Week 21 Chemistry Mole Conversions Molar Mass Percent









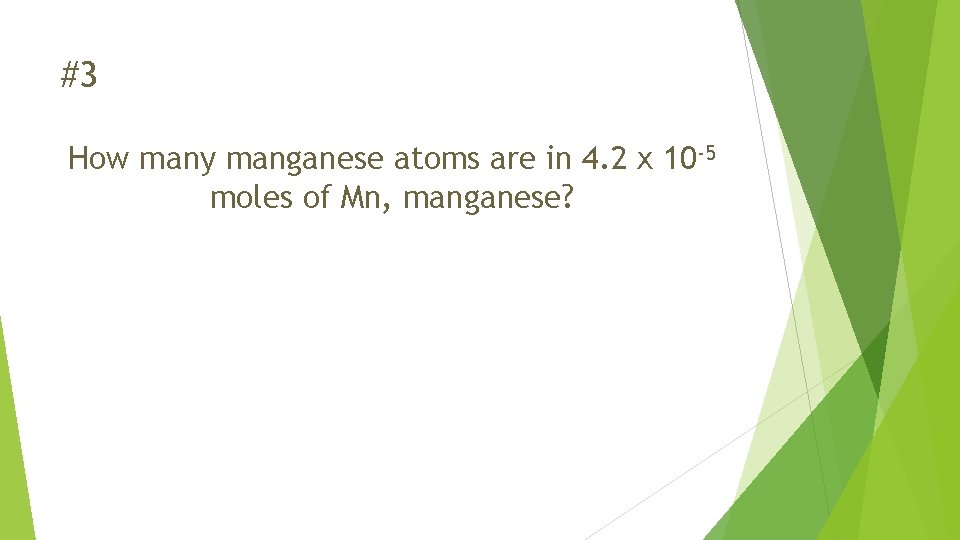





















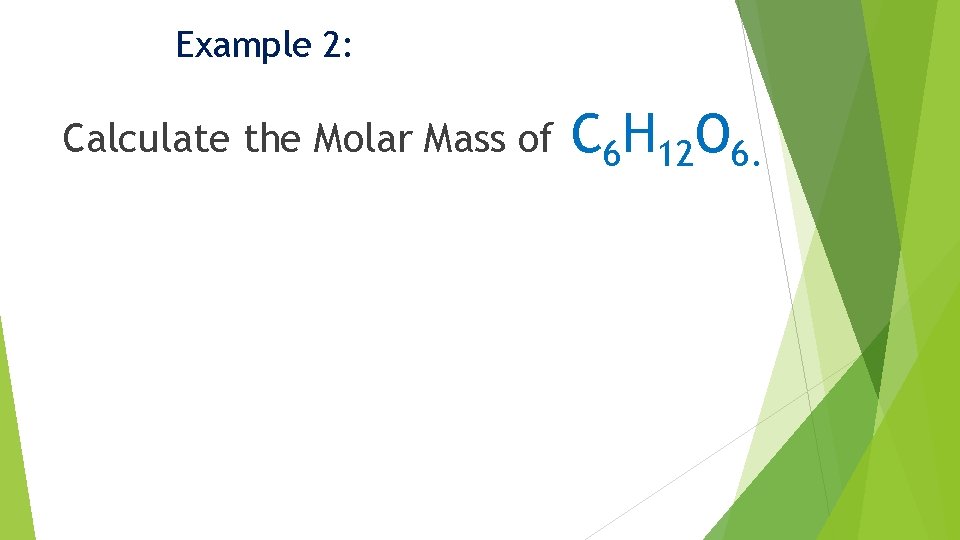





- Slides: 53

Week 21 Chemistry Mole Conversions, Molar Mass, Percent Composition

Warm Up: 4 Minutes Stay in your own seat Write the Learning Target You should be working SILENTLY How many molecules are in 2. 0 moles of Carbon Dioxide? Hint: 1 mole = _______ particles?

Agenda Warm Up: 7 Minutes Notes/Examples: Guided 15 minutes Practice: 12 minutes Independent Closing: Practice: 15 minutes 4 Minutes

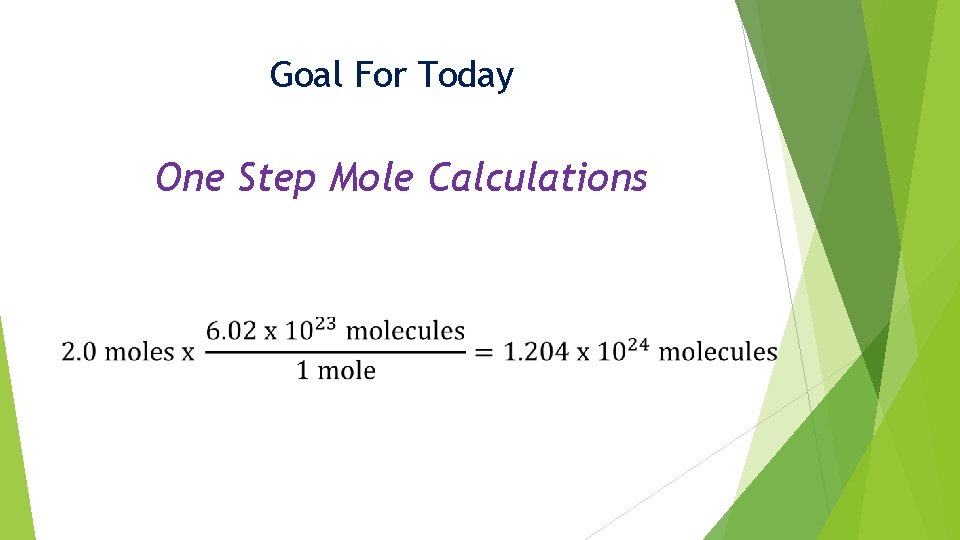
Goal For Today One Step Mole Calculations

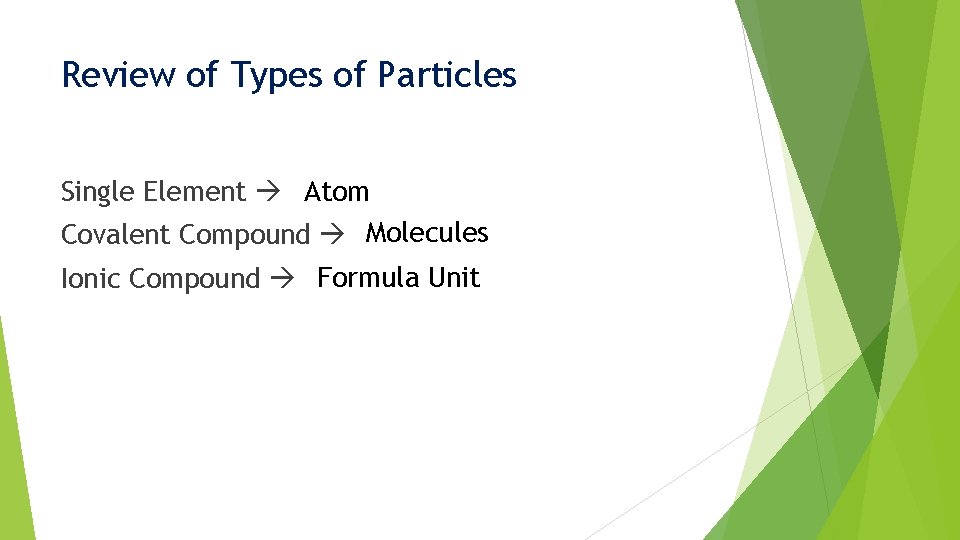
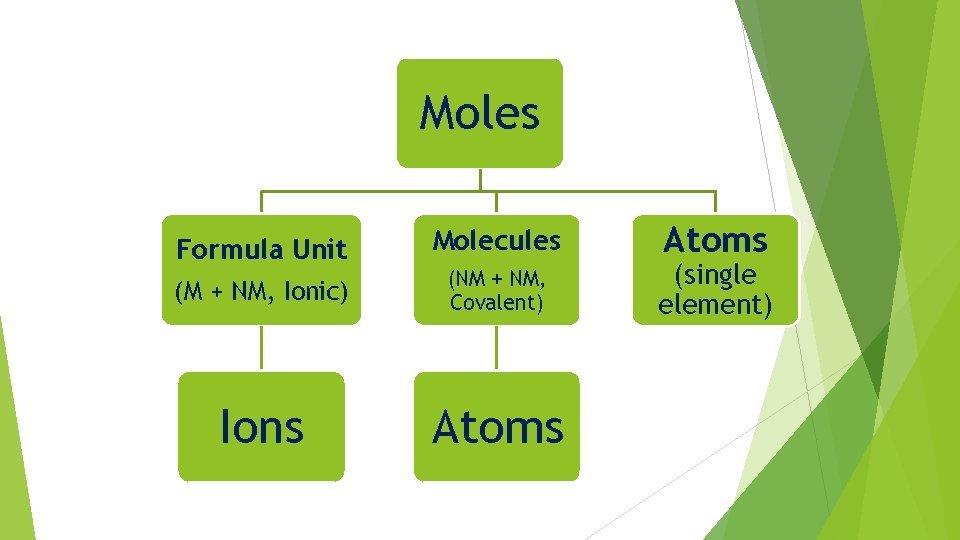
Review of Types of Particles Single Element Atom Covalent Compound Molecules Ionic Compound Formula Unit

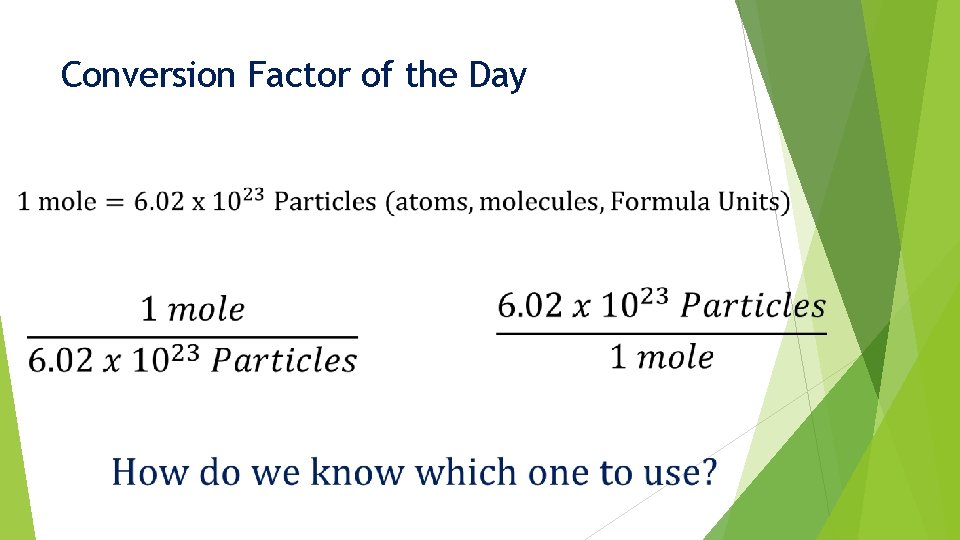
Conversion Factor of the Day

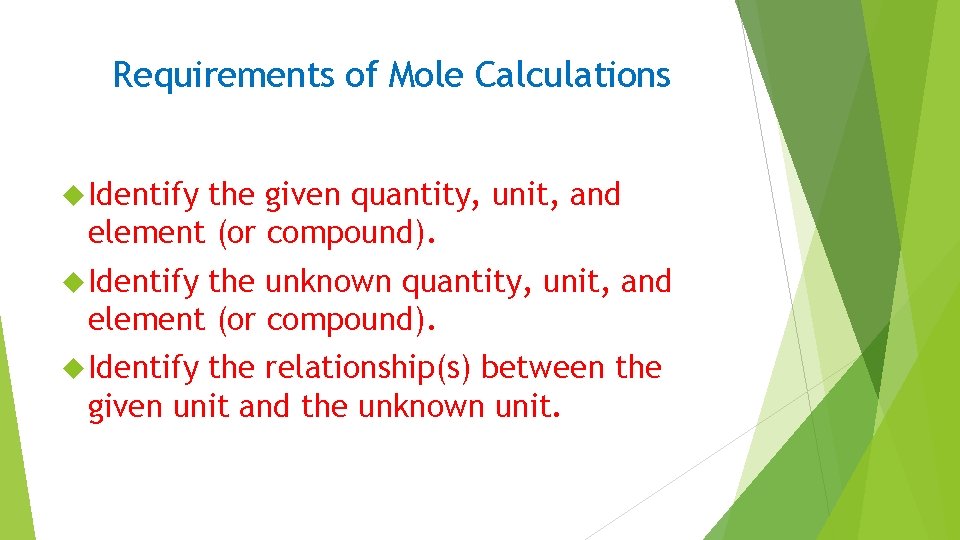
Requirements of Mole Calculations Identify the given quantity, unit, and element (or compound). Identify the unknown quantity, unit, and element (or compound). Identify the relationship(s) between the given unit and the unknown unit.

Example 1 How many atoms does 12. 0 moles of He represent?

Example 2 How many moles does 1. 1 x 1021 molecules of Carbon Dioxide (CO 2) represent?

Example 3 How many formula units are in 5. 1 moles of Calcium Hydroxide, Ca(OH)2?

Guided Practice Expectations As Mr. Ghosh projects the question, silently read the question. (17 seconds) When Mr. Ghosh calls Team, discuss with your teammates to solve the question correctly. (81 seconds) Be ready to share your response when Mr. Ghosh calls SWAG.

#1 How many molecules are in 0. 723 moles of Dihydrogen Monoxide, H 2 O?

#2 How many moles are in 2. 3 x 1025 formula units of Potassium Permanganate, KMn. O 4?
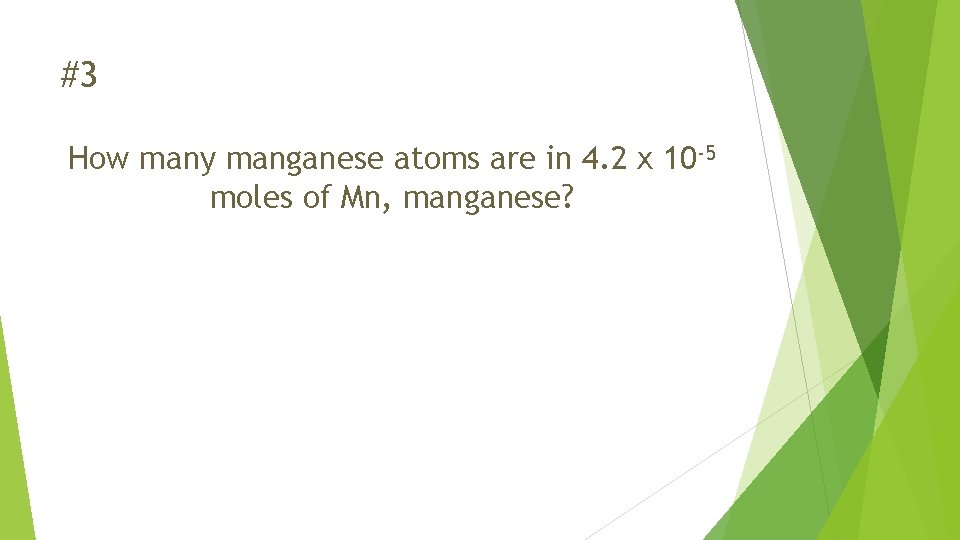
#3 How many manganese atoms are in 4. 2 x 10 -5 moles of Mn, manganese?

Independent Practice 85% Practice makes Perfect

Closing What relationships are necessary to perform mole calculations?

Warm Up: 4 Minutes Stay in your own seat You should be working SILENTLY How many moles are in 6. 42 x 1028 atoms of gold? No more than 4 people per row Write the Learning Target

Agenda Warm Up: 7 Minutes Expectations for Stations: 4 minutes Stations Review: 40 minutes Closing: 2 Minutes

How Will Stations Work? ? You will be placed with a group of other scholars to complete four stations on the following topics: Ø Station 1: Mole Calculations Ø Station 2: Writing Formulas Ø Station 3: Writing Names of Compounds Ø Station You Need: • Periodic Table • Notes 4: Quantifying Atoms and Ions in Molecules and Formula Units

How Will Stations Work? ? You will spend 10 minutes at each station working each problem as a group on scratch paper. You will write your FINAL answer on the answer document. When time is called, you will leave your current station and move on to the next station You will have 10 seconds to move to the next station

Closing Why did we review these concepts? is it important that everybody master these objectives?

About your seats Seats were chosen to help you focus and succeed in class Grades You If You and behavior were factors must sit in your assigned seat every day not, I will mark you absent may NEVER move the seats around. Seats are arranged to make sure everyone can see the board

Warm Up: 4 Minutes Stay in your own seat You should be working SILENTLY How many molecules are in 2. 0 moles of Carbon Dioxide (CO 2)? How many oxygen atoms are in one molecule of Carbon Dioxide (CO 2)? Write the Learning Target

Agenda Seating Warm Chart: 3 minutes Up: 7 Minutes Notes/Examples: Guided 15 minutes Practice: 12 minutes Independent Closing: Practice: 12 minutes 4 Minutes
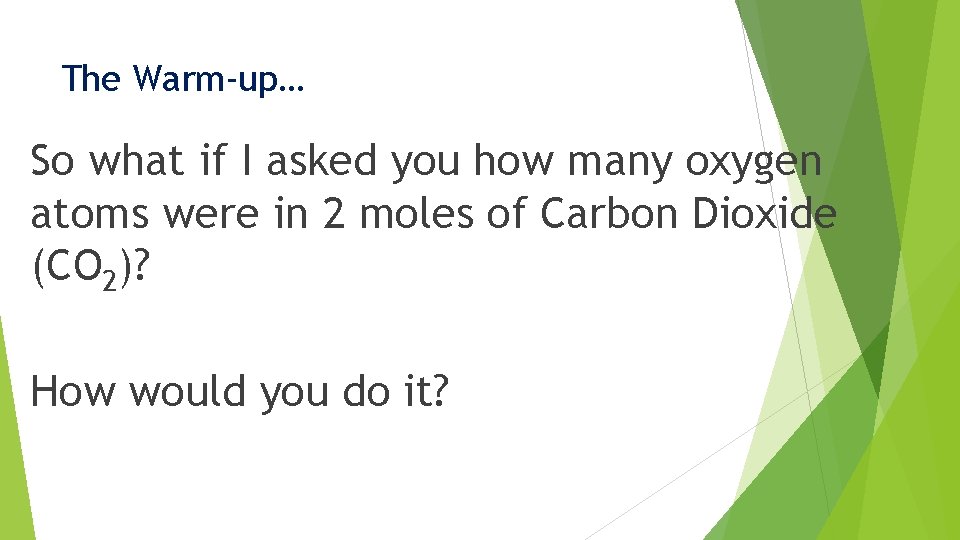
The Warm-up… So what if I asked you how many oxygen atoms were in 2 moles of Carbon Dioxide (CO 2)? How would you do it?

Question… What is 2 x 3? 6 What is 6 x 4? 24 How did we do this? What is 2 x 3 x 4? 24

Goal For Today Multi Step Mole Calculations

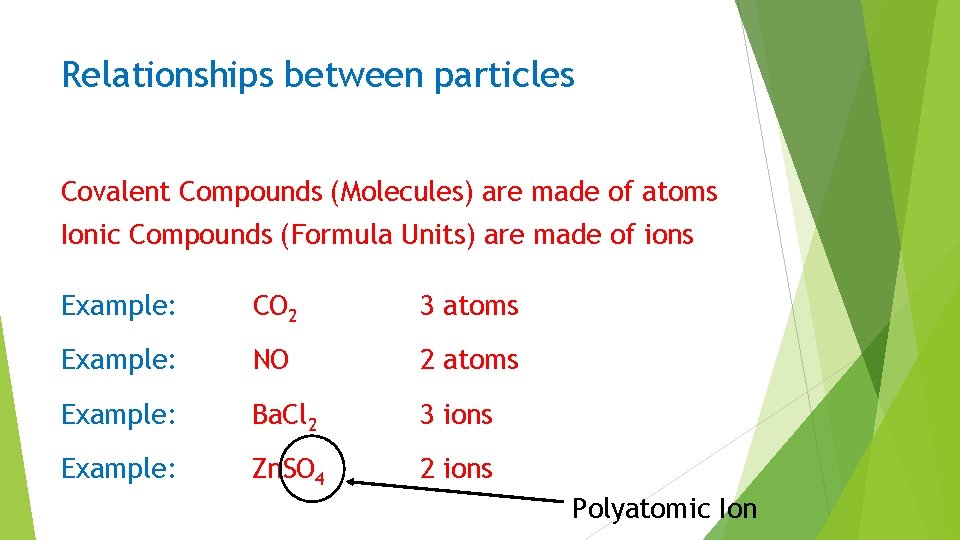
Relationships between particles Covalent Compounds (Molecules) are made of atoms Ionic Compounds (Formula Units) are made of ions Example: CO 2 3 atoms Example: NO 2 atoms Example: Ba. Cl 2 3 ions Example: Zn. SO 4 2 ions Polyatomic Ion

Reminder: Requirements of Mole Calculations Identify the given quantity, unit, and element (or compound). Identify the unknown quantity, unit, and element (or compound). Identify the relationship(s) between the given unit and the unknown unit.

Moles Formula Unit Molecules (M + NM, Ionic) (NM + NM, Covalent) Ions Atoms (single element)

Example 1 How many atoms does 12. 0 moles of Sulfur Dioxide (SO 2) represent?

Example 2 How many hydroxide ions are in 5. 1 moles of Calcium Hydroxide, Ca(OH)2?

Guided Practice Expectations As Mr. Ghosh projects the question, silently read the question. (17 seconds) When Mr. Ghosh calls Team, discuss with your teammates to solve the question correctly. (81 seconds) Be ready to share your response when Mr. Ghosh calls SWAG.

#1 How many ions are in 2. 3 formula units of Potassium Permanganate, KMn. O 4?

#2 How many oxygen atoms are in 0. 723 moles of Dihydrogen Monoxide, H 2 O?

#3 How many zinc ions are in 4. 2 x 10 -5 moles of Zinc Chloride (Zn. Cl 2)?

Independent Practice 85% Practice makes Perfect

Closing What relationships are necessary to perform mole calculations?

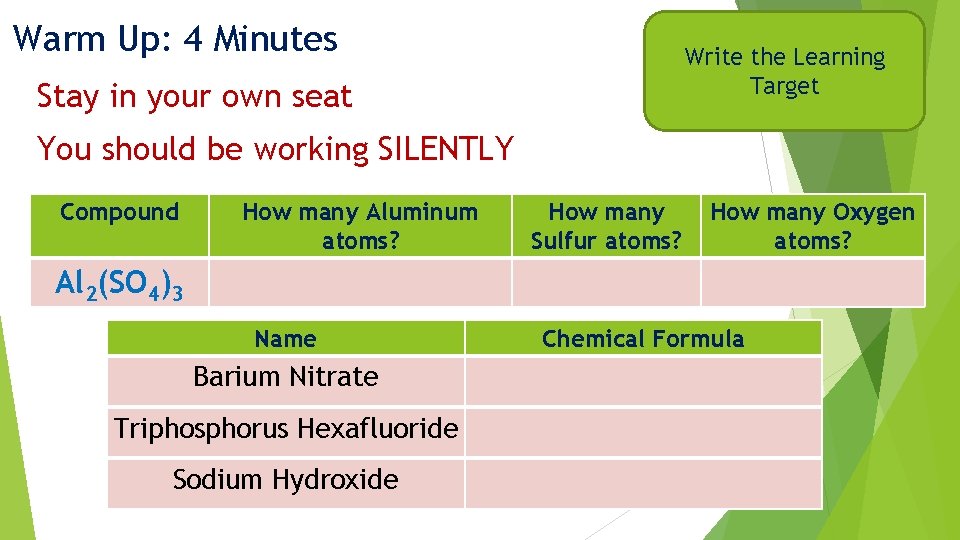
Warm Up: 4 Minutes Write the Learning Target Stay in your own seat You should be working SILENTLY Compound How many Aluminum atoms? How many Sulfur atoms? How many Oxygen atoms? Al 2(SO 4)3 Name Barium Nitrate Triphosphorus Hexafluoride Sodium Hydroxide Chemical Formula

Agenda Warm Up: 7 Minutes Notes/Examples: Guided 13 minutes Practice: 10 minutes Independent Closing: Practice: 13 minutes 2 Minutes

What is Molar Mass? The mass (in grams) of one mole of the substance

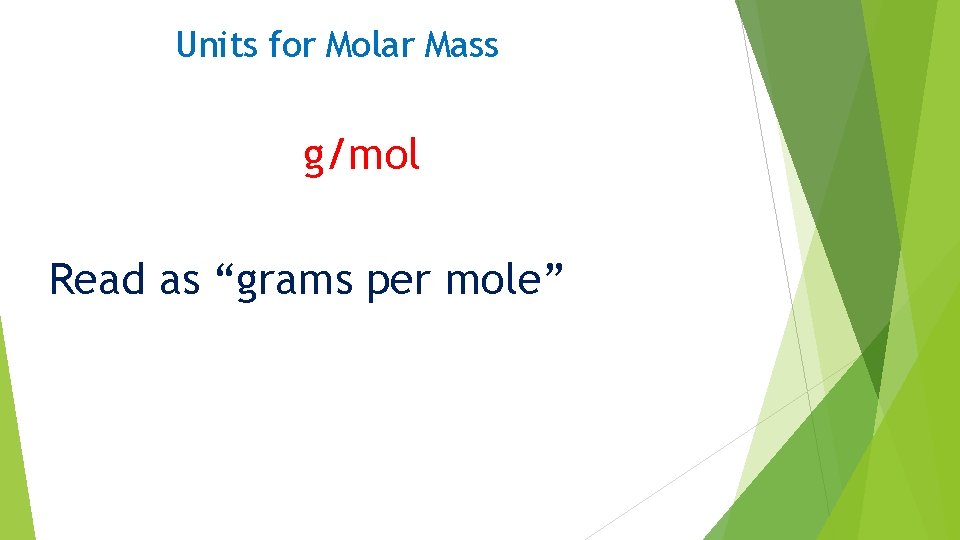
Units for Molar Mass g/mol Read as “grams per mole”

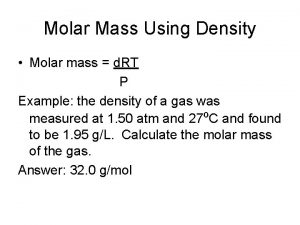
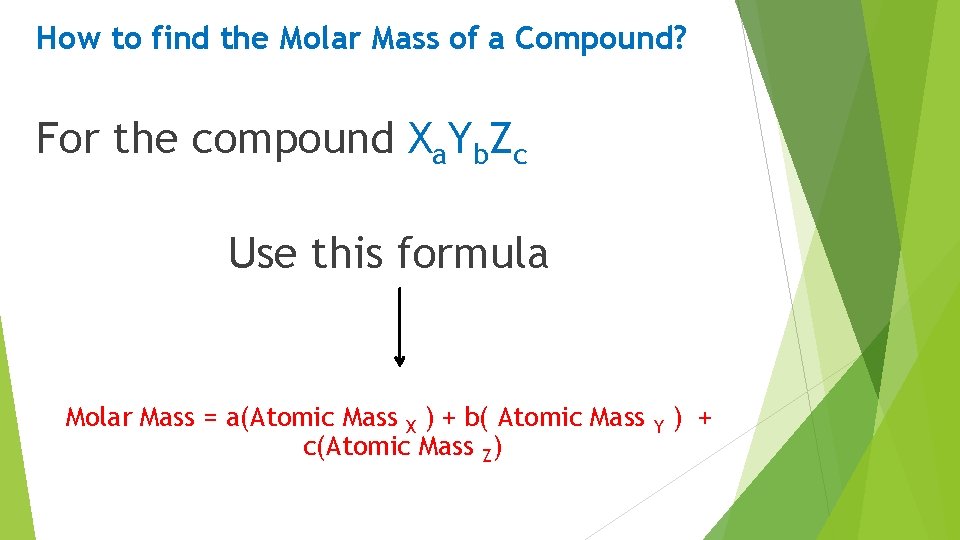
How to find the Molar Mass of a Compound? For the compound Xa. Yb. Zc Use this formula Molar Mass = a(Atomic Mass X ) + b( Atomic Mass Y ) + c(Atomic Mass Z)

Example 1: Calculate the Molar Mass of Na. Br
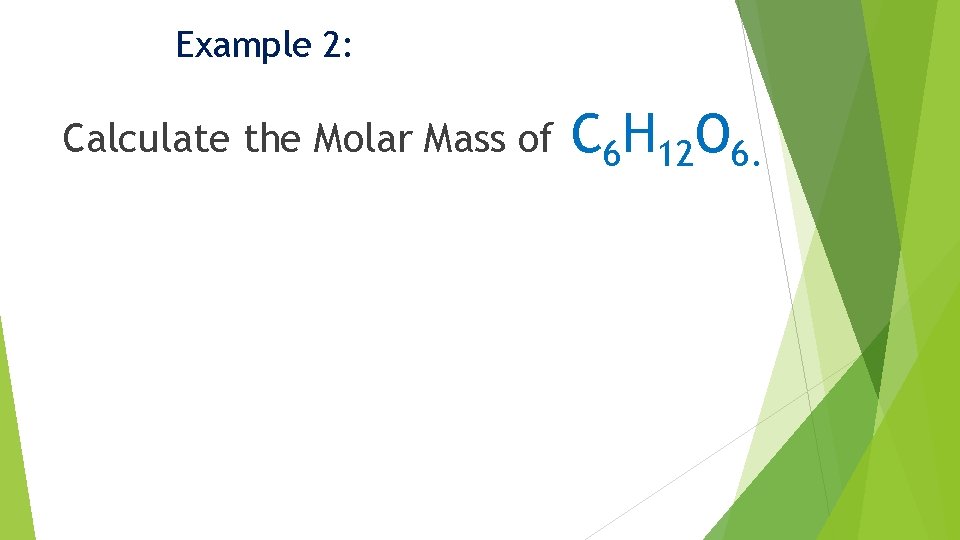
Example 2: Calculate the Molar Mass of C 6 H 12 O 6.

Example 3: Calculate the Molar Mass of Al 2(SO 4)3

Expectations for Guided Practice Take the first 13 seconds to write the chemical formula or name. With your shoulder partner, take 61 seconds to find the molar mass. When Mr. Ghosh calls SWAG, be ready to share your answers out loud.

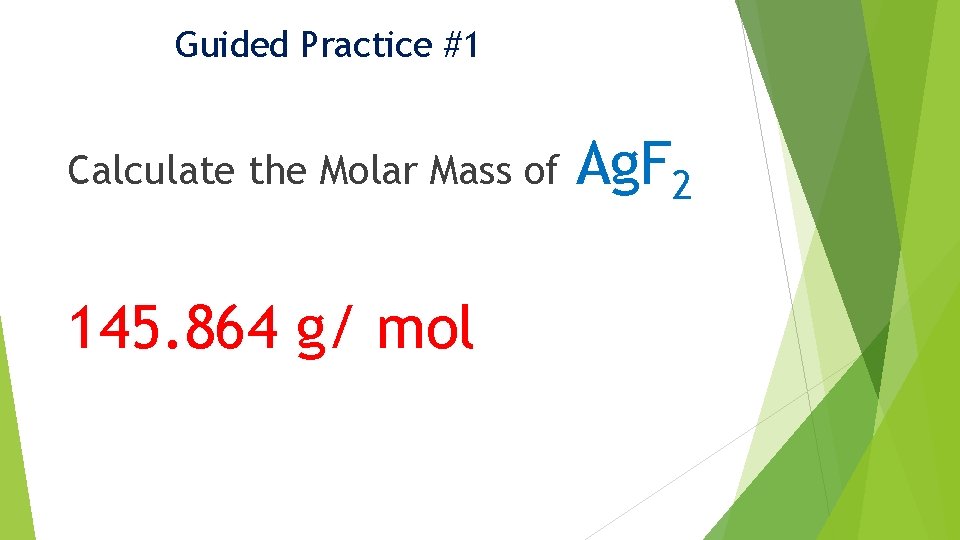
Guided Practice #1 Calculate the Molar Mass of 145. 864 g/ mol Ag. F 2

Guided Practice #2 Calculate the Molar Mass of Calcium Nitrate. Ca(NO 3)2 164. 086 g/ mol

Guided Practice #3 Calculate the Molar Mass of Tetracarbon Trioxide. C 4 O 3 96. 041 g/mol

Independent Practice 85% Practice makes Perfect

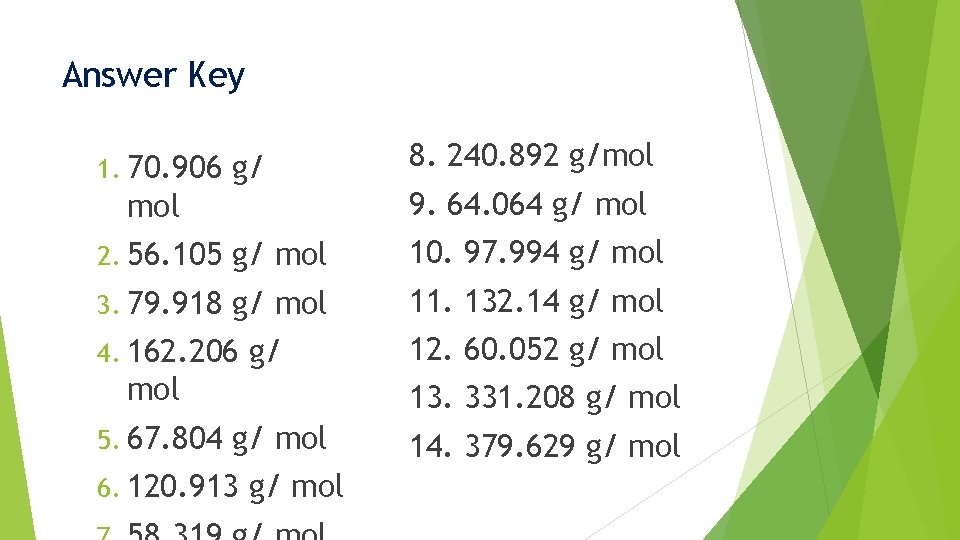
Answer Key g/ 8. 240. 892 g/mol 2. 56. 105 g/ mol 10. 97. 994 g/ mol 3. 79. 918 g/ mol 11. 132. 14 g/ mol 1. 70. 906 mol 4. 162. 206 g/ mol 5. 67. 804 9. 64. 064 g/ mol 12. 60. 052 g/ mol 13. 331. 208 g/ mol 6. 120. 913 g/ mol 14. 379. 629 g/ mol

Closing In terms of a percentage, does the molar mass represent the part or the whole?
 Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Mountains into molehills mass-mole conversions answers
Mountains into molehills mass-mole conversions answers How to convert grams to moles
How to convert grams to moles Mole mass and mole volume relationships
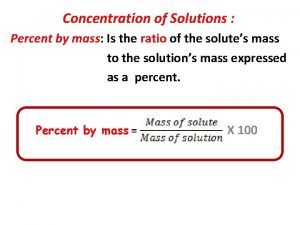
Mole mass and mole volume relationships How to find percent concentration
How to find percent concentration Percent concentration formula
Percent concentration formula Volume to mols
Volume to mols How to convert moles to grams
How to convert moles to grams Molar mass def
Molar mass def Units for molar mass
Units for molar mass Formula mass vs molar mass
Formula mass vs molar mass Atomic mass vs molar mass
Atomic mass vs molar mass Mole conversion task cards answers
Mole conversion task cards answers Conversions examples
Conversions examples Molar conversions ch 3 & 7 answers
Molar conversions ch 3 & 7 answers Two-step molar conversions worksheet answers
Two-step molar conversions worksheet answers Gram to gram conversion
Gram to gram conversion Mole mole factor
Mole mole factor Molar mass of sucrose
Molar mass of sucrose Stoichiometry mole-mole
Stoichiometry mole-mole M/v to g/l
M/v to g/l Frequency missing
Frequency missing Dimensional analysis
Dimensional analysis Week by week plans for documenting children's development
Week by week plans for documenting children's development What's a mole chemistry
What's a mole chemistry Molar road map
Molar road map Mole formule
Mole formule Mole hill chemistry
Mole hill chemistry Chapter 10 the mole study guide
Chapter 10 the mole study guide Mole bridge chemistry
Mole bridge chemistry Mole bridge chemistry
Mole bridge chemistry Cookie stoichiometry
Cookie stoichiometry Stoichiometry airbag lab answer key
Stoichiometry airbag lab answer key Chapter 10 study guide the mole
Chapter 10 study guide the mole Let's make some cookies
Let's make some cookies Moles to grams
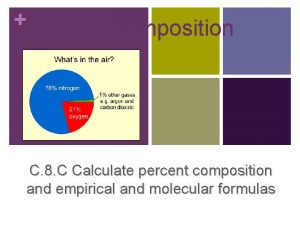
Moles to grams Percent composition
Percent composition How to calculate percent error in chemistry
How to calculate percent error in chemistry How to calculate percent error chemistry
How to calculate percent error chemistry Negative percent error
Negative percent error How to calculate percent error in chemistry
How to calculate percent error in chemistry How to calculate yield in chemistry
How to calculate yield in chemistry Percent yield efficiency
Percent yield efficiency Molar relationships chemistry
Molar relationships chemistry One mole of pennies
One mole of pennies Convert from mass to moles
Convert from mass to moles Mole by volume
Mole by volume Mole to mass
Mole to mass Formula mass vs gram formula mass
Formula mass vs gram formula mass Percent by mass solution
Percent by mass solution How to convert percent to molarity
How to convert percent to molarity How to get mass percent
How to get mass percent Part/whole x 100
Part/whole x 100 Mass percent formula
Mass percent formula