The Mole and Molar Mass The Mole 1










- Slides: 16

The Mole and Molar Mass

• • The Mole 1 dozen = 12 eggs 1 ream = 500 sheets of paper 1 hat trick = 3 goals 1 mole = 6. 022 x 1023 of something – Or 602, 200, 000, 000 Also known as “Avogadro’s number”

The Mole • 1 mole of hydrogen = 6. 022 x 1023 atoms • 1 mole of carbon = 6. 022 x 1023 atoms • 1 mole of gold = 6. 022 x 1023 atoms • 1 mole of calcium = 6. 022 x 1023 atoms • See the pattern?

Understanding Check Suppose we invented a new collection unit called a “mep”. One mep contains 8 objects. A. How many paper clips in 1. 0 mep? B. How many oranges in 2. 0 meps? C. How many meps contain 40 gummy bears?

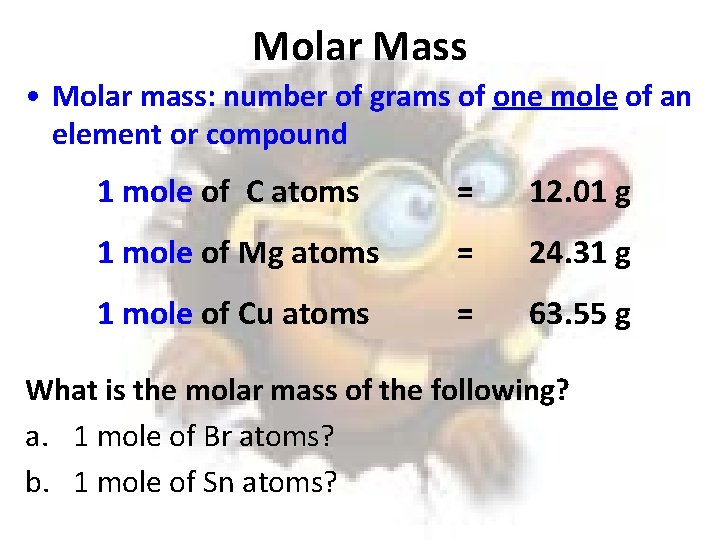
Molar Mass • Molar mass: number of grams of one mole of an element or compound 1 mole of C atoms = 12. 01 g 1 mole of Mg atoms = 24. 31 g 1 mole of Cu atoms = 63. 55 g What is the molar mass of the following? a. 1 mole of Br atoms? b. 1 mole of Sn atoms?

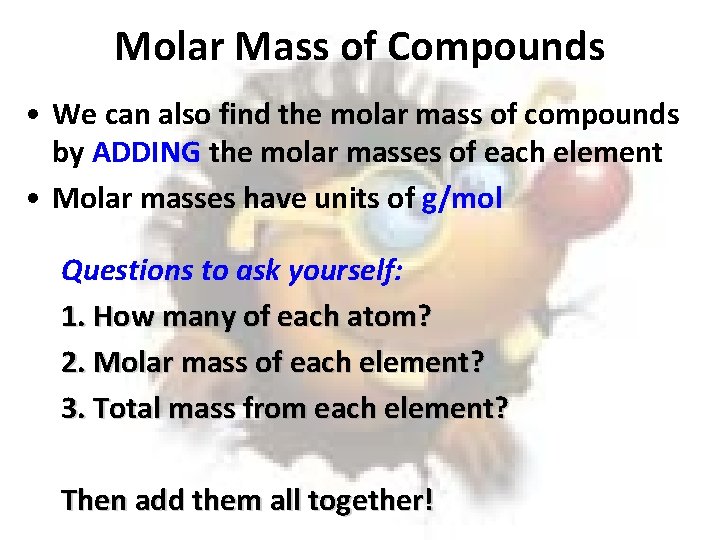
Molar Mass of Compounds • We can also find the molar mass of compounds by ADDING the molar masses of each element • Molar masses have units of g/mol Questions to ask yourself: 1. How many of each atom? 2. Molar mass of each element? 3. Total mass from each element? Then add them all together!

Molar Mass of Compounds Example #1 Find the molar mass of magnesium chloride, Mg. Cl 2 Questions to ask yourself: 1. How many of each atom? 2. Molar mass of each element? 3. Total mass from each element? Then add them all together!

Molar Mass of Compounds Example #2 Find the molar mass of calcium hydroxide. Questions to ask yourself: 1. How many of each atom? 2. Molar mass of each element? 3. Total mass from each element? Then add them all together!

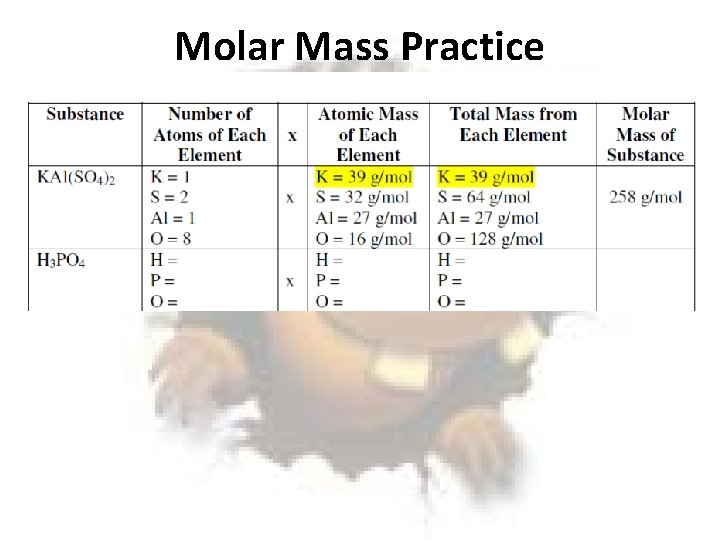
Molar Mass Practice

More Practice with Molar Mass For each of the compounds below, determine the number of atoms for each element: 1. Cu 3 PO 4 2. Fe 2(SO 2)3 3. potassium phosphide

More Practice with Molar Mass For each of the compounds below, determine the molar mass of the compound: 1. Mn. Br 2 2. Sr(NO 3)2

Calculations with Molar Mass molar mass Grams Moles

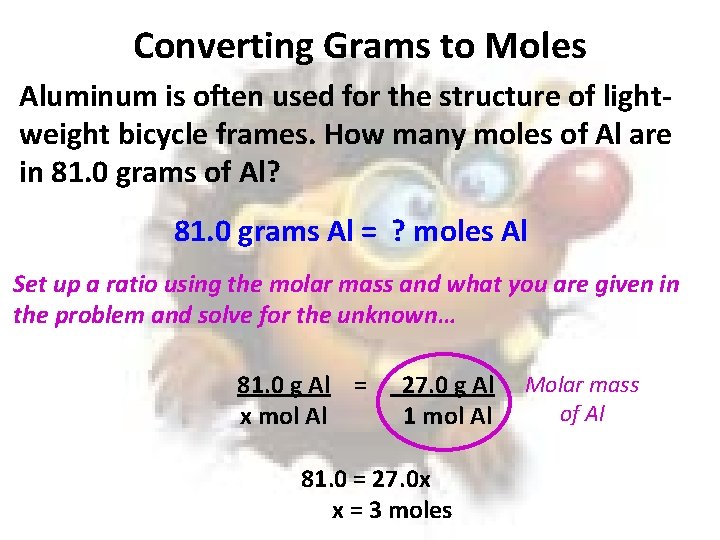
Converting Grams to Moles Aluminum is often used for the structure of lightweight bicycle frames. How many moles of Al are in 81. 0 grams of Al? 81. 0 grams Al = ? moles Al Set up a ratio using the molar mass and what you are given in the problem and solve for the unknown… 81. 0 g Al = x mol Al 27. 0 g Al 1 mol Al 81. 0 = 27. 0 x x = 3 moles Molar mass of Al

Converting Grams to Moles Practice How many moles does a 25. 2 gram sample of Ca. Cl 2 contain?

Converting Moles to Grams Example The decomposition of hydrogen peroxide (H 2 O 2) provides sufficient energy to launch a rocket. How many grams of H 2 O 2 are needed for rocket launch that requires 5. 00 moles H 2 O 2? 5. 00 moles H 2 O 2 = ? g H 2 O 2

Converting Moles to Grams Practice Aluminum satellite dishes are resistant to corrosion because the aluminum reacts with oxygen in the air to form a coating of aluminum oxide (Al 2 O 3). This tough, resistant coating prevents any further corrosion. What is the mass of 9. 45 mol of Al 2 O 3?