UCLA FUND MANAGER TRAINING Office of Clinical Research





















































- Slides: 78

UCLA FUND MANAGER TRAINING Office of Clinical Research David Geffen School of Medicine November 12 th, 2019

4 160+ 1200+ Hospitals Medical Practices Active Clinical Research Studies

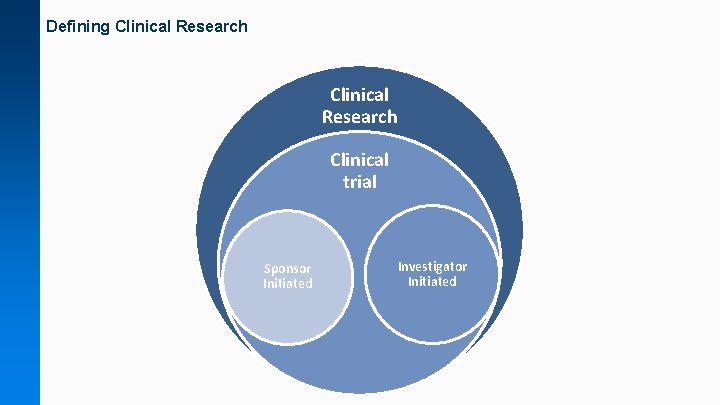
Defining Clinical Research Clinical trial Sponsor Initiated Investigator Initiated

What is a Clinical Trial? Definition Clinical Trials Definition (National Institute of Health): “A type of clinical study in which participants are assigned to groups that receive one or more intervention/treatment (or no intervention) so that researchers can evaluate the effects of the interventions on biomedical or health-related outcomes. The assignments are determined by the study's protocol. Participants may receive diagnostic, therapeutic, or other types of interventions. ” • Types of intervention • Protocol author • Funding source Visit the Research. Go website for more information

Clinical Trials Types of intervention and Examples Drugs or biologics • Investigational New Drug (IND) or IND exempt Medical devices • Investigational Device Exemption (IDE), 510 k approved Procedures • Surgical technics Diagnostic services • Imaging modalities

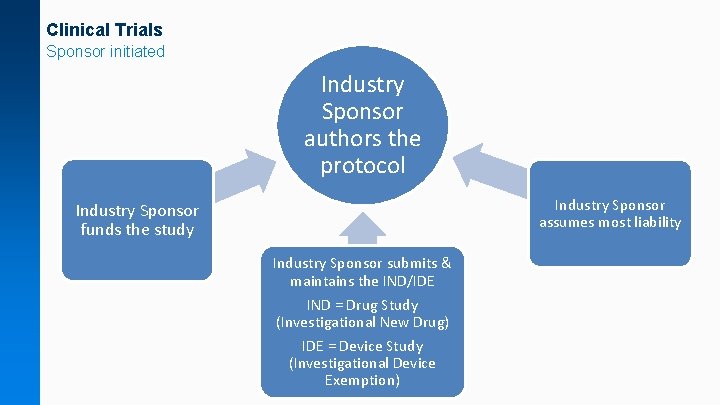
Clinical Trials Sponsor initiated Industry Sponsor authors the protocol Industry Sponsor assumes most liability Industry Sponsor funds the study Industry Sponsor submits & maintains the IND/IDE IND = Drug Study (Investigational New Drug) IDE = Device Study (Investigational Device Exemption)

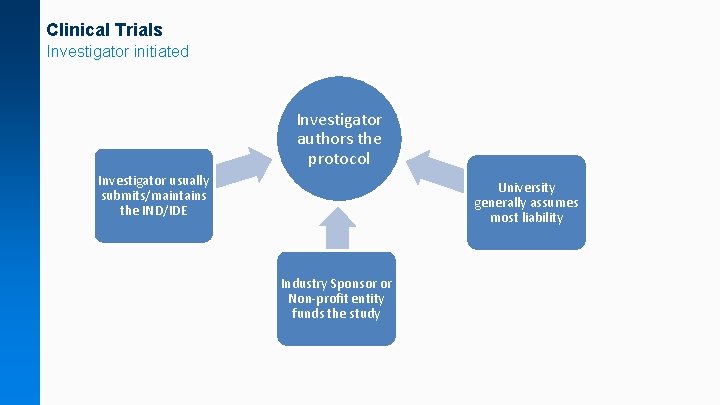
Clinical Trials Investigator initiated Investigator authors the protocol Investigator usually submits/maintains the IND/IDE University generally assumes most liability Industry Sponsor or Non-profit entity funds the study

Clinical Trials Indirect Costs Facilities & Administrative (F&A) costs, aka: “overhead” or “indirect costs” Industry-funded “Clinical Trials” (both, sponsor-authored & investigator-authored): • ALL COSTS subject to 26% indirect cost rate (with the exception of mandatory IRB fees) Non-profit-funded “Clinical Trials” (investigator-authored): • Varies based on contract but typically PATIENT CARE COSTS subject to 56% indirect cost rate (with the exception of mandatory IRB fees Other Clinical Research conducted on campus (e. g. does NOT meet UCLA definition of a “Clinical Trial”): • Select costs (“Modified Total Direct Costs”) subject to 56% indirect cost rate.

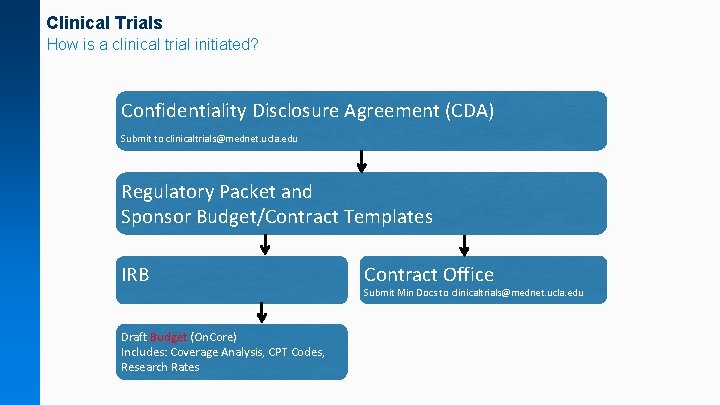
Clinical Trials How is a clinical trial initiated? Confidentiality Disclosure Agreement (CDA) Submit to clinicaltrials@mednet. ucla. edu Regulatory Packet and Sponsor Budget/Contract Templates IRB Draft Budget (On. Core) Includes: Coverage Analysis, CPT Codes, Research Rates Contract Office Submit Min Docs to clinicaltrials@mednet. ucla. edu

Research. Connect Study Activation Workflow Parallel Processes: IRB, Budgeting, Contract/Award, Servicing Department Approvals


QUICK REVIEW: IRB Submission Triggers Research. Connect Activation Responsible Party: Study Team Web. IRB § 2. 3: “Methods/Procedures-Descriptors” • • • Determines which studies may be in-scope for On. Core construction. In-Scope studies are automatically sent to On. Core via an IRB-On. Core interface. “Yes” response triggers § 2. 4 – coverage analysis/billing question (more on that later)

QUICK REVIEW: IRB Submission & Research. Connect Triggers Responsible Party: Study Team Key Lessons Learned • • Inaccurate web. IRB responses and/or information cause study activation delays. Incorrectly marking “no” to web. IRB § 2. 3 prevents the study from opening for enrollment in Care. Connect. Incorrect consent cost language and/or inconsistency between consent/budget/contract can cause study activation delays. . Missing or inaccurate study documents uploaded to web. IRB may cause study activation delays.


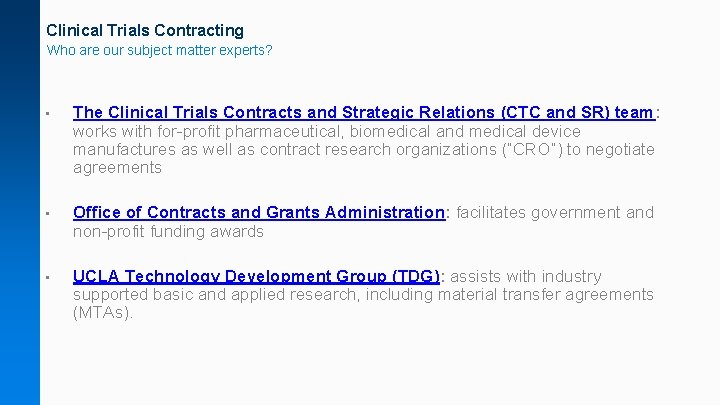
Clinical Trials Contracting Who are our subject matter experts? • The Clinical Trials Contracts and Strategic Relations (CTC and SR) team: works with for-profit pharmaceutical, biomedical and medical device manufactures as well as contract research organizations (“CRO”) to negotiate agreements • Office of Contracts and Grants Administration: facilitates government and non-profit funding awards • UCLA Technology Development Group (TDG): assists with industry supported basic and applied research, including material transfer agreements (MTAs).

Clinical Trials Contracting Industry Sponsored trials – CTC&SR

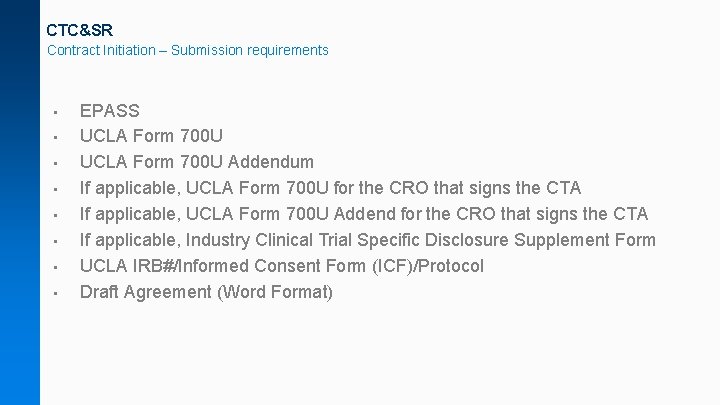
CTC&SR Contract Initiation – Submission requirements • • EPASS UCLA Form 700 U Addendum If applicable, UCLA Form 700 U for the CRO that signs the CTA If applicable, UCLA Form 700 U Addend for the CRO that signs the CTA If applicable, Industry Clinical Trial Specific Disclosure Supplement Form UCLA IRB#/Informed Consent Form (ICF)/Protocol Draft Agreement (Word Format)

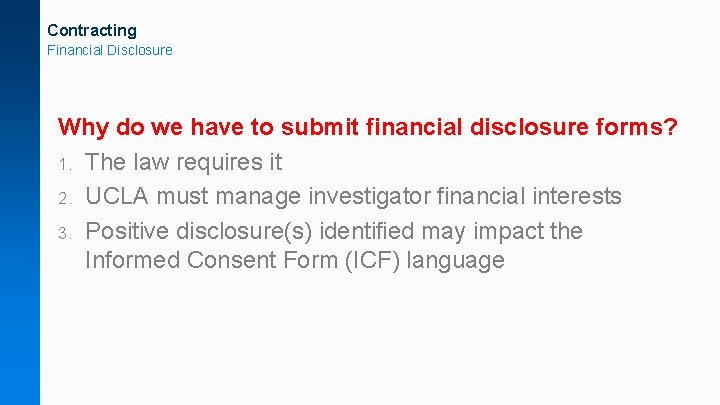
Contracting Financial Disclosure Why do we have to submit financial disclosure forms? 1. The law requires it 2. UCLA must manage investigator financial interests 3. Positive disclosure(s) identified may impact the Informed Consent Form (ICF) language

Contract Financial Disclosure

Contracting Financial Disclosures are effective for a 12 -month period: • 700 -U: report 12 months prior • 700 -U Addendum: report 12 months prior & future CIRC only meets once a month: • Contracts office must receive disclosure documents at least three (3) business days prior to CIRC deadline. • Missed deadline may cause a significant delay (30 days min)

Question & Discussion True or False: Financial Disclosure Forms are only completed during study start-up. FALSE In the event a contract amendment replaces the Principal Investigator, or increases funding for the study, new disclosures will need to be submitted.

Question & Discussion True or False: Financial Disclosure Forms should only be completed for the Sponsor. FALSE In the event a Contract Research Organization (CRO) is executing the agreement, disclosure forms must be completed for the Sponsor and CRO.


On. Core Intake Processes Responsible Party: FCA 1. If the study is in-scope for Research. Connect, then a study “shell” is automatically created via an IRB-On. Core interface. – 2. On. Core Intake Team uploads core study doc’s from web. IRB into On. Core – – 3. 4. Study “shell” contains basic study info. such as PI, coordinator, study title, etc. Protocol and draft consent(s) investigator brochure, lab/drug/device manuals (if available) On. Core Intake Team determines if an itemized budget is required, and if so, assigns the study budget/calendar for construction. On. Core Intake Team enters additional study information into On. Core – – Funding type and research charge master rate base (industry vs. non-profit) Applicable servicing departments (Radiology, Investigational Pharmacy, Lab/Pathology)


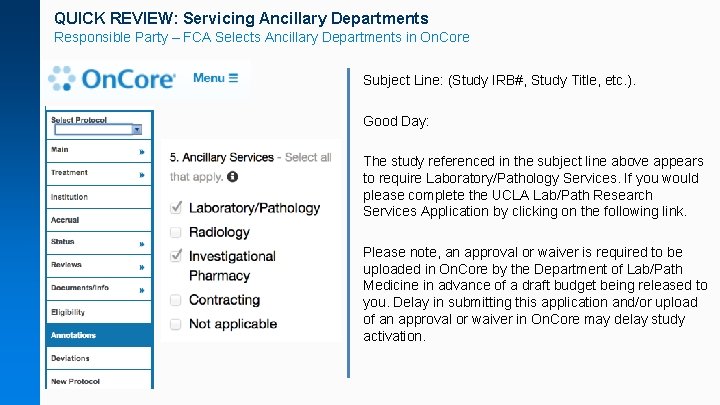
QUICK REVIEW: Servicing Ancillary Departments Responsible Party – FCA Selects Ancillary Departments in On. Core Subject Line: (Study IRB#, Study Title, etc. ). Good Day: The study referenced in the subject line above appears to require Laboratory/Pathology Services. If you would please complete the UCLA Lab/Path Research Services Application by clicking on the following link. Please note, an approval or waiver is required to be uploaded in On. Core by the Department of Lab/Path Medicine in advance of a draft budget being released to you. Delay in submitting this application and/or upload of an approval or waiver in On. Core may delay study activation.


On. Core Budget/Calendar Construction & Validation Responsible Party: FCA As a default, itemized budgets are constructed for most studies. Some exceptions may include: 1 2 Registry & Observational Studies Research-Only billing studies (e. g. no patient/insurance study-related billing and no contract requirement for itemized sponsor invoicing) – Most use cases will be non-profit or internally funded research-only studies.

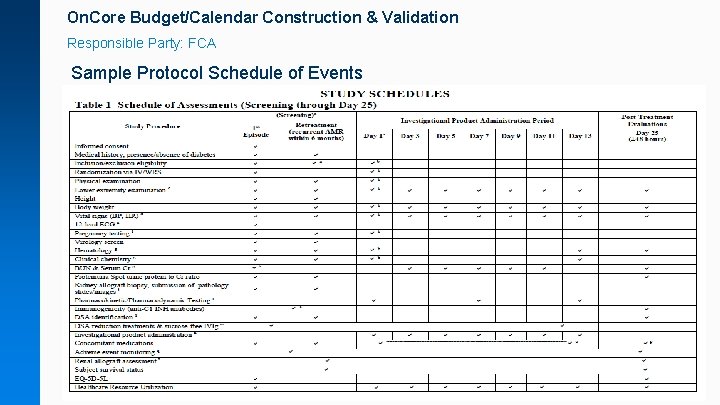
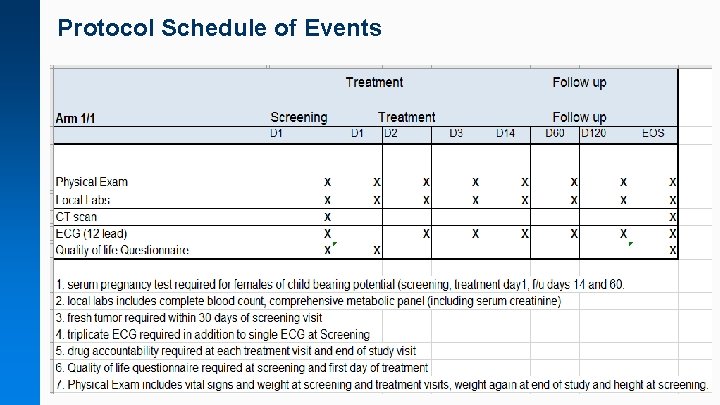
On. Core Budget/Calendar Construction & Validation Responsible Party: FCA Sample Protocol Schedule of Events

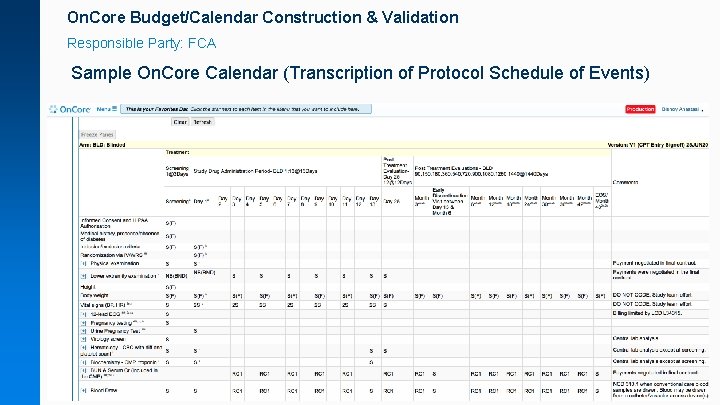
On. Core Budget/Calendar Construction & Validation Responsible Party: FCA Sample On. Core Calendar (Transcription of Protocol Schedule of Events)

On. Core Budget/Calendar Construction & Validation Responsible Party: Study team Sponsor draft budgets help FCA craft a calendar that more accurately reflects the sponsor payments terms: • Internal or sponsor trial feasibility budgets • Draft budgets provided as part of the regulatory package • Research-Only billing studies (e. g. no patient/insurance studyrelated billing and no contract requirement for itemized sponsor invoicing) • Most use cases will be non-profit or internally funded researchonly studies.


Insurance Coverage Analysis Responsible Party: FCA What is Coverage Analysis? • Adopted as Federal law in 2000(National Coverage Decision NCD 310. 1) for studies that intend to bill any study-related services to insurance (particularly to the Centers for Medicare and Medicaid Services). • Medicare requires a three-step process for coverage analysis: 1. 2. 3. Is the study a “Qualifying Trial” that may potentially pursue reimbursement? If a study a “Qualifying Trial, ” then we evaluate National and Local Coverage Determinations and nationally recognized specialty guidelines to determine which study-related services may be billable to insurance as “Routine Costs” vs. researchdriven services that should be paid for by study funding. Do Medicare rules allow coverage for the study-specific “Routine Costs. ”

Insurance Coverage Analysis Responsible Party: FCA Why Should we Perform Coverage Analysis? • • Enables Medicare/Medicaid beneficiaries to participate in studies that require insurance billing. May allow us to bill some study-related services to insurance that would not have otherwise been billable. Mitigates under-budgeting and potential insurance denials. Required by law, and failure to do so & bill appropriately has resulted in large False Claims Act settlements with leading medical centers. – CMS requires National Clinical Trial numbers and research billing modifiers designating “Qualifying Trials” & “Routine Costs” be placed on insurance claims.

Insurance Coverage Analysis Responsible Party: FCA How Does Coverage Analysis Impact Billing? “Routine Costs” research billing modifiers are required on many insurance claims (as well as National Clinical Trials numbers): • • Conventional care typically provided absent the clinical trial (Q 1) Provision/administration of the investigational service (e. g. IV infusion of an investigational chemotherapy agent) (Q 1) Items or services need for the safety monitoring and/or prevention of complications (Q 1) Approved investigational item (e. g. investigational drug or device) (Q 0)

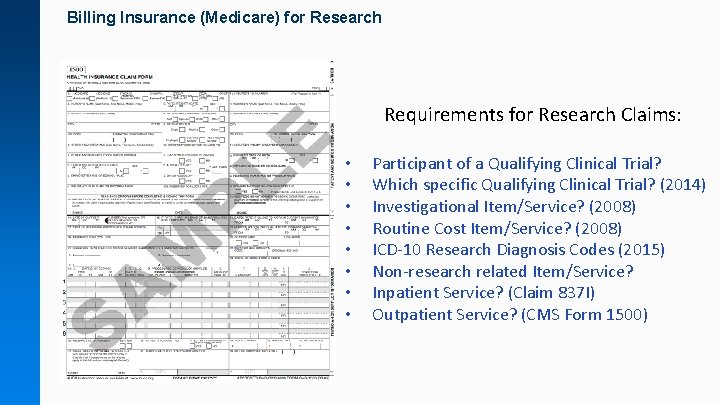
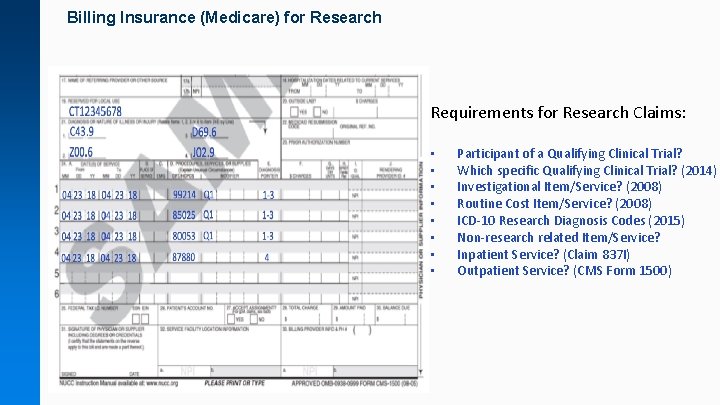
Billing Insurance (Medicare) for Research Requirements for Research Claims: • • Participant of a Qualifying Clinical Trial? Which specific Qualifying Clinical Trial? (2014) Investigational Item/Service? (2008) Routine Cost Item/Service? (2008) ICD-10 Research Diagnosis Codes (2015) Non-research related Item/Service? Inpatient Service? (Claim 837 I) Outpatient Service? (CMS Form 1500)

Billing Insurance (Medicare) for Research Requirements for Research Claims: • • Participant of a Qualifying Clinical Trial? Which specific Qualifying Clinical Trial? (2014) Investigational Item/Service? (2008) Routine Cost Item/Service? (2008) ICD-10 Research Diagnosis Codes (2015) Non-research related Item/Service? Inpatient Service? (Claim 837 I) Outpatient Service? (CMS Form 1500)

Insurance Coverage Analysis Responsible Party: FCA How Do We Know Which Studies Need Coverage Analysis? “Yes” response to web. IRB § 2. 3 triggers coverage analysis question (§ 2. 4): • • • Corresponding consent cost language provided directly in web. IRB (right panel). Insurance coverage analysis is only performed when study-related services are intended to be billed to patients/insurance (e. g. “No” response to web. IRB § 2. 4 below). If all study-required services will be paid for by study funding (e. g. no patient/insurance billing and “Yes” is selected for web. IRB § 2. 4), constructed budget directs all study services to study account and no time is wasted on coverage analysis.


Certified Procedural Coding Responsible Party: FCA for hem-onc &neuro-onc; CRBP for others Certified Procedural Coding (CPTs/HCPCS) Occurs after a calendar has been constructed & billing designations (“Routine Costs” vs. research-funded service) are entered in On. Core by coverage analysts. • Certified Coders associate procedural codes (CPTs/HCPCS) to all protocolrequired patient care costs in On. Core. – • Examples of patient care costs: blood draw, local labs, ECG, physician consult (physical exam + med history + vitals), CT/MRI, etc. UCLA’s research charge master is integrated into On. Core & populates baserates for all patient care costs that will be paid for by study funding. – – Studies are associated to either an industry or non-profit funded charge master. Study teams are required to ensure negotiated/contracted budgets cover these baserates (may otherwise cause activation delays – more on the QA process later).

Draft Budget Release to Study Teams Study Team review of completed budget & initiate sponsor negotiations, if applicable.


Draft Budget Release Responsible Party: FCA Customized Excel Budgets Released by FCA to Study Teams DEPENDENCIES: ü IRB application submitted by Study Team ü Ancillary applications are submitted by Study Team ü Calendar constructed by Forte ü Calendar validated and enhanced by FCA ü Coverage Analysis completed by FCA ü Certified Procedural Coding completed by FCA/CRBP ü Ancillary approvals/waivers uploaded in On. Core by Ancillaries

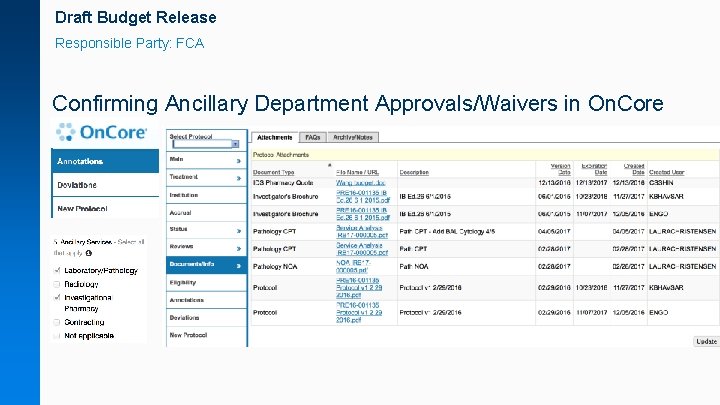
Draft Budget Release Responsible Party: FCA Confirming Ancillary Department Approvals/Waivers in On. Core

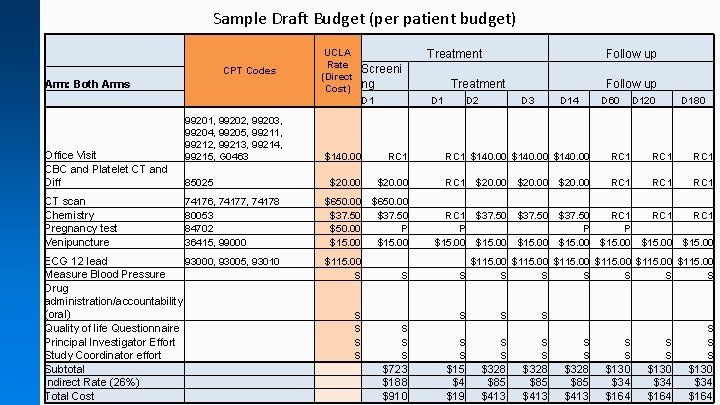
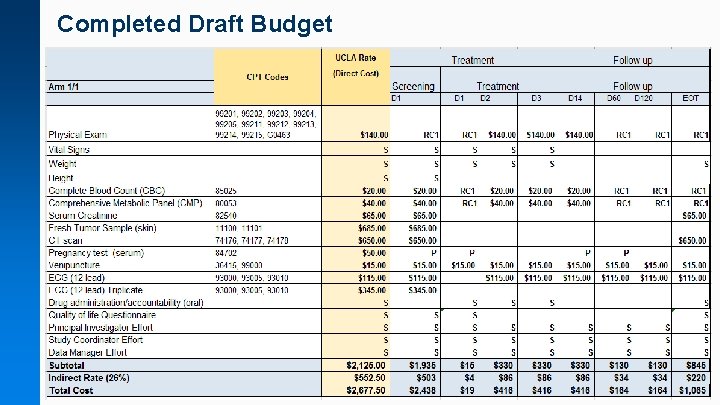
Sample Draft Budget (per patient budget) UCLA Rate (Direct Cost) CPT Codes Arm: Both Arms Office Visit CBC and Platelet CT and Diff CT scan Chemistry Pregnancy test Venipuncture Treatment Screeni ng D 1 99201, 99202, 99203, 99204, 99205, 99211, 99212, 99213, 99214, 99215, G 0463 85025 74176, 74177, 74178 80053 84702 36415, 99000 ECG 12 lead 93000, 93005, 93010 Measure Blood Pressure Drug administration/accountability (oral) Quality of life Questionnaire Principal Investigator Effort Study Coordinator effort Subtotal Indirect Rate (26%) Total Cost Treatment D 1 D 2 Follow up D 3 D 14 D 60 D 120 D 180 $140. 00 RC 1 $140. 00 RC 1 $20. 00 RC 1 $650. 00 $37. 50 $50. 00 P $15. 00 Follow up RC 1 $37. 50 RC 1 P P $15. 00 $15. 00 $115. 00 S S $115. 00 S S S S S S S $723 $188 $910 $15 $4 $19 S S S $328 $85 $413 S S $130 $34 $164 S S S $130 $34 $164

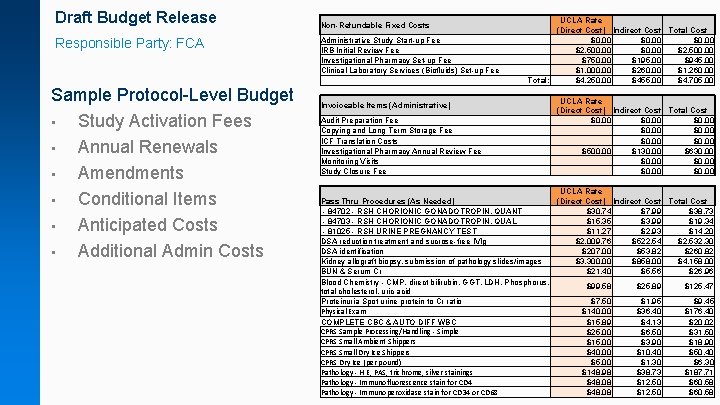
Draft Budget Release Responsible Party: FCA Sample Protocol-Level Budget • Study Activation Fees • Annual Renewals • Amendments • Conditional Items • Anticipated Costs • Additional Admin Costs Non-Refundable Fixed Costs Administrative Study Start-up Fee IRB Initial Review Fee Investigational Pharmacy Set-up Fee Clinical Laboratory Services (Biofluids) Set-up Fee Invoiceable Items (Administrative) Audit Preparation Fee Copying and Long Term Storage Fee ICF Translation Costs Investigational Pharmacy Annual Review Fee Monitoring Visits Study Closure Fee UCLA Rate (Direct Cost) Indirect Cost Total Cost $0. 00 $2, 500. 00 $750. 00 $195. 00 $945. 00 $1, 000. 00 $260. 00 $1, 260. 00 Total: $4, 250. 00 $455. 00 $4, 705. 00 UCLA Rate (Direct Cost) Indirect Cost Total Cost $0. 00 $0. 00 $500. 00 $130. 00 $630. 00 $0. 00 UCLA Rate Pass Thru Procedures (As Needed) (Direct Cost) Indirect Cost Total Cost - 84702 - RSH CHORIONIC GONADOTROPIN, QUANT $30. 74 $7. 99 $38. 73 - 84703 - RSH CHORIONIC GONADOTROPIN, QUAL $15. 35 $3. 99 $19. 34 - 81025 - RSH URINE PREGNANCY TEST $11. 27 $2. 93 $14. 20 DSA reduction treatment and sucrose-free IVIg $2, 009. 76 $522. 54 $2, 532. 30 DSA identification $207. 00 $53. 82 $260. 82 Kidney allograft biopsy, submission of pathology slides/images $3, 300. 00 $858. 00 $4, 158. 00 BUN & Serum Cr $21. 40 $5. 56 $26. 96 Blood Chemistry - CMP, direct bilirubin, GGT, LDH, Phosphorus, $99. 58 $25. 89 $125. 47 total cholesterol, uric acid Proteinuria Spot urine protein to Cr ratio $7. 50 $1. 95 $9. 45 Physical Exam $140. 00 $36. 40 $176. 40 COMPLETE CBC & AUTO DIFF WBC $15. 89 $4. 13 $20. 02 CPRS Sample Processing/Handling - Simple $25. 00 $6. 50 $31. 50 CPRS Small Ambient Shippers $15. 00 $3. 90 $18. 90 CPRS Small Dry Ice Shippers $40. 00 $10. 40 $50. 40 CPRS Dry Ice (per pound) $5. 00 $1. 30 $6. 30 Pathology - H E, PAS, trichrome, silver stainings $148. 98 $38. 73 $187. 71 Pathology - Immunofluorescence stain for CD 4 $48. 08 $12. 50 $60. 58 Pathology - Immunoperoxidase stain for CD 34 or CD 68 $48. 08 $12. 50 $60. 58

TRAINING EXERCISE – DRAFT BUDGET REVIEW Applied Learning

Protocol Schedule of Events

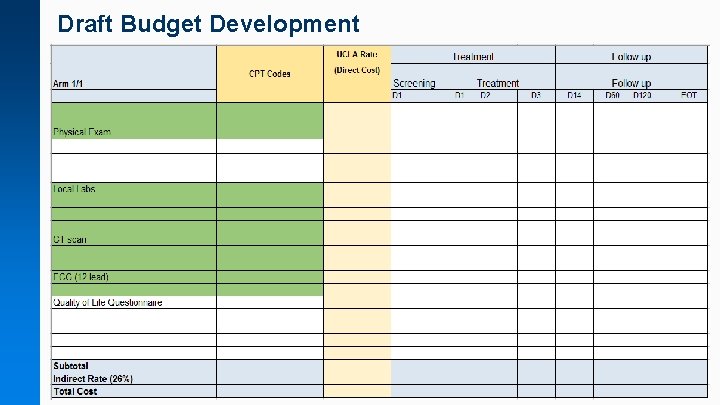
Draft Budget Development

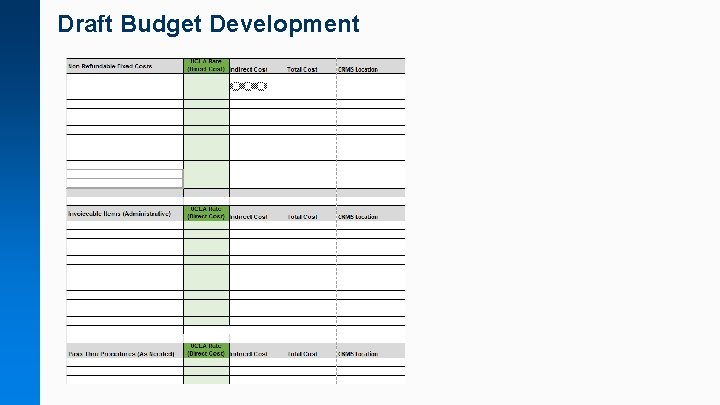
Draft Budget Development

Draft Budget Review & Negotiation Responsible Party: Study Team Budgeting Tasks Download and Review the Draft Budget in On. Core; • Ensure Draft Budget is an accurate representation of the current study protocol: ü ü ü All protocol required study visits are accurately represented. All protocol required services are accurately represented. All administrative & personnel costs required for study performance are included. • ü All study activation, amendments and annual renewal costs are included. • ü IRB and ancillary department activation and renewal fees, protocol amendments, contract amendments, coverage analysis, etc. All conditional/invoiceable costs are included. • ü Principal Investigator, Sub-Investigator, Nurse, Study Coordinator, Regulatory Coordinator, Data Management, Research Assistant, Statistician, etc. Unscheduled visits, repeat clinical services, anticipated adverse events, medwatch/IND safety reports, etc. Additional administrative costs you may consider: • Monitoring visit fees, monitor change fees, document storage fees, study auditing costs (not for cause), facsimile, printing, copying and redaction, etc.

Completed Draft Budget


Demonstration of On. Core Draft Budget & Study Team Budget Review Locating draft budgets in On. Core; Study Team responsibilities; Budget Negotiation Best Practices

BUDGETING RECOMMENDATIONS LESSONS LEARNED

Draft Budget Review & Negotiation Responsible Party: Study Team Common Budgeting/Negotiation Challenges Sponsor/CRO states: • “You are (by far) the most expensive site. The rates you are requesting violate the Federal Anti-Kickback Statute as well as our Corporate Integrity Agreement with the Federal Government. ” Appropriate Site Response?

Draft Budget Review & Negotiation Responsible Party: Study Team Common Budgeting/Negotiation Challenges Sponsor/CRO states: • “Your budget is not Fair Market Value compliant. ” Appropriate Site Response?

Draft Budget Review & Negotiation Responsible Party: Study Team Common Budgeting/Negotiation Challenges Sponsor/CRO states: • “We can only agree to pay you Medicare reimbursement rates as we have offered all other participating sites. ” Appropriate Site Response?

Draft Budget Review & Negotiation Responsible Party: Study Team Common Budgeting/Negotiation Challenges Sponsor/CRO states: • “Your administrative, startup and personnel are considered industry standard costs of doing business. Our policy does not allow reimbursement for these items. ” Appropriate Site Response? UCOP 95 -05

Draft Budget Review & Negotiation Responsible Party: Study Team Common Budgeting/Negotiation Challenges Sponsor/CRO states: • “If you remove the cost of the pregnancy test and reduce the cost of the ECG, we would be willing to accept the increased cost for your personnel fees” Appropriate Site Response?

Draft Budget Review & Negotiation Responsible Party: Study Team Common Budgeting/Negotiation Challenges Sponsor/CRO states: • “We can agree to pay you the total cost per patient you are requesting if you can move 50% of your screening visit costs to the end of study visit. ” Appropriate Site Response?

Draft Budget Review & Negotiation Responsible Party: Study Team Common Budgeting/Negotiation Challenges Draft budget rate may appear higher than expected: • Some patient care costs can vary significantly in cost from one setting to another (e. g. hospital vs. clinic rates). – • If the PI can ensure all study services will only occur in a single setting, CRBP can help update the coding and pricing in On. Core. Please note – in the event study encounters occur in the more expensive setting, the study account can still be charged the higher rates. Some studies only require either a technical or professional service, but not both (e. g. performing an ECG, but not needing to have a physician interpret and report the results). – If the PI can ensure all study subjects will only require either a technical or professional service, but not both, CRBP can help update the coding and pricing in On. Core. Please note – in the event study encounters order both services, the study account can be charged both fees. Global Service $180 Technical component $110 Professional component $70


Study Team Budget Approval & Upload in On. Core & Study Activation QA Responsible Party: Study Team & Research QA Team Once Study Teams have downloaded the Draft Budget in On. Core, and approved and/or negotiated the Budget (as applicable), Study Teams must upload the approved/negotiated budget into On. Core for Research QA review and approval. • Study Teams must ensure negotiated budgets comply with UCLA Research Pricing Policy 915. 1 and that budgeted rates do not fall below the rates provided to them in the Draft Budget. . – • Some non-profit awards may not include an itemized budget listing individual rates for patient care costs (which may be okay). Please upload the draft budget provided to you to indicate your approval of the On. Core budget. FCA provides study budget development and negotiation services on a sales & service basis. Please contact Coverage. Analysis@mednet. ucla. edu for info.

Study Team Budget Approval & Upload in On. Core & Study Activation QA Responsible Party: Study Team & Research QA Team reviews IRB-approved consent(s), contract(s)/award(s), and On. Core budget to ensure conformity and consistency. • Confirms the itemized costs, as well as per subject and study level costs are consistent in the contract and On. Core budget. • Confirms the billing designations in On. Core are consistent with the terms described in the contract/award and approved consent form(s). • Confirms that the negotiated rates (if applicable) meet UCLA Research Pricing Policy 915. 1. • Confirms that all ancillary department approvals/waivers have been obtained. PLEASE NOTE: Studies may not open for enrollment in Care. Connect without Research QA Team review and approval.

Pitfalls To Avoid Accelerating Study Activation • • • Incorrect and/or incomplete responses, information and/or documents provided to IRB and/or servicing departments. Failure or delay in engaging servicing ancillary departments. Failure or delay in submitting contracting documents to contracting offices, as applicable (CTC-SR, OCGA, or TDG). Failure or delay in responding to inquiries from administrative research units (CRIS, FCA, CRBP, Care. Connect, etc. ). Failure to negotiate itemized rates in accordance with UCLA Research Pricing Policy 915. 1. Inconsistency(ies) between the On. Core budget, contract/award, and approved consents. – Particularly, costs for participation, subject injury, remuneration, and potential benefits sections of the consent.

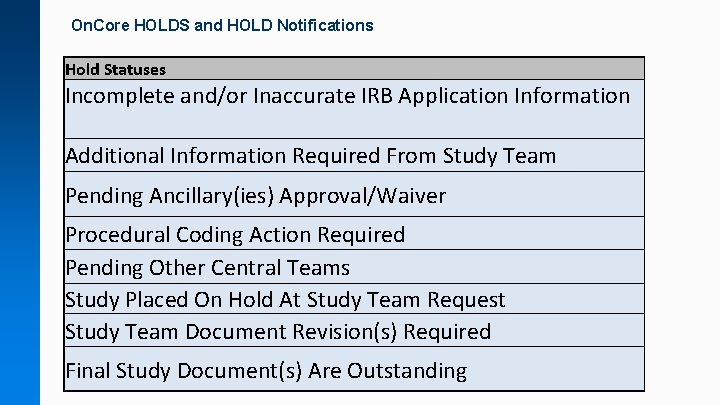
On. Core HOLDS and HOLD Notifications Hold Statuses Incomplete and/or Inaccurate IRB Application Information Additional Information Required From Study Team Pending Ancillary(ies) Approval/Waiver Procedural Coding Action Required Pending Other Central Teams Study Placed On Hold At Study Team Request Study Team Document Revision(s) Required Final Study Document(s) Are Outstanding

Sample On-Hold Notification This study has been placed on hold for build in On. Core for the reason indicated below. If you have any questions about this notification, please reply to this email or call 310 -267 -2273 (7 -CARE). Thank you, CRIS Help Desk Clinical Research Information Systems | UCLA Office of Clinical Research 10911 Weyburn Avenue, Suite 300, Los Angeles, CA 90024 email: CRIShelpdesk@mednet. ucla. edu Protocol: 17 -001321 Short Title: On Hold Fake Study Title: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial of Something Not Real Dept: MEDICINE-GASTROENTEROLOGY Status Date: 2017 -10 -11 Status Reason: Pending Ancillary(ies) Approval/Waiver Status Comments: Pending Pathology/Laboratory & Radiology approval or waiver.

Draft Budget Review & Negotiation Responsible Party: Study Team Additional Resources Coming Soon (via www. researchgo. ucla. edu): Study Startup & Administrative Costs Executive Memorandum • Detailed explanation of standard UCLA Health System clinical trial startup costs and administrative costs required for most industry sponsored trials. Clinical Research Participant Insurance Pre-Authorization Template Clinical Research Participant Insurance Denial Response/Appeal Template Financial Reports Reconciling Front-End Budgeting (coding/pricing) with Back-End Charges

Post-award Identifying potential issues Reconciliation of Patient Care Costs • All study patient bills & charges must be reviewed for accuracy. • Contact billing office(s) for service areas and request that listing of charges be sent for review and approval PRIOR to bills being charged to the study. • Review charges and compare with the Coverage Analysis Matrix to determine if the charges were paid for by the sponsor or if they should be billed to insurance as Routine Care. • Advise billing office(s) how each charge should be processed, and if it is paid for the by the sponsor, at what rate it should be charged. • There are 2 Billing Offices: Hospital/Technical vs. Professional charges

Post-award Identifying potential issues Invoicing • Studies must be invoiced in accordance with the payment schedule in the contract. • Payment schedules are unique for each study.

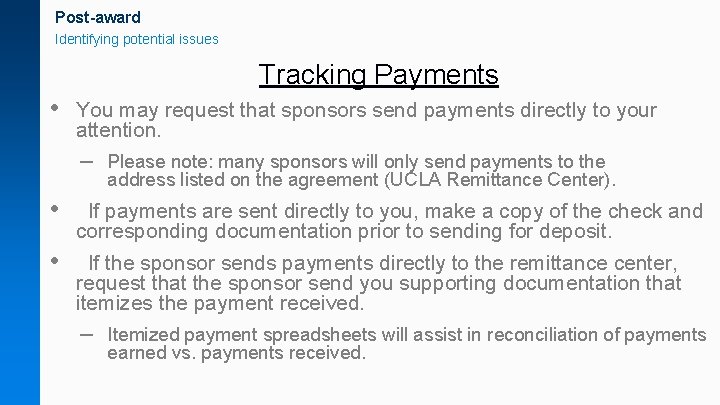
Post-award Identifying potential issues Tracking Payments • You may request that sponsors send payments directly to your attention. – Please note: many sponsors will only send payments to the address listed on the agreement (UCLA Remittance Center). • If payments are sent directly to you, make a copy of the check and corresponding documentation prior to sending for deposit. • If the sponsor sends payments directly to the remittance center, request that the sponsor send you supporting documentation that itemizes the payment received. – Itemized payment spreadsheets will assist in reconciliation of payments earned vs. payments received.

Post-award Policy 913 • If an unexpected balance remains after close-out of an expired or terminated fixed-price or fixed-rate contract or nonrefundable grant, at the Principal Investigator’s request, applicable indirect costs will be applied to the balance and the remaining amount may be converted to unrestricted funds to be made available to the Principal Investigator. • To close a contract & transfer the unexpected balance to Policy 913 (fund 69970), EFM will require confirmation for the following: • ALL work has been completed & any applicable reports have been submitted. • ALL study costs have been appropriately charged to the award fund. • ALL invoices have been submitted. • ALL anticipated payments have been received. • No other expenses are expected to hit the ledger (e. g. payroll & other expenses) http: //www. adminpolicies. ucla. edu/APP/Number/913

Post-award Policy 913 If the remaining balance is more than 25% of the total award, the following is required: • Principal Investigator must provide written justification explaining the balance (carbon copy your MSO and Division Chief on the e-mail to EFM). • Once EFM has received the required information, EFM will close the fund and transfer the unexpended balance to fund 69970. http: //www. adminpolicies. ucla. edu/APP/Number/913

Post-award Policy 913 • Important Points to Remember: • No expenses are allowed that originally occurred outside contract approval dates. • EFM will transfer the TOTAL cost balance to 69970 and the Dean’s Office will recharge the indirect costs quarterly. • Example: Total Unexpended Balance is $1, 575 (inclusive of $325 indirect costs) • $1, 250 (Direct) + $325 (Indirect) = $1, 575 total (EFM will deposit entire amount into fund 69970 • However, Dean’s Office will recharge (debit) your account $325 indirect costs. SO DON’T SPEND IT!! http: //www. adminpolicies. ucla. edu/APP/Number/913

Draft Budget Review & Negotiation Responsible Party: Study Team Additional Resources Coming Soon (via www. researchgo. ucla. edu): Study Startup & Administrative Costs Executive Memorandum • Detailed explanation of standard UCLA Health System clinical trial startup costs and administrative costs required for most industry sponsored trials. Clinical Research Participant Insurance Pre-Authorization Template Clinical Research Participant Insurance Denial Response/Appeal Template Financial Reports Reconciling Front-End Budgeting (coding/pricing) with Back-End Charges

THERE IS NO FINISH LINE! Always Seeking Opportunities for Improvement & Appreciate Your Feedback

Questions? General Inquiries: Coverage. Analysis@mednet. ucla. edu Additional Information: www. Research. Go. UCLA. edu
 Ucla fau
Ucla fau Ucla epass
Ucla epass Sling health ucla
Sling health ucla Asbmt clinical research training course
Asbmt clinical research training course Mrc clinical research training fellowship
Mrc clinical research training fellowship Imprest system and fluctuating
Imprest system and fluctuating Citi training ucla
Citi training ucla Ucla autism center of excellence
Ucla autism center of excellence Senior manager vs general manager
Senior manager vs general manager Portfolio manager synergy manager parental developer
Portfolio manager synergy manager parental developer Readyset ohsu
Readyset ohsu Wealtheoffice
Wealtheoffice Clinical investigator training program
Clinical investigator training program Basic clinical observations training
Basic clinical observations training Student doctor method of clinical training
Student doctor method of clinical training Oracle clinical training ppt
Oracle clinical training ppt Grant implementation plan
Grant implementation plan Beroepsprofiel office manager
Beroepsprofiel office manager Position of office manager
Position of office manager Front office processes
Front office processes Beroepsprofiel office manager
Beroepsprofiel office manager Organization chart of large hotel
Organization chart of large hotel What is socra
What is socra Scientia clinical research
Scientia clinical research Research design in clinical psychology
Research design in clinical psychology Pi clinical research consultancy
Pi clinical research consultancy Alcoac
Alcoac Role of statistician in clinical trials
Role of statistician in clinical trials Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Kavi institute of clinical research
Kavi institute of clinical research Aros klinik
Aros klinik Translating research findings to clinical nursing practice
Translating research findings to clinical nursing practice Jasper clinical research
Jasper clinical research Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Clinical research support services
Clinical research support services Clinical research definition
Clinical research definition Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Drcr.net
Drcr.net Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Questra clinical research
Questra clinical research Foundations of clinical research applications to practice
Foundations of clinical research applications to practice Iwr clinical trial
Iwr clinical trial Hedge fund research inc
Hedge fund research inc Achronims
Achronims Epfr fund flows
Epfr fund flows Veritas volume manager tutorial
Veritas volume manager tutorial Notam manager training
Notam manager training Dft street manager
Dft street manager Federal notam system
Federal notam system Ctsc training
Ctsc training Uq research data manager
Uq research data manager Office and factory
Office and factory Ms office 2007 training
Ms office 2007 training Wvu research office
Wvu research office Training is expensive without training it is more expensive
Training is expensive without training it is more expensive Perbedaan on the job training dan off the job training
Perbedaan on the job training dan off the job training Aggression replacement training facilitator training
Aggression replacement training facilitator training Ucla logon
Ucla logon Ucla mecn personal statement
Ucla mecn personal statement Ucla school of nursing requirements
Ucla school of nursing requirements Ucla nursing application
Ucla nursing application Alex bui ucla
Alex bui ucla Ucla endowed chairs
Ucla endowed chairs Ucla dom dra
Ucla dom dra Dr gelabert ucla
Dr gelabert ucla Ucla epass
Ucla epass Anova stata ucla
Anova stata ucla Dehong xu
Dehong xu Michael rosove
Michael rosove Dgsom financial aid
Dgsom financial aid Rosenfeld library ucla
Rosenfeld library ucla Ucla cs33
Ucla cs33 John cho ucla
John cho ucla Scala ucla npi
Scala ucla npi Timothy fong ucla
Timothy fong ucla Ucla cemrp
Ucla cemrp Horace mann ucla community school
Horace mann ucla community school Ucla admission requirements transfer
Ucla admission requirements transfer