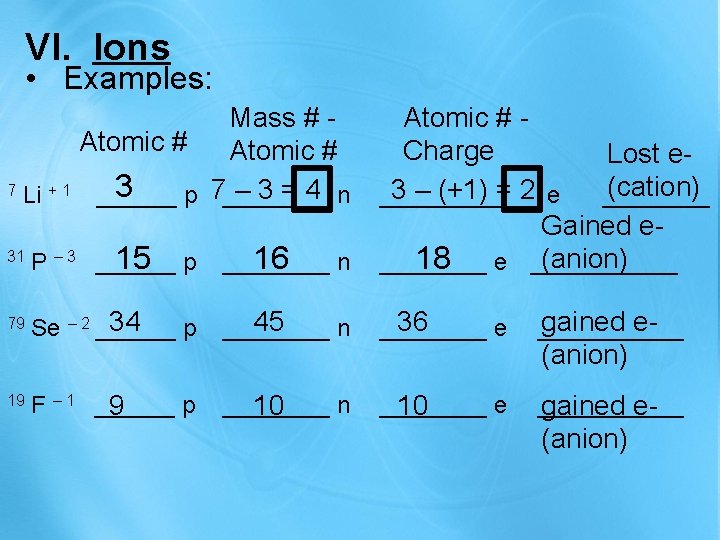
VI. Ions • Examples: 31 15 p P – 3 ______ 16 n ____ Atomic # Charge Lost e(cation) 3 – (+1) = 2 e ______ Gained e(anion) 18 e ______ 79 34 Se – 2 ______ p 45 ____ n 36 ____ e gained e______ F – 1 ______ p 9 ____ n 10 ____ e 10 ______ gained e- 7 Li + 1 19 Mass # Atomic # 3 – 3=4 n ______ p 7____ (anion)

MOVING BACK TO SECTION III

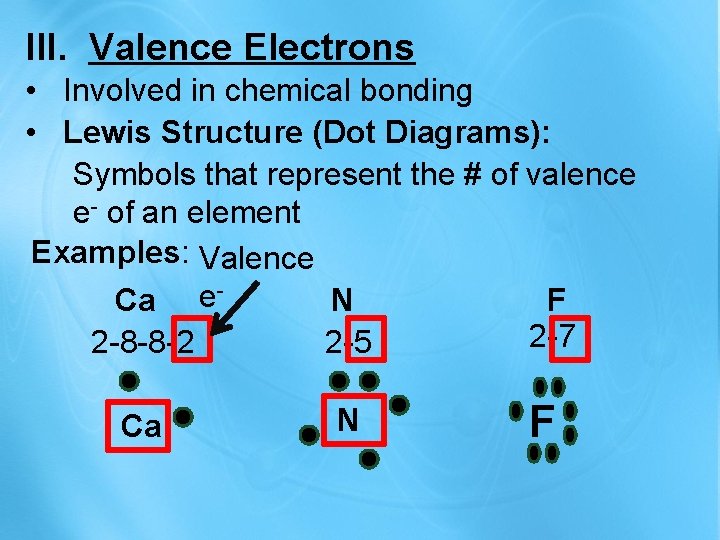
III. Valence Electrons • Involved in chemical bonding • Lewis Structure (Dot Diagrams): Symbols that represent the # of valence e- of an element Examples: Valence Ca e. N F 2 -7 2 -8 -8 -2 2 -5 Ca N F

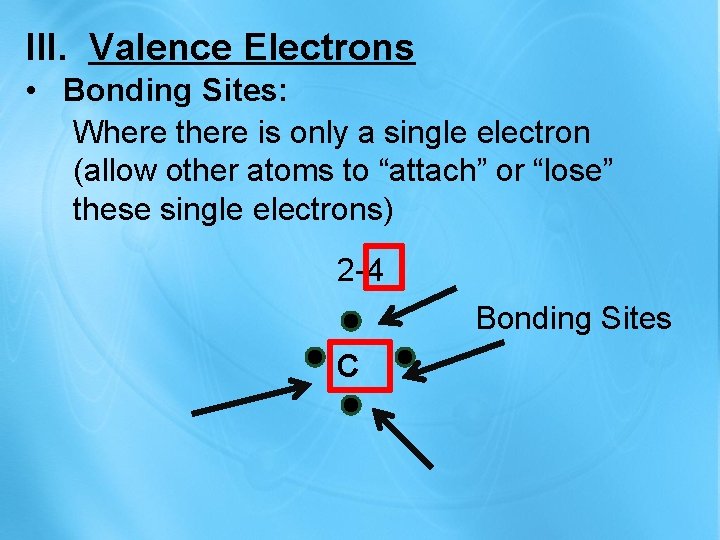
III. Valence Electrons • Bonding Sites: Where there is only a single electron (allow other atoms to “attach” or “lose” these single electrons) 2 -4 Bonding Sites C

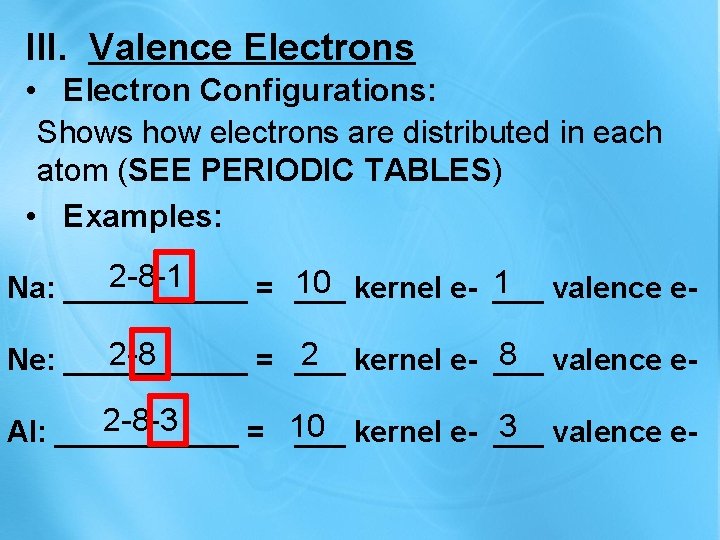
III. Valence Electrons • Electron Configurations: Shows how electrons are distributed in each atom (SEE PERIODIC TABLES) • Examples: 2 -8 -1 10 1 Na: ______ = ___ kernel e- ___ valence e 2 -8 2 8 Ne: ______ = ___ kernel e- ___ valence e 2 -8 -3 10 3 Al: ______ = ___ kernel e- ___ valence e-

III. Valence Electrons • Excited State Electrons and Emission Spectrum: A method used to identify unknown elements How electrons become excited (youtube) How spectra is produced (youtube) Periodic Table of Emission/Absorption Spectrum

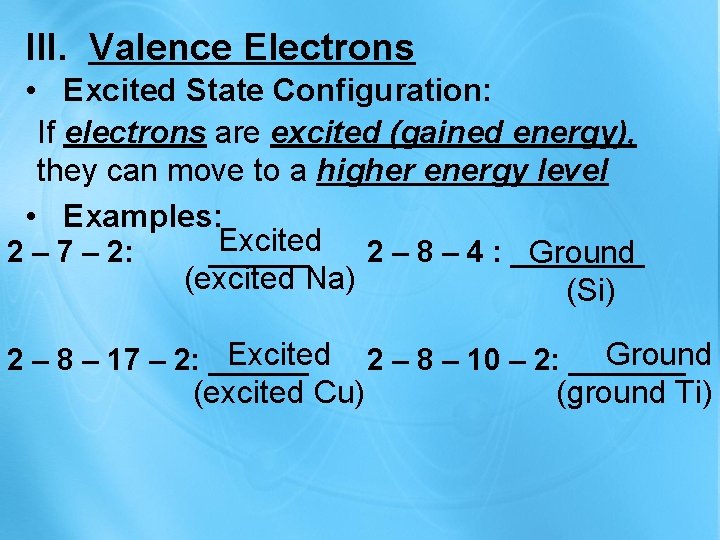
III. Valence Electrons • Excited State Configuration: If electrons are excited (gained energy), they can move to a higher energy level • Examples: Excited 2 – 8 – 4 : ____ 2 – 7 – 2: ______ Ground (excited Na) (Si) Excited 2 – 8 – 17 – 2: ______ (excited Cu) Ground 2 – 8 – 10 – 2: _______ (ground Ti)

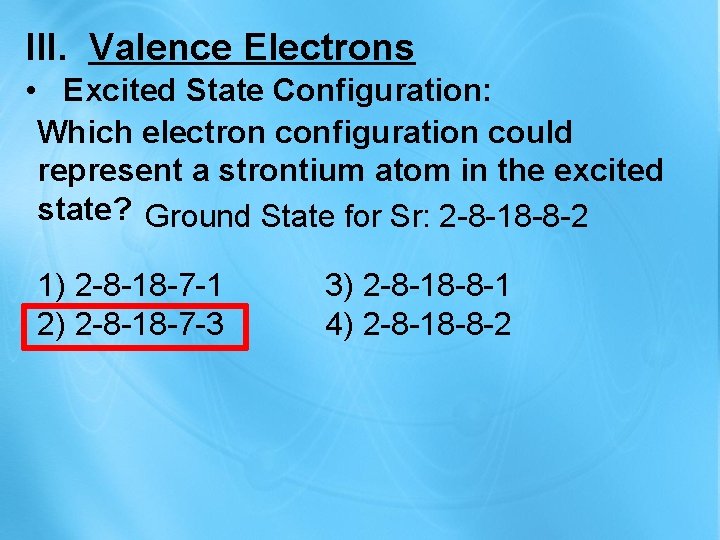
III. Valence Electrons • Excited State Configuration: Which electron configuration could represent a strontium atom in the excited state? Ground State for Sr: 2 -8 -18 -8 -2 1) 2 -8 -18 -7 -1 3) 2 -8 -18 -8 -1 2) 2 -8 -18 -7 -3 4) 2 -8 -18 -8 -2