Chapter 6 The Mole Molar Mass Mole Relationships









- Slides: 27

Chapter 6 The Mole Molar Mass Mole Relationships in Chemical Equations Mass Calculations for Reactions 1

1 trio Collection Terms = 3 singers 1 six-pack Cola = 6 cans Cola drink 1 dozen donuts = 12 donuts 1 gross of pencils = 144 pencils 2

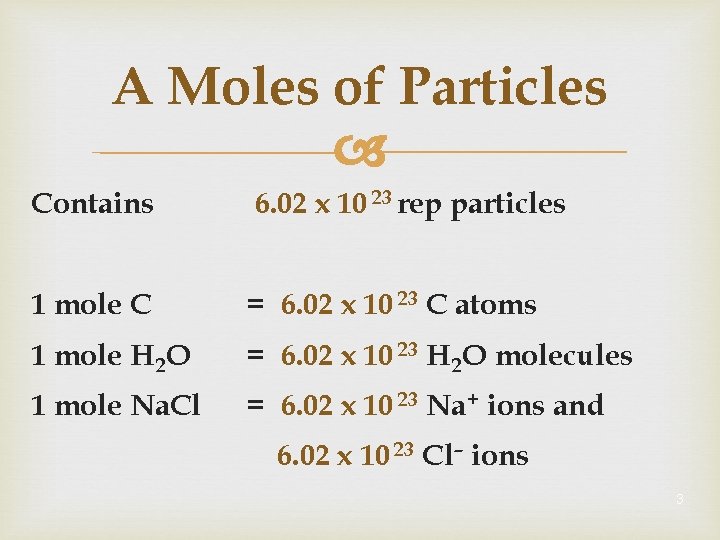
A Moles of Particles Contains 6. 02 x 10 23 rep particles 1 mole C = 6. 02 x 10 23 C atoms 1 mole H 2 O = 6. 02 x 10 23 H 2 O molecules 1 mole Na. Cl = 6. 02 x 10 23 Na + ions and 6. 02 x 10 23 Cl– ions 3

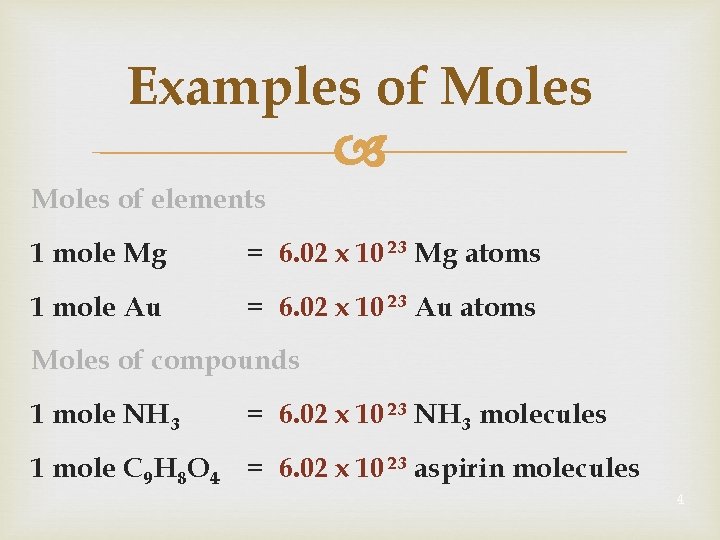
Examples of Moles of elements 1 mole Mg = 6. 02 x 10 23 Mg atoms 1 mole Au = 6. 02 x 10 23 Au atoms Moles of compounds 1 mole NH 3 = 6. 02 x 10 23 NH 3 molecules 1 mole C 9 H 8 O 4 = 6. 02 x 10 23 aspirin molecules 4

Avogadro’s Number 6. 02 x 10 particles 23 1 mole or 1 mole 6. 02 x 10 23 particles 5

Learning Check Suppose we invented a new collection unit called a mep. One mep contains 8 objects. A. How many paper clips in 1 mep? 1) 1 2) 4 3) 8 B. How many oranges in 2. 0 meps? 1) 4 2) 8 3) 16 C. How many meps contain 40 gummy bears? 1) 5 2) 10 3) 20 6

Solution Suppose we invented a new collection unit called a mep. One mep contains 8 objects. A. How many paper clips in 1 mep? 3) 8 B. How many oranges in 2. 0 meps? 3) 16 C. How many meps contain 40 gummy bears? 1) 5 7

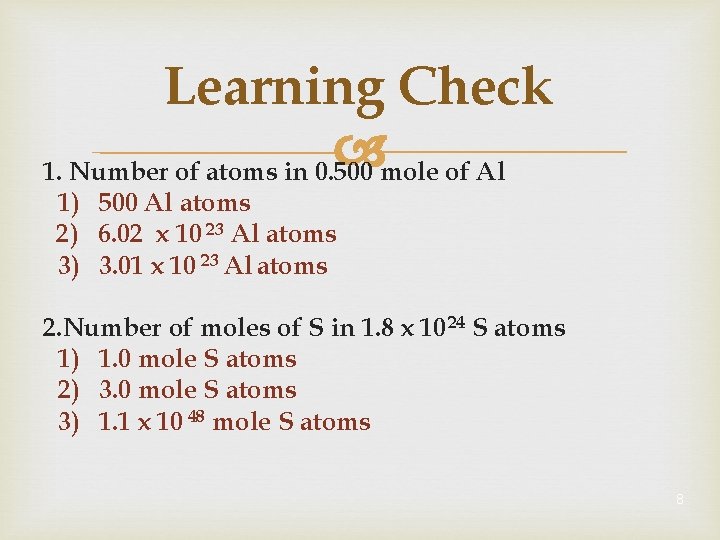
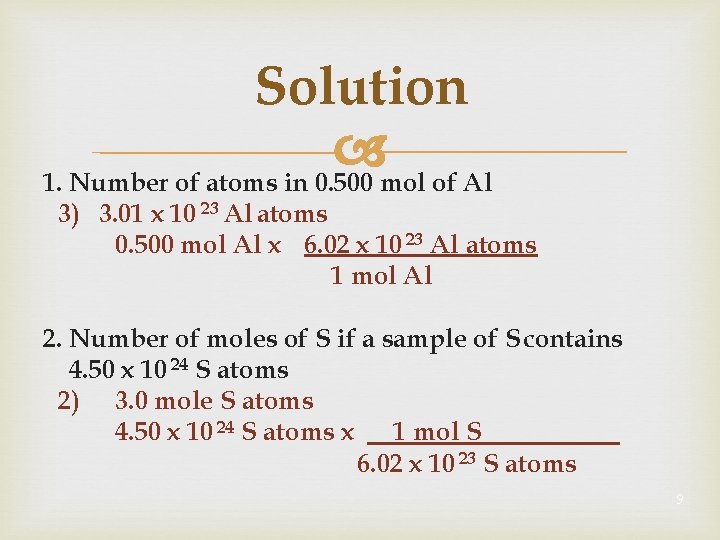
Learning Check 1. Number of atoms in 0. 500 mole of Al 1) 500 Al atoms 2) 6. 02 x 10 23 Al atoms 3) 3. 01 x 10 23 Al atoms 2. Number of moles of S in 1. 8 x 10 24 S atoms 1) 1. 0 mole S atoms 2) 3. 0 mole S atoms 3) 1. 1 x 10 48 mole S atoms 8

Solution 1. Number of atoms in 0. 500 mol of Al 3) 3. 01 x 10 23 Al atoms 0. 500 mol Al x 6. 02 x 10 23 Al atoms 1 mol Al 2. Number of moles of S if a sample of S contains 4. 50 x 10 24 S atoms 2) 3. 0 mole S atoms 4. 50 x 10 24 S atoms x 1 mol S 6. 02 x 10 23 S atoms 9

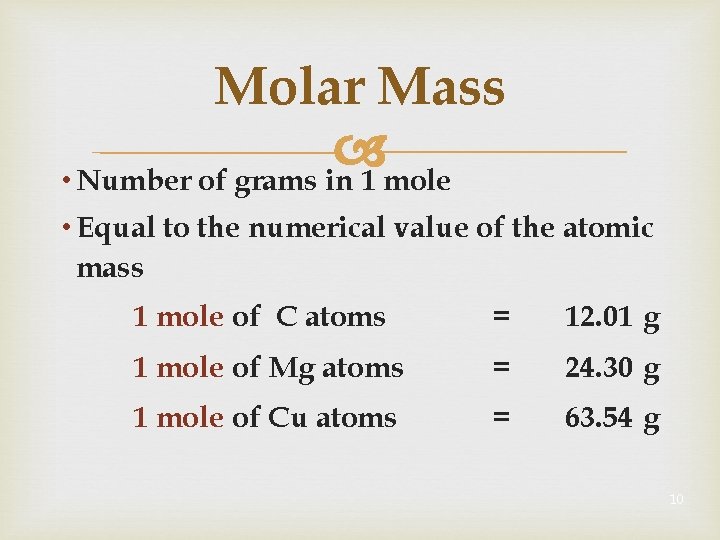
Molar Mass • Number of grams in 1 mole • Equal to the numerical value of the atomic mass 1 mole of C atoms = 12. 01 g 1 mole of Mg atoms = 24. 30 g 1 mole of Cu atoms = 63. 54 g 10

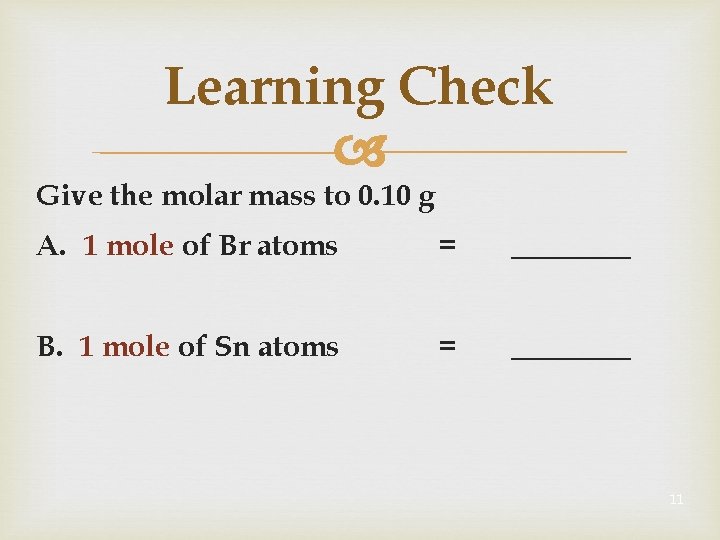
Learning Check Give the molar mass to 0. 10 g A. 1 mole of Br atoms = ____ B. 1 mole of Sn atoms = ____ 11

Solution Give the molar mass to 0. 10 g A. 1 mole of Br atoms = 79. 90 g/mole B. 1 mole of Sn atoms = 118. 71 g/mole 12

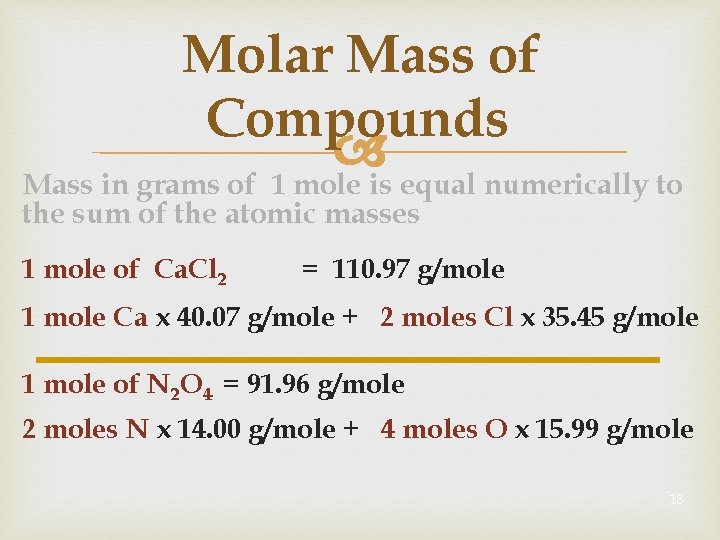
Molar Mass of Compounds Mass in grams of 1 mole is equal numerically to the sum of the atomic masses 1 mole of Ca. Cl 2 = 110. 97 g/mole 1 mole Ca x 40. 07 g/mole + 2 moles Cl x 35. 45 g/mole 1 mole of N 2 O 4 = 91. 96 g/mole 2 moles N x 14. 00 g/mole + 4 moles O x 15. 99 g/mole 13

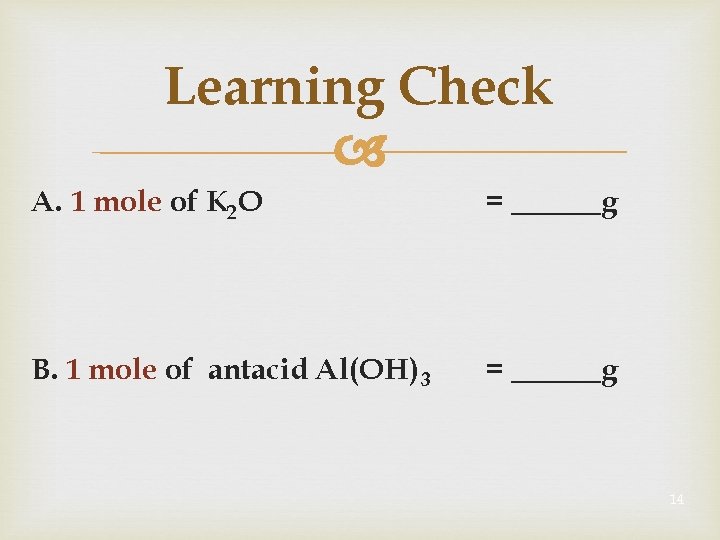
Learning Check A. 1 mole of K 2 O = ______g B. 1 mole of antacid Al(OH) 3 = ______g 14

Solution A. 1 mole of K 2 O = 94. 19 g 2 K x 39. 10 g/mole + 1 O x 15. 99 g/mole B. 1 mole of antacid Al(OH) 3 = 77. 95 g 1 Al x 26. 98 g/mole + 3 O x 15. 99 g/mole + 3 H x 1. 00 15

Learning Check Prozac, C 17 H 18 F 3 NO, is a widely used antidepressant that inhibits the uptake of serotonin by the brain. It has a molar mass of 1) 40. 0 g/mole 2) 262 g/mole 3) 309. 13 g/mole 16

Solution Prozac, C 17 H 18 F 3 NO, is a widely used antidepressant that inhibits the uptake of serotonin by the brain. It has a molar mass of 3) 309. 13 g/mole 17 C (12. 01) + 18 H (1. 00) + 3 F (18. 99) + 1 N (14. 00) + 1 O (15. 99) 17

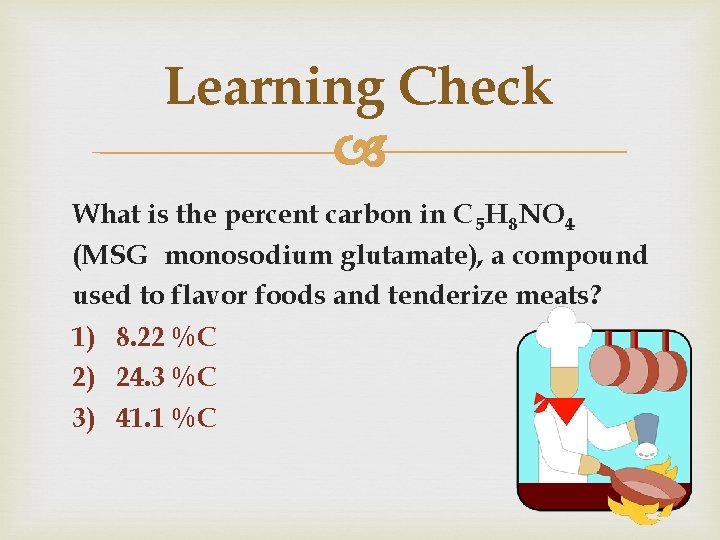
Learning Check What is the percent carbon in C 5 H 8 NO 4 (MSG monosodium glutamate), a compound used to flavor foods and tenderize meats? 1) 8. 22 %C 2) 24. 3 %C 3) 41. 1 %C 18

Solution Molar mass = 146. 0 g/mole % = total g C x 100 total g compound = 60. 0 g C 146. 0 g MSG x 100 = 41. 1% C 19

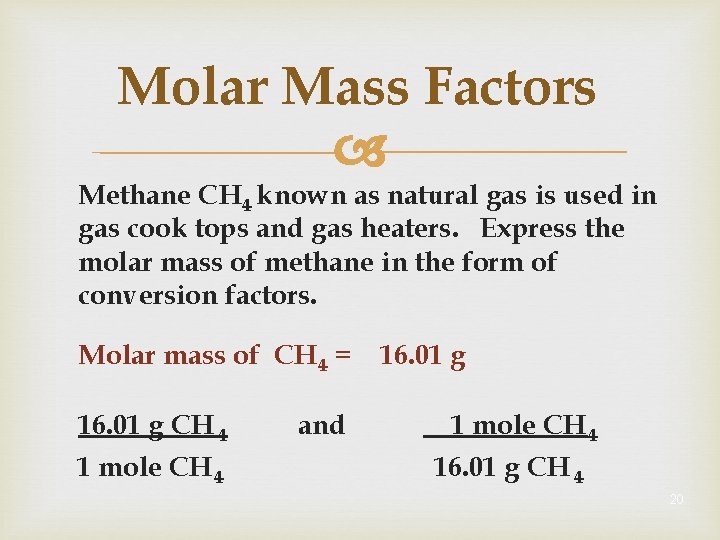
Molar Mass Factors Methane CH 4 known as natural gas is used in gas cook tops and gas heaters. Express the molar mass of methane in the form of conversion factors. Molar mass of CH 4 = 16. 01 g CH 4 1 mole CH 4 and 16. 01 g 1 mole CH 4 16. 01 g CH 4 20

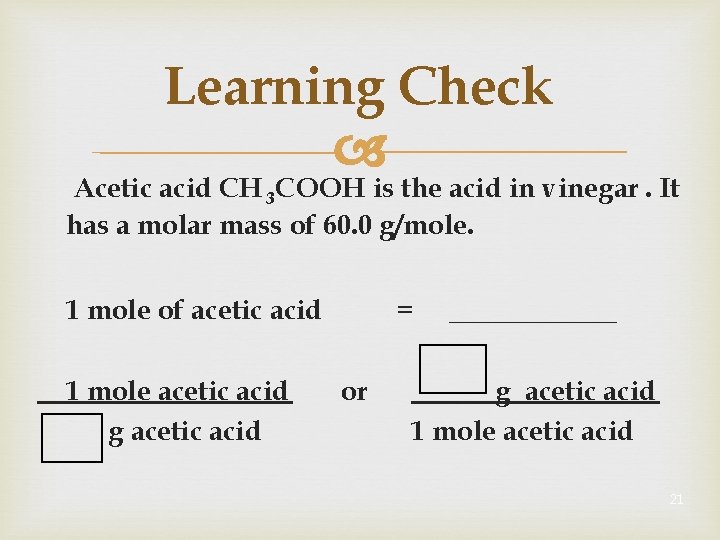
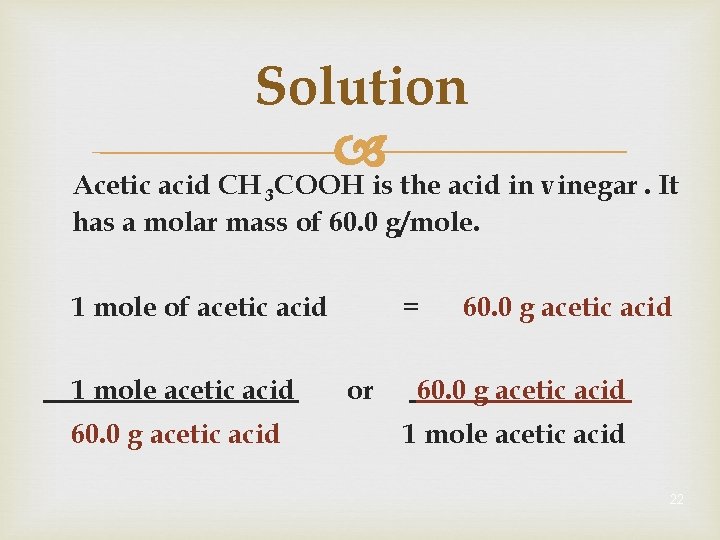
Learning Check Acetic acid CH 3 COOH is the acid in vinegar. It has a molar mass of 60. 0 g/mole. 1 mole of acetic acid 1 mole acetic acid g acetic acid = or ______ g acetic acid 1 mole acetic acid 21

Solution Acetic acid CH 3 COOH is the acid in vinegar. It has a molar mass of 60. 0 g/mole. 1 mole of acetic acid 1 mole acetic acid 60. 0 g acetic acid = or 60. 0 g acetic acid 1 mole acetic acid 22

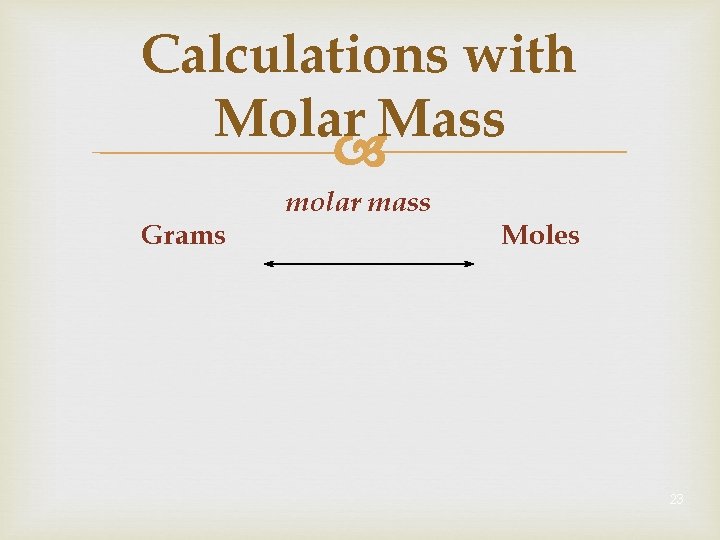
Calculations with Molar Mass Grams molar mass Moles 23

Moles and Grams Aluminum is often used for the structure of light-weight bicycle frames. How many grams of Al are in 3. 00 moles of Al? 3. 00 moles Al ? g Al 24

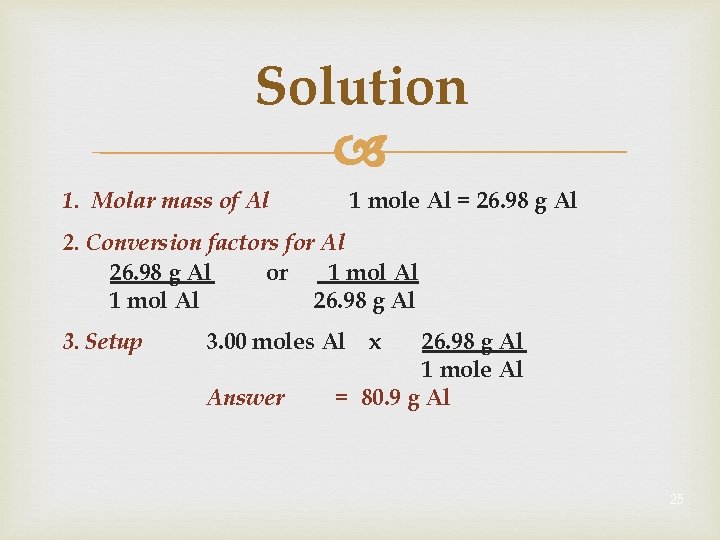
Solution 1 mole Al = 26. 98 g Al 1. Molar mass of Al 2. Conversion factors for Al 26. 98 g Al or 1 mol Al 26. 98 g Al 3. Setup 3. 00 moles Al Answer x 26. 98 g Al 1 mole Al = 80. 9 g Al 25

Learning Check The artificial sweetener aspartame (Nutri. Sweet) formula C 14 H 18 N 2 O 5 is used to sweeten diet foods, coffee and soft drinks. How many moles of aspartame are present in 225 g of aspartame? 26

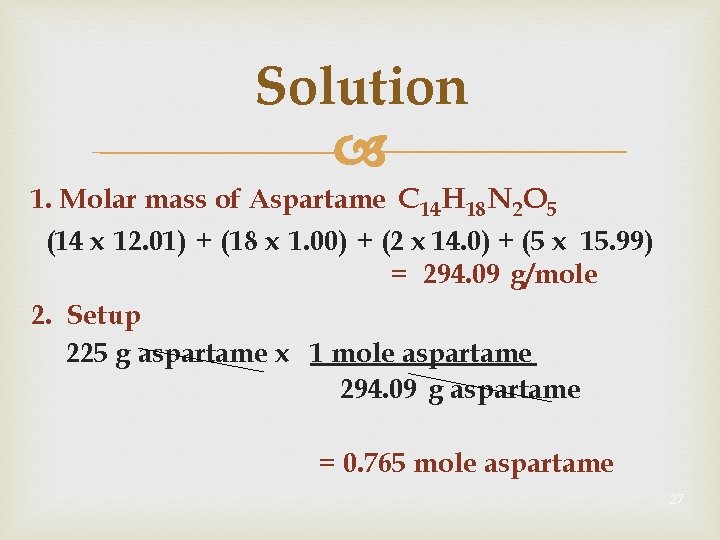
Solution 1. Molar mass of Aspartame C 14 H 18 N 2 O 5 (14 x 12. 01) + (18 x 1. 00) + (2 x 14. 0) + (5 x 15. 99) = 294. 09 g/mole 2. Setup 225 g aspartame x 1 mole aspartame 294. 09 g aspartame = 0. 765 mole aspartame 27