The Mole and Molar Mass The Mole Chemistrys





- Slides: 13

The Mole and Molar Mass

The Mole, Chemistry's Mascot

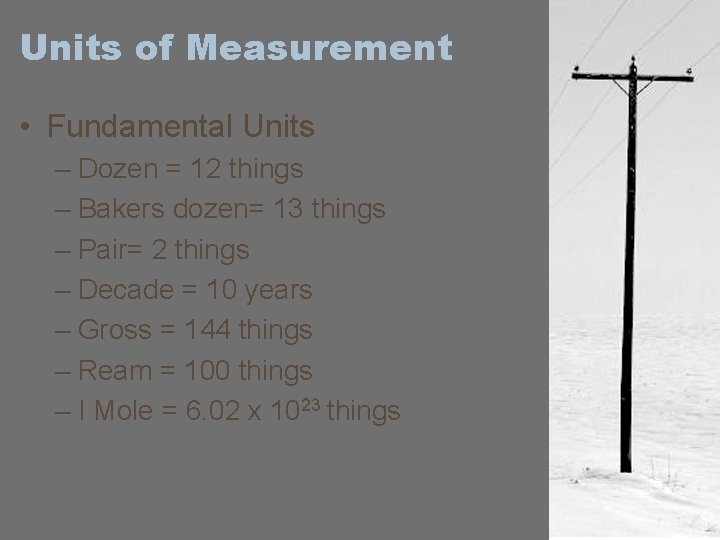
Units of Measurement • Fundamental Units – Dozen = 12 things – Bakers dozen= 13 things – Pair= 2 things – Decade = 10 years – Gross = 144 things – Ream = 100 things – I Mole = 6. 02 x 1023 things

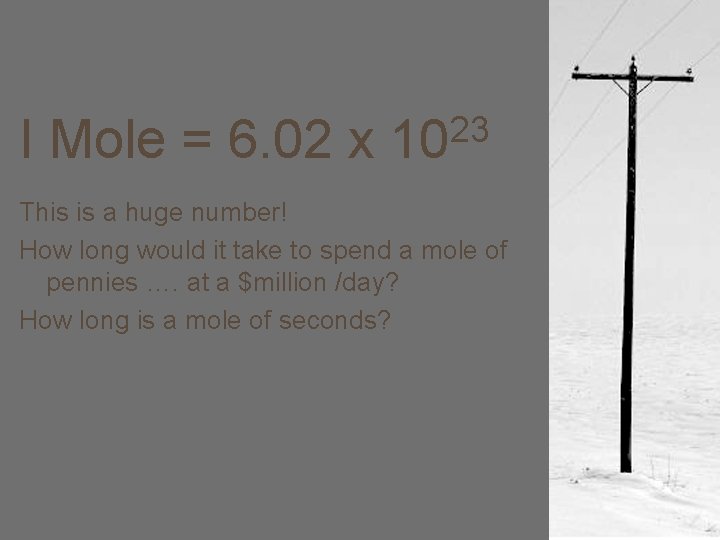
I Mole = 6. 02 x 23 10 This is a huge number! How long would it take to spend a mole of pennies …. at a $million /day? How long is a mole of seconds?

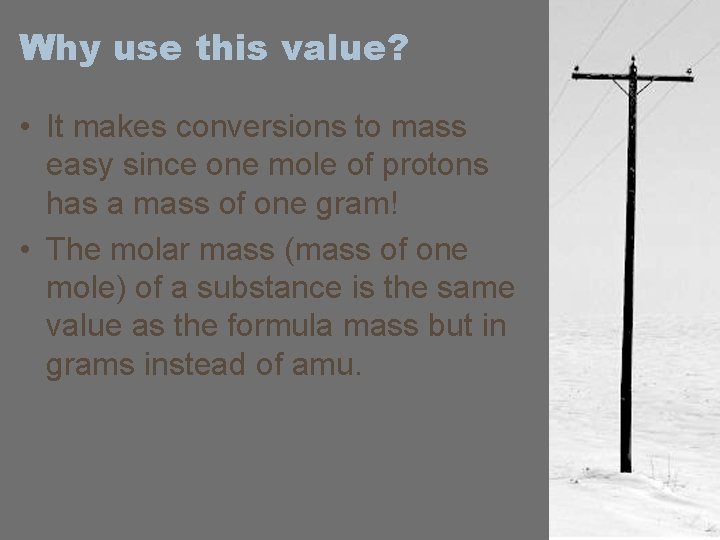
Why use this value? • It makes conversions to mass easy since one mole of protons has a mass of one gram! • The molar mass (mass of one mole) of a substance is the same value as the formula mass but in grams instead of amu.

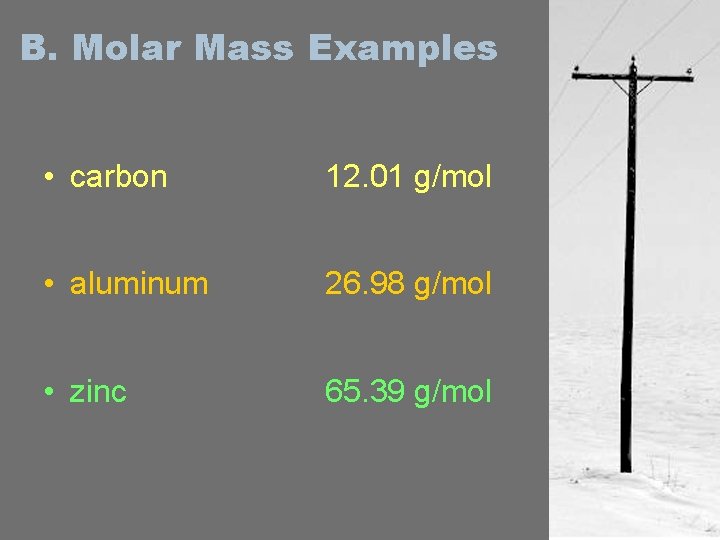
B. Molar Mass Examples • carbon 12. 01 g/mol • aluminum 26. 98 g/mol • zinc 65. 39 g/mol

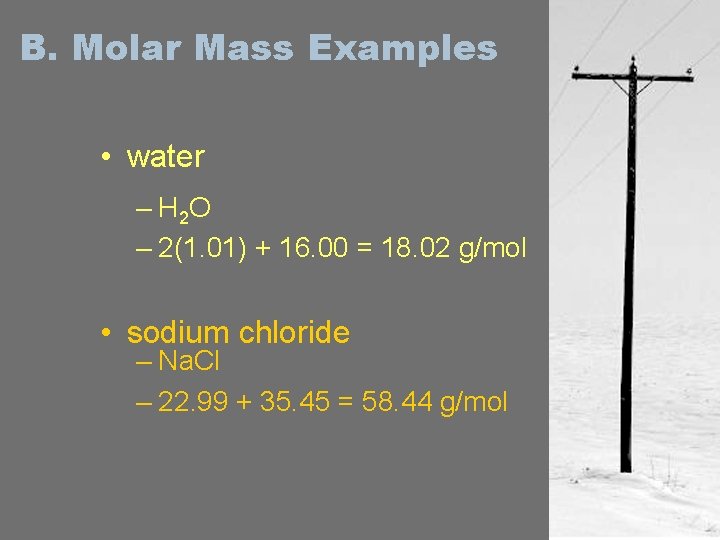
B. Molar Mass Examples • water – H 2 O – 2(1. 01) + 16. 00 = 18. 02 g/mol • sodium chloride – Na. Cl – 22. 99 + 35. 45 = 58. 44 g/mol

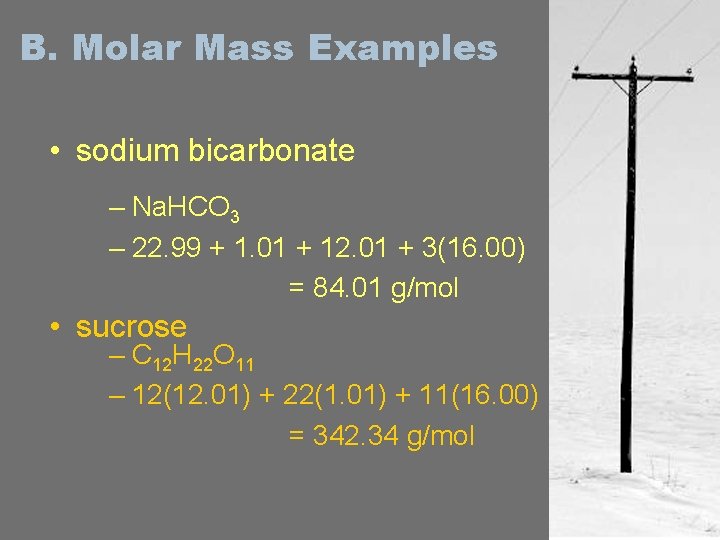
B. Molar Mass Examples • sodium bicarbonate – Na. HCO 3 – 22. 99 + 1. 01 + 12. 01 + 3(16. 00) = 84. 01 g/mol • sucrose – C 12 H 22 O 11 – 12(12. 01) + 22(1. 01) + 11(16. 00) = 342. 34 g/mol

Measuring with Moles • The Mole is a counting unit – Called the chemist’s dozen – Used to count number of • Atoms • Molecules (nm + nm) or (m + nm) – We will count both atoms and molecules

Conversions with Moles • How do you find mass of one mole of substance? • We can do this if we know the formula mass • When we do this we get the MOLAR MASS – The mass in grams of 1 mole of substance is equal to the formula mass – Units: grams/mol

Conversions with Moles Mass Molar mass = number of moles

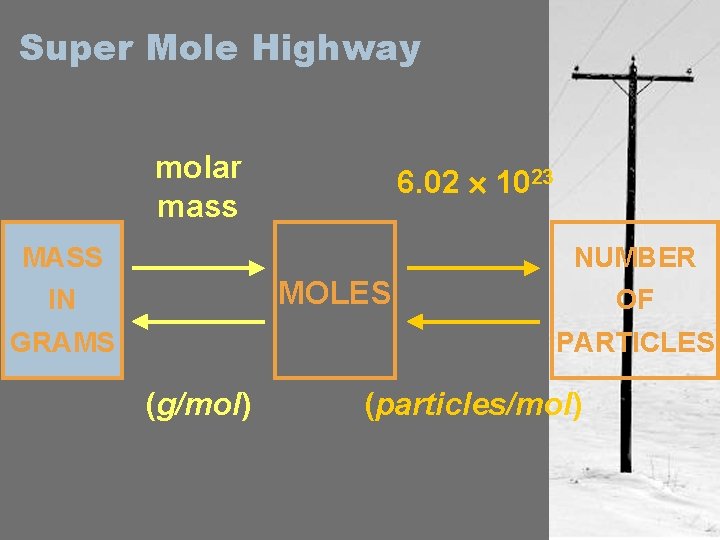
Super Mole Highway • So Far we Can Convert Between – Moles – Grams – Particles • The Mole Highway will help us convert from one to another

Super Mole Highway molar mass 6. 02 1023 MASS NUMBER MOLES IN GRAMS OF PARTICLES (g/mol) (particles/mol)