Ch 3 7 The Mole I Molar Conversions



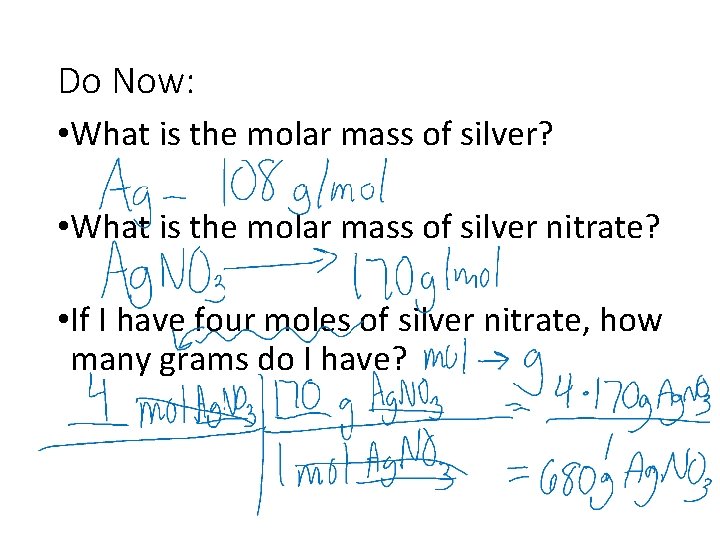

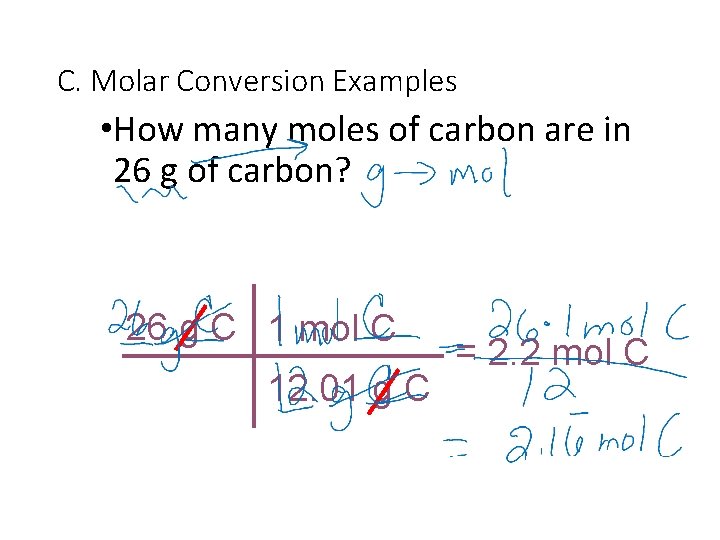


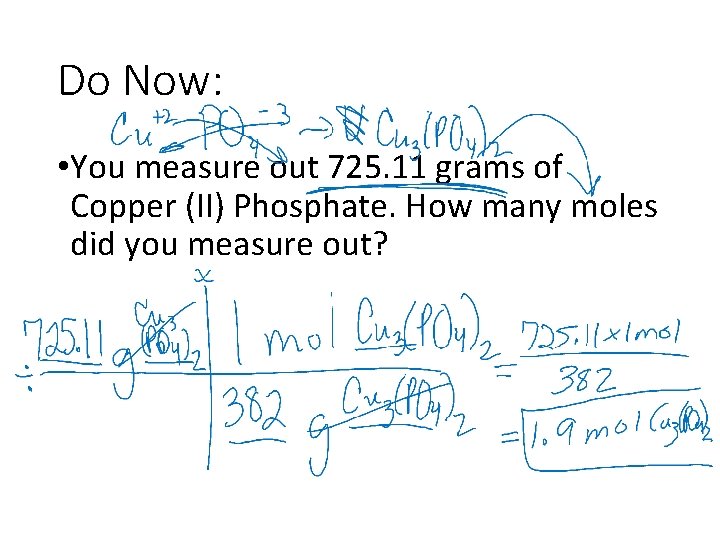










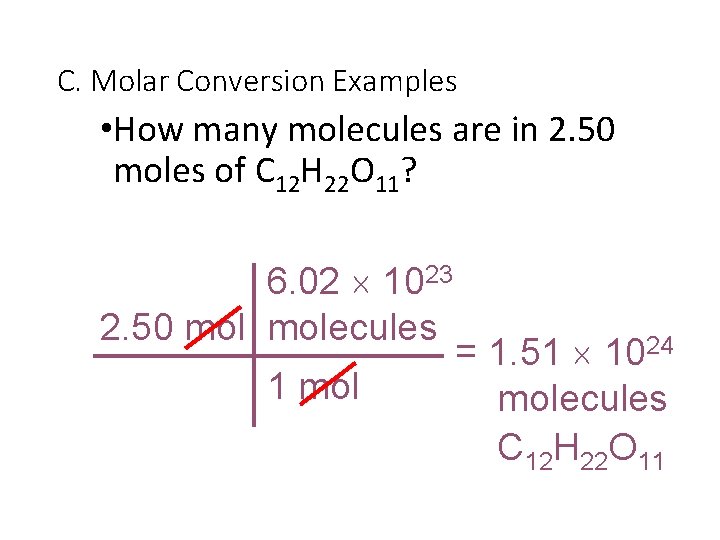























- Slides: 63

Ch. 3 & 7 – The Mole I. Molar Conversions (p. 80 -85, 221 -226) I II IV

Do Now: • Share your paragraph explaining the “why” of the flame test lab with a partner!

Consider This: • How many atoms do chemists need to have in order to measure a quantity of an atom? Do you believe that the same number is always needed? Explain.

A. What is the Mole? • A counting number (like a dozen) • Avogadro’s number (NA) • 1 mol = 6. 02 1023 items A large amount!!!!

n 1 mole of hockey pucks would equal the mass of the moon! n 1 mole of basketballs would fill a bag the size of the earth! • 1 mole of pennies would cover the Earth 1/4 mile deep!

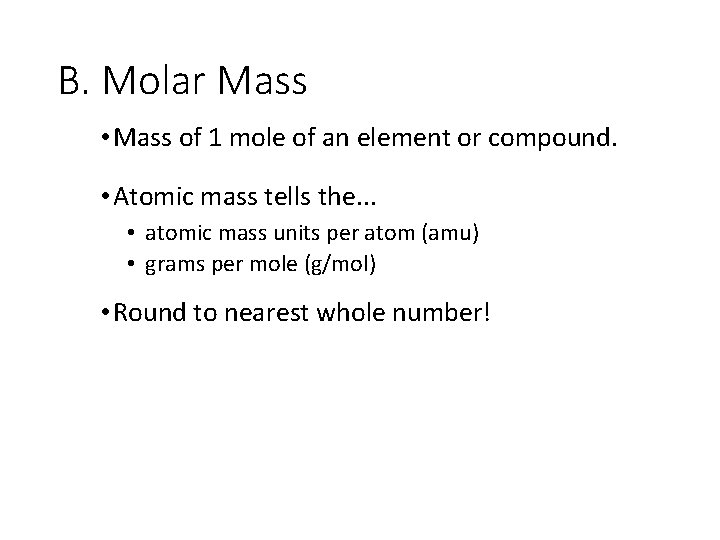
B. Molar Mass • Mass of 1 mole of an element or compound. • Atomic mass tells the. . . • atomic mass units per atom (amu) • grams per mole (g/mol) • Round to nearest whole number!

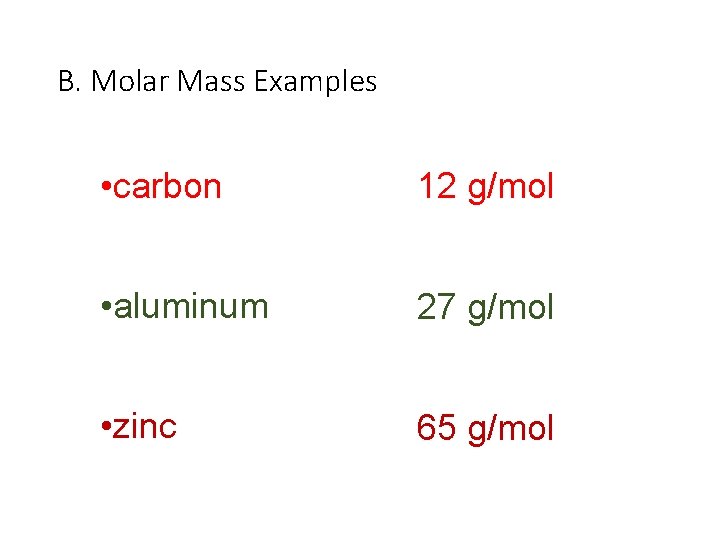
B. Molar Mass Examples • carbon 12 g/mol • aluminum 27 g/mol • zinc 65 g/mol

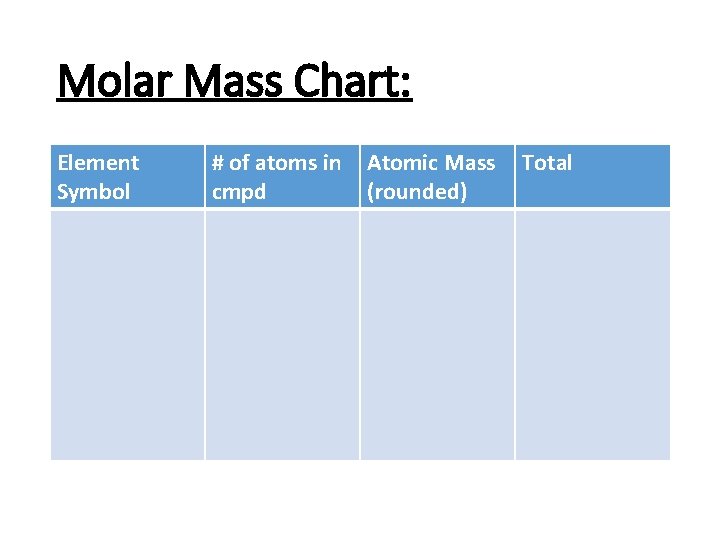
Molar Mass Chart: Element Symbol # of atoms in cmpd Atomic Mass (rounded) Total

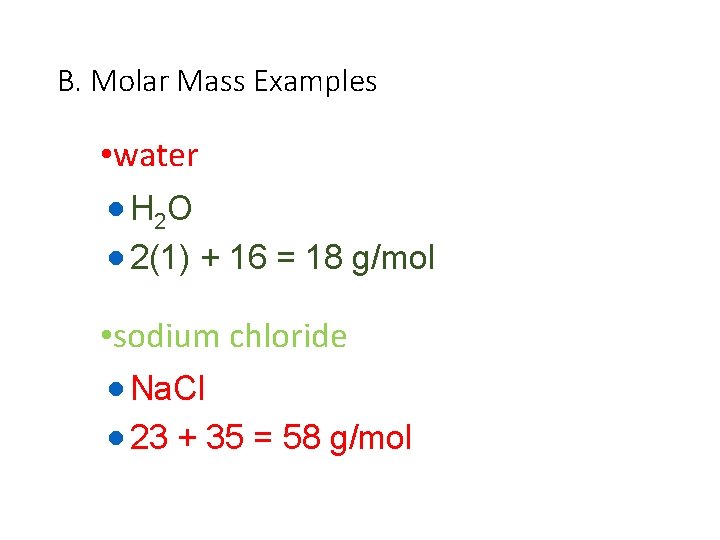
B. Molar Mass Examples • water · H 2 O · 2(1) + 16 = 18 g/mol • sodium chloride · Na. Cl · 23 + 35 = 58 g/mol

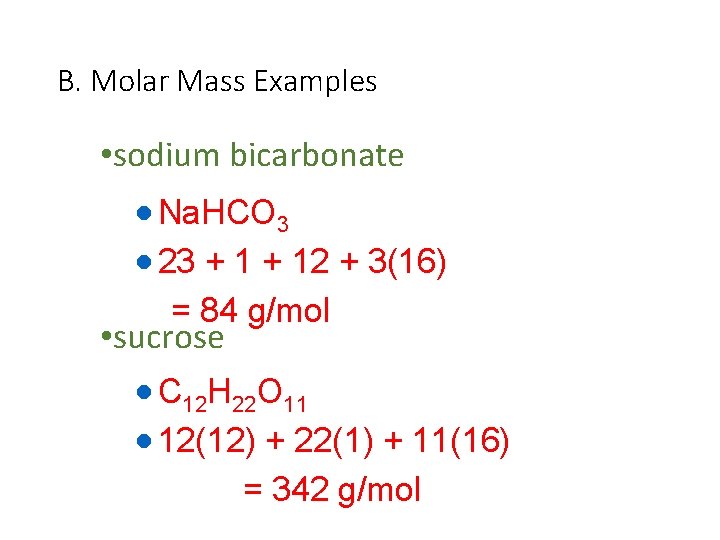
B. Molar Mass Examples • sodium bicarbonate · Na. HCO 3 · 23 + 12 + 3(16) = 84 g/mol • sucrose · C 12 H 22 O 11 · 12(12) + 22(1) + 11(16) = 342 g/mol

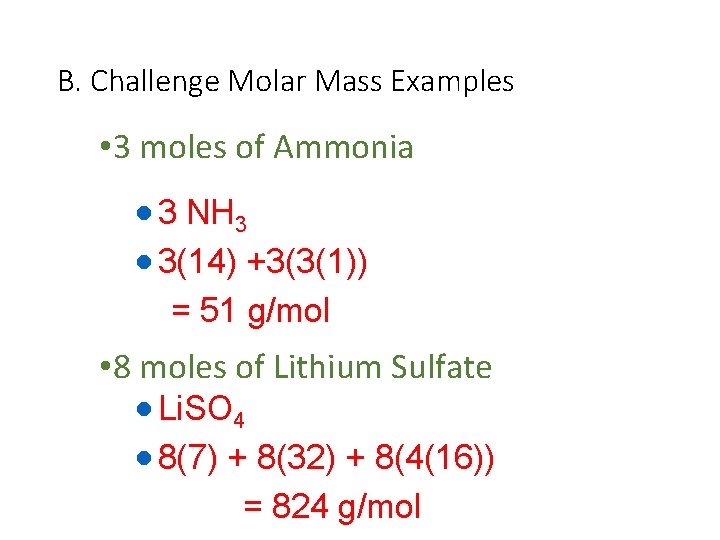
B. Challenge Molar Mass Examples • 3 moles of Ammonia · 3 NH 3 · 3(14) +3(3(1)) = 51 g/mol • 8 moles of Lithium Sulfate · Li. SO 4 · 8(7) + 8(32) + 8(4(16)) = 824 g/mol

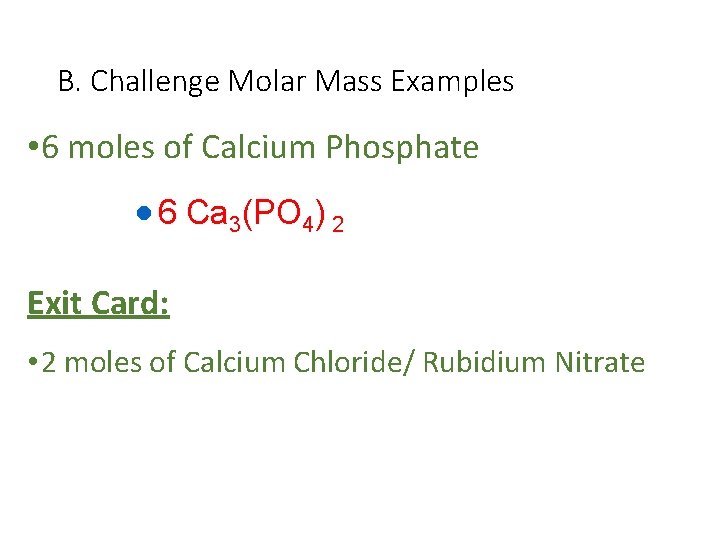
B. Challenge Molar Mass Examples • 6 moles of Calcium Phosphate · 6 Ca 3(PO 4) 2 Exit Card: • 2 moles of Calcium Chloride/ Rubidium Nitrate
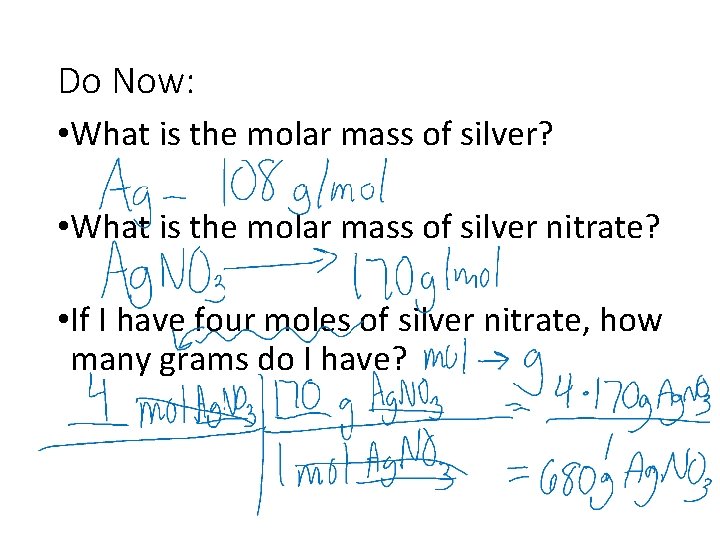
Do Now: • What is the molar mass of silver? • What is the molar mass of silver nitrate? • If I have four moles of silver nitrate, how many grams do I have?

C. Molar Conversion Examples Use this set up for mol to g REMEMBER: 1 st—Molar Mass chart; 2 nd –multiply by the number of moles! __mol____g ____ 1 mol ____ =

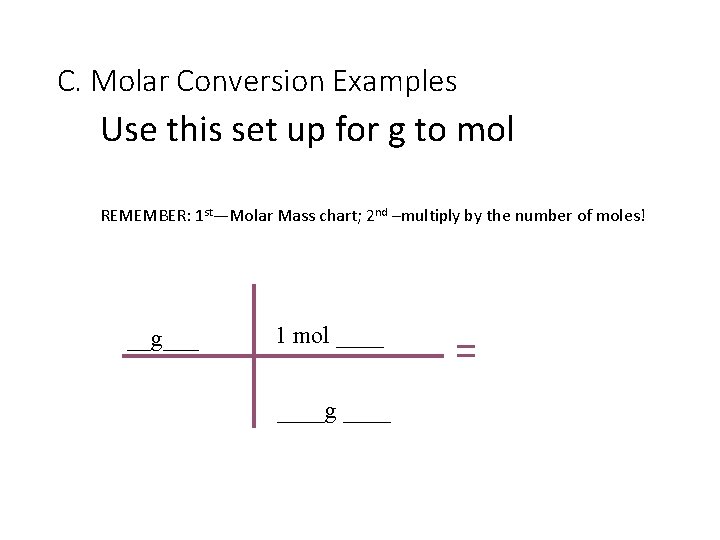
C. Molar Conversion Examples Use this set up for g to mol REMEMBER: 1 st—Molar Mass chart; 2 nd –multiply by the number of moles! __g___ 1 mol ____g ____ =
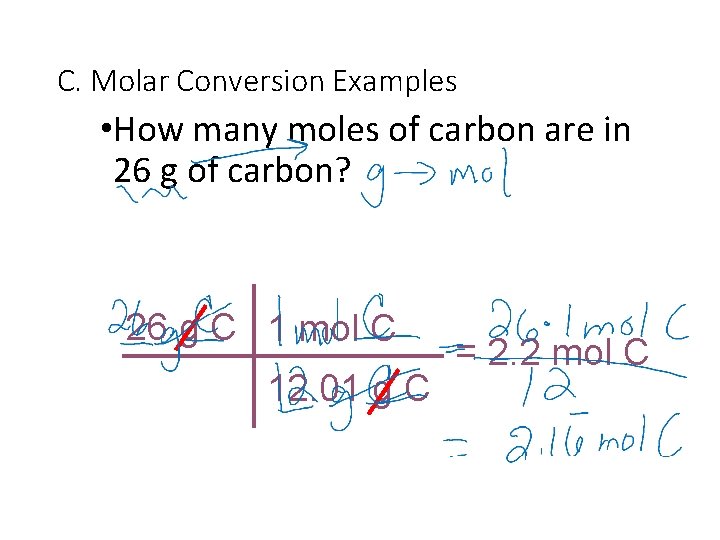
C. Molar Conversion Examples • How many moles of carbon are in 26 g of carbon? 26 g C 1 mol C 12. 01 g C = 2. 2 mol C

C. Molar Conversion Examples • How many grams of cobalt nitrate are in 2 moles? REMEMBER: 1 st—create the chart; 2 nd –multiply by the number of moles! =

Your Turn: • Spend the rest of the period working on the handout, you received at the door. • I will be monitoring and helping as needed!
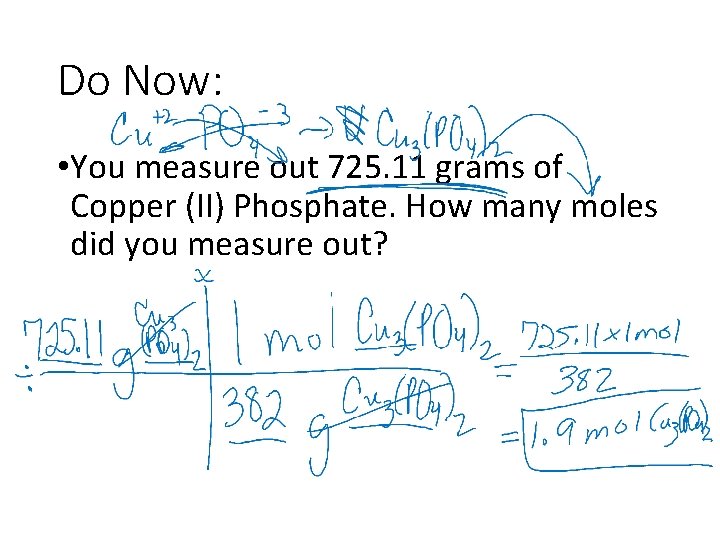
Do Now: • You measure out 725. 11 grams of Copper (II) Phosphate. How many moles did you measure out?

REMINDER: • The Fall Final Exam (which counts as two test grades is NEXT Thursday). • Take a moment to generate a list of ALL the topics that we have studied over the last semester!

Topic List • Sig Figs & Sci. Notation • Phase Diagrams • Matter Classification (mix, ele, etc. ) • Density • Periodic Trends • Intensive/Extensive Prop. • Unit Conversion • Physical/Chemical Change • Atom’s History • Atomic Structure (p, n, e) • Periodic Table • Electrons (behavior, orbitals, configuration) • Bonding (I, C, M) • Naming (Ionic & Covalent) • Writing Formulas • Molar Mass Calculation

Want 10 Bonus Points? • Create a “Review Packet” • Must Contain: • Both Molar Mass wkshts from this week • A check off sheet, explaining how you studied each topic, SIGNED by a parent/guardian.

For the Special Lab next Friday • Bring a fillable Christmas Ornament • Some sort of figurine • Cool present for a younger sibling/ yourself! • Can’t get the stuff? • You’ll do another lab.

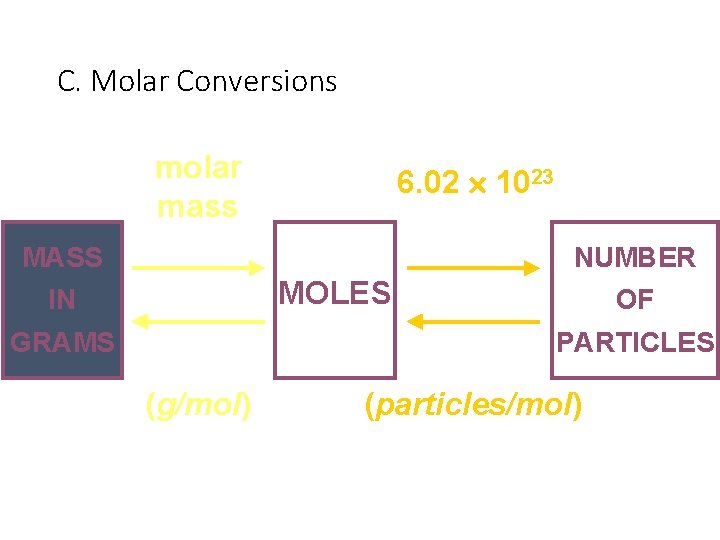
C. Molar Conversions molar mass 6. 02 1023 MASS NUMBER MOLES IN GRAMS OF PARTICLES (g/mol) (particles/mol)

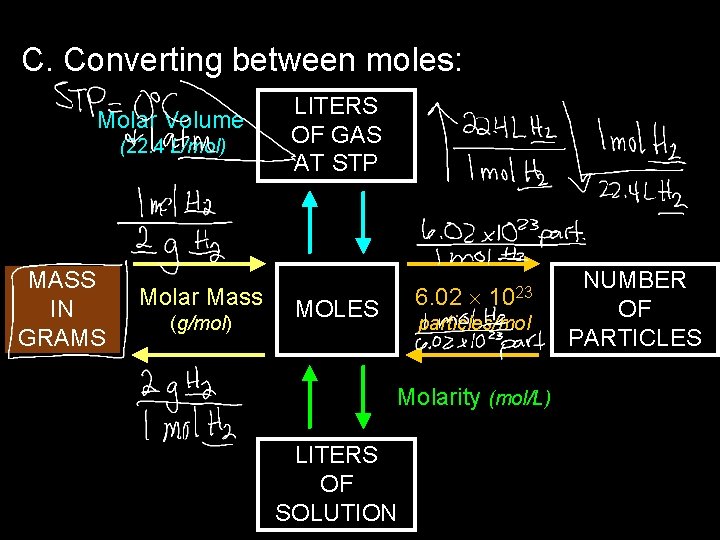
C. Converting between moles: Molar Volume (22. 4 L/mol) MASS IN GRAMS Molar Mass (g/mol) LITERS OF GAS AT STP 6. 02 MOLES 1023 particles/mol Molarity (mol/L) LITERS OF SOLUTION NUMBER OF PARTICLES

Do Now: • How many grams are in 7. 28 mol of Al. PO 4? Show your work!

Very Quickly…. • Let’s set up this lab. • On your Styrofoam cup, write your name. • Sharing scissors, cut your pipe cleaner into fourths (fold in half twice then cut at all folds) • Twist into snowflake design • Get string from me (find a pencil/pen too!)

Do Now: • How many molecules are in 4. 00 moles of glucose?

Alumimole Lab • Get into groups of three—assign each member a role: recorder, materials manager, and speaker • Listen to all directions. • You only have 15 minutes to complete the lab!

Alumimole Procedure • Determine the molar mass of aluminum: _____g/1 mol • Acquire exactly 0. 1 mol of aluminum foil • Create a mole (animal) model • Explain how you determined the molar mass of aluminum in a sentence. • How many grams would be in 1. 5 mol of Aluminum? • In 22 mol of Aluminum? • How many atoms were in the amount of Al you got? Show all work!

Check out your Snowflake… • Done? • Spray with acrylic spray and take home. • Note quite—let’s finish your set up. Periods 4 -6.

In the time remaining… • Work on your molar conversion worksheets. • Need help, ask a classmate!
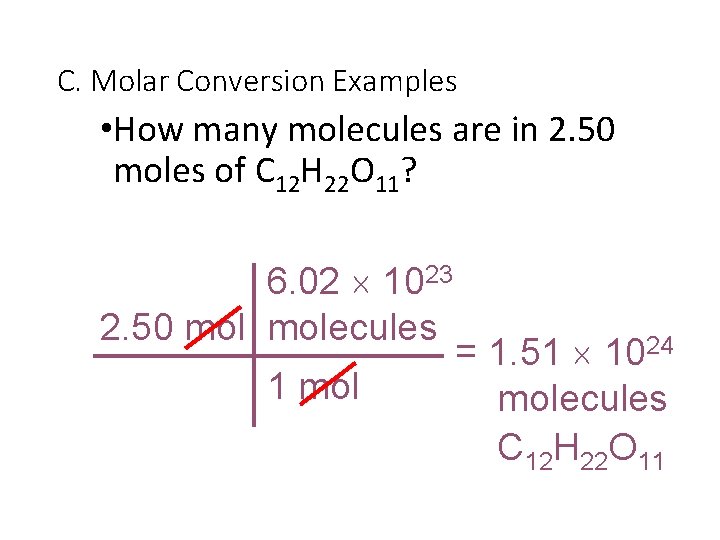
C. Molar Conversion Examples • How many molecules are in 2. 50 moles of C 12 H 22 O 11? 6. 02 1023 2. 50 molecules 1 mol = 1. 51 1024 molecules C 12 H 22 O 11

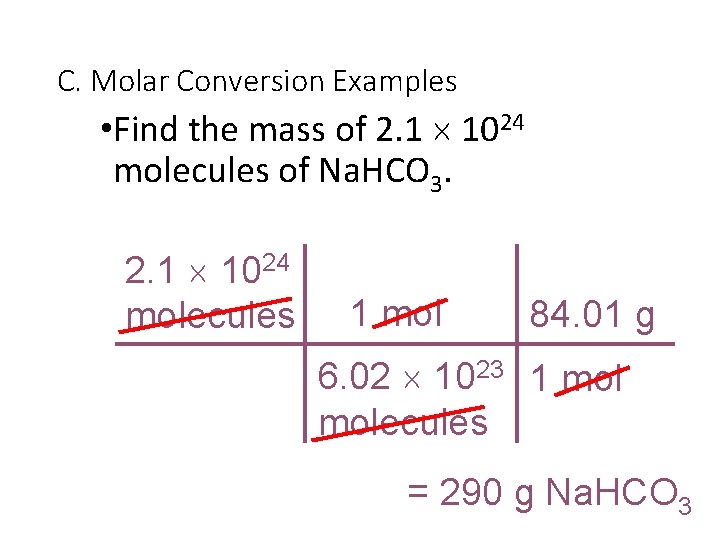
C. Molar Conversion Examples • Find the mass of 2. 1 1024 molecules of Na. HCO 3. 2. 1 1024 molecules 1 mol 84. 01 g 6. 02 1023 1 molecules = 290 g Na. HCO 3

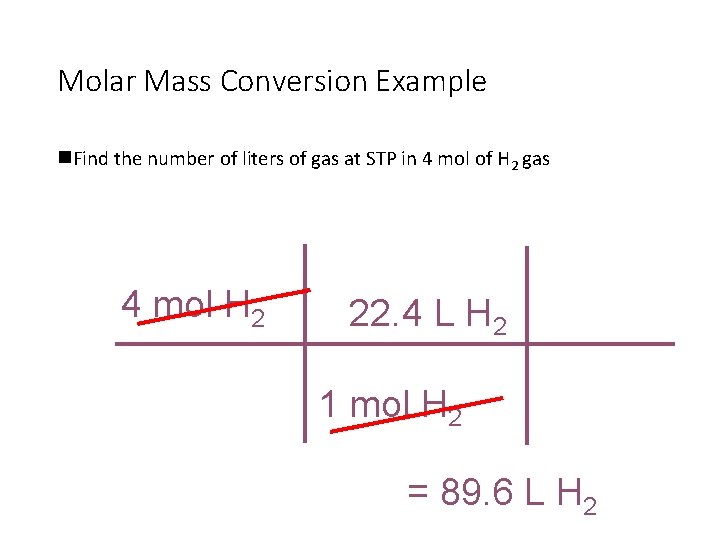
Molar Mass Conversion Example n. Find the number of liters of gas at STP in 4 mol of H 2 gas 4 mol H 2 22. 4 L H 2 1 mol H 2 = 89. 6 L H 2

More Practice: • If there are 9. 6 x 10^15 particles of sugar in a solution then how many moles of sugar are there?

More Practice • When there are 0. 0314 moles of candy canes how many candy canes are there?

More Practice • What volume (L) is 0. 00353 moles of He gas at 0ºC and 1. 0 atm?

More (IV) Practice • If we have 0. 072 g of Fe. Cl 3 then how many moles are there?

Do Now:

Do Now: • Be certain to continue working on your homework! • Need help? Come to tutoring… • Pull out a pencil, piece of scratch paper, your periodic table, and a calculator that is ONLY a calculator!

SLO Administration: • Diagnostic • If you don’t know, don’t answer it will alter your data • Do the best you can and TAKE your time—you do KNOW some of this! • Good Luck!

Do Now: • Solve the following molar mass/ conversion problems: • Molar Mass of Ca. Cl 2 • How many grams are in 7. 3 mol of Ti(NO 3)2? • How many mols are in 123. 8 L of H 2 gas at STP? • How many grams of Fe. Cl 3 are in 7. 214 1024 molecules?

Last Minute—Student Presentations • Pull out your timeline and your pencil • Let’s be attentive quickly…So we can play!

The Return of… • Two Groups • Get Ready to determine the “Molar Massters” of the class! • Write your answer on a whiteboard—all must have the correct answer in a group to earn the point! • Flip over to show your classmates • Ready…set…let’s start!

Let’s Play! 1. Number of atoms in 0. 500 mole of Al a) 500 Al atoms b) 6. 02 x 1023 Al atoms c) 3. 01 x 1023 Al atoms 2. Number of moles of S in 6. 02 x 1023 S atoms a) 1. 0 mole S atoms b) 3. 0 mole S atoms c) 1. 1 x 1048 mole S atoms

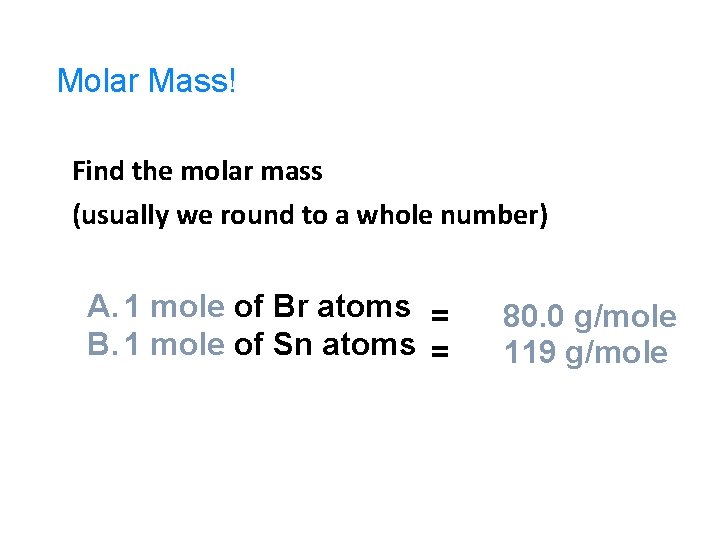
Molar Mass! Find the molar mass (usually we round to a whole number) A. 1 mole of Br atoms = B. 1 mole of Sn atoms = 80. 0 g/mole 119 g/mole

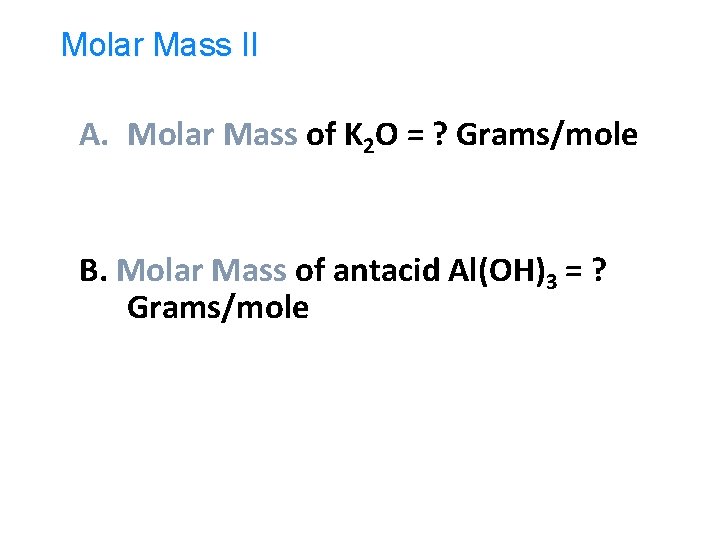
Molar Mass II A. Molar Mass of K 2 O = ? Grams/mole B. Molar Mass of antacid Al(OH)3 = ? Grams/mole

Molar Mass Challenge Prozac, C 17 H 18 F 3 NO, is a widely used antidepressant that inhibits the uptake of serotonin by the brain. Find its molar mass.

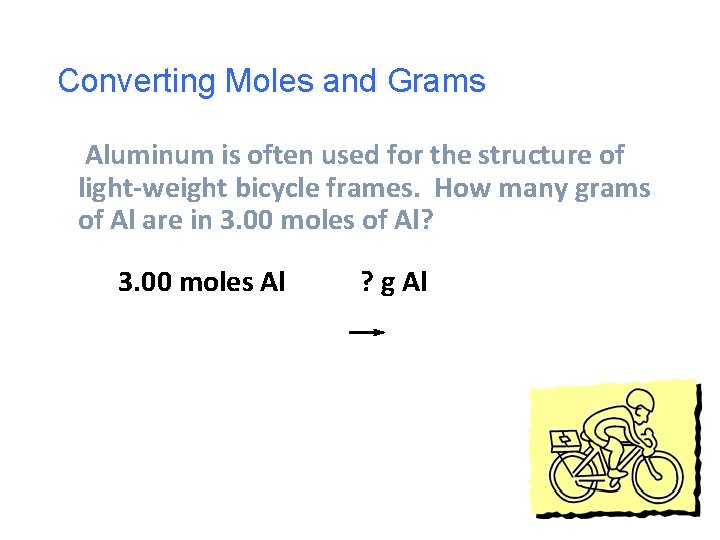
Converting Moles and Grams Aluminum is often used for the structure of light-weight bicycle frames. How many grams of Al are in 3. 00 moles of Al? 3. 00 moles Al ? g Al

Converting II The artificial sweetener aspartame (Nutra. Sweet) formula C 14 H 18 N 2 O 5 is used to sweeten diet foods, coffee and soft drinks. How many moles of aspartame are present in 225 g of aspartame?

Do Now: Create a word splash for “Mole” Mole Attach related words to the end of each line—do as many as you can!

Lab—all about the mole! • Read the directions at each station before you begin. • Follow them fully and record your data! • Complete all calculations after measuring EVERYTHING! • Measurements must be completed in 4 minutes or less!

Remember tutoring! 2: 30 to 3: 15 Today!!!

Do Now: • Describe how you would solve for the molar mass of H 2 O 2. • Describe how you set up a conversion problem—how do you know what unit should be on the top? The bottom?

Quick Quiz • 15 minutes to complete the quiz! • Show all work to earn full credit and TRY every problem • All other quiz/test procedures apply as usual!

Let’s get excited about… The Science Fair

Science Fair Lab Info: • Groups of no more than THREE! • Final project product (ppt) must be turned in by December 8 th. • This nine weeks you must generate a testable and SIGNIFICANT hypothesis, a list of materials and resources needed, a procedure as well as an image that will represent your experimental design! • Recommendation—start a google doc just for this project! Share it with me: stephanie. n. morris@gmail. com

Today: • Goal: Generate a testable and significant hypothesis that follows the format below: • If we ___________ we believe that we will find that ________________________ because _________. This study is of great significance because_______________________________. Resource 1: (no. coms allowed) Resource 2: (use your phones) Resource 3: (skim the articles ONLY)

Idea Splash: Topical Science Fair Ideas

Do a little research: • ID your partners (if you want them—you can’t go to state or the international fair with partners) • Choose your idea from our IDEA SPLASH • Research that topic • Keywords: [insert topic name] studies, [insert topic name] experiments, [insert topic name] news • Try out sites like: Google Scholar, Jstor, etc. (websites created for scholarly work and the scholars who read them!)

Set up your hypothesis: If we ___________ we believe that we will find that ________________________ because _________. This study is of great significance because_________________________________ _. Resource 1: (no. coms allowed) Resource 2: (use your phones) Resource 3: (skim the articles ONLY)

Closing: • Show me your work as I monitor the room and find a SAFE place for it to remain until MONDAY! • MONDAY—you will work on your materials and procedure needed!