Society of Clinical Research Associates SOCRA Clinical Site
















- Slides: 18

Society of Clinical Research Associates (SOCRA): Clinical Site Coordinator/Manager Workshop November 9 -10, 2017 San Diego, CA Heather M. Quinlan-Baron, Grants Manager, Endocrinology February 26, 2018

What is The Society of Clinical Research Associates (SOCRA) is a non-profit, charitable and educational membership organization committed to providing education, certification, and networking opportunities to all persons involved in clinical research activities. SOCRA, the premier educational organization for oncology site coordinators, has now emerged as a leading educational organization for clinical researchers in all therapeutic areas, supporting industry, government and academia.

• Incorporated in 1991, with local chapters across the U. S. and Canada, along with international chapters in Belgium, Brazil, Poland Saudi Arabia • Membership includes conferences, workshops, a journal and online learning • Annual conference takes place in the Fall of each year • Workshops on such topics as Clinical Investigators and GCP; Drug Development and Clinical Science; Project Management; and Advanced Site Management: Finance and Productivity • Certified Clinical Research Professional (CCRP) program § Industry-recognized certification, with review course and examination offering

Why did I attend the Clinical Site Coordinator/Manager Workshop? Came from a research background before working at BU/BMC and hoping to become more involved with ongoing clinical trials in a research versus solely financial capacity Working on developing a Clinical Trials Unit in our Section Expand my knowledge regarding research study visit design and implementation Become more familiar with the regulatory/IRB process Stop bothering my colleague, Ashley Mc. Carthy, all the time!

Workshop Presenters Both research nurses and CCRP certified, with combined 62 years of clinical research experience From Canada (Calgary and Toronto) One working for the Center for Addiction and Mental Health in Toronto, the other in an independent clinical research consulting organization Very knowledgeable, especially strong on GCP regulations, informed consent, and source documents

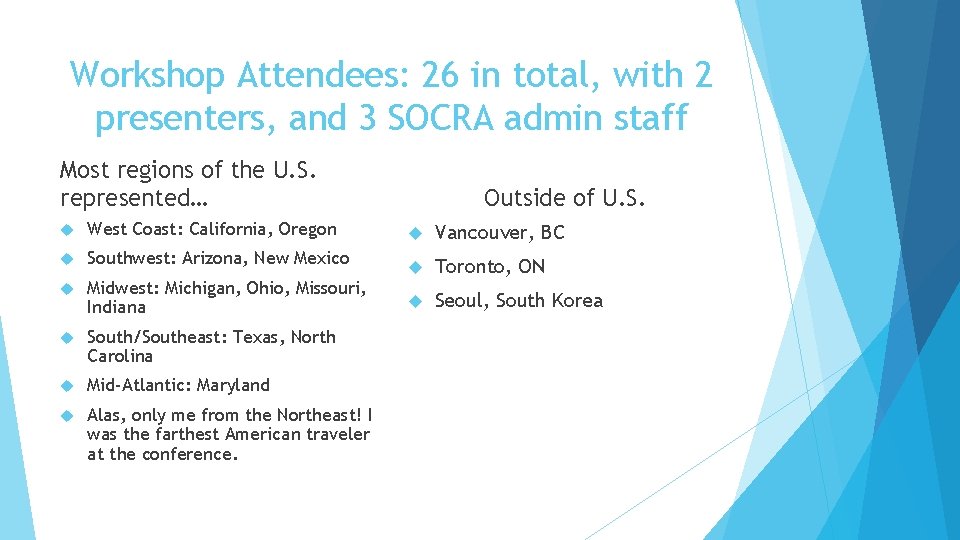
Workshop Attendees: 26 in total, with 2 presenters, and 3 SOCRA admin staff Most regions of the U. S. represented… Outside of U. S. West Coast: California, Oregon Vancouver, BC Southwest: Arizona, New Mexico Midwest: Michigan, Ohio, Missouri, Indiana Toronto, ON Seoul, South Korea South/Southeast: Texas, North Carolina Mid-Atlantic: Maryland Alas, only me from the Northeast! I was the farthest American traveler at the conference.

Workshop Topics and Agenda The Regulatory Environment Good Clinical Practice at the Research Site Informed Consent Process Safety for the Research Subject Study Implementation Monitoring Visits Audits and Inspections Continuous Quality Improvement

So, what exactly is GCP and why is it so important? Good Clinical Practice (GCP): A standard for the design, conduct, performance, monitoring, auditing, recording, analyses, and reporting of clinical trials that provides assurance that the data and reported results are credible and accurate, and that the rights, integrity, and confidentiality of trial subjects are protected Major theme of the workshop and discussed throughout each topic

The Regulatory Environment Evolution of GCP § Experimentation/research atrocities during WWII lead to Nuremberg Code in 1947 § Thalidomide (1959 -1962) resulted in amendment to the FDA Cosmetics Act in 1962 § Declaration of Helsinki: adopted in 1964, statement of ethical principles and guidance for physicians and others conducting research § Tuskegee Syphilis study (1932 -1972): subjects not informed of purpose of study, treatment deliberately withheld § Belmont report (1979): informed consent, and special protection for those with diminished autonomy § International Council on Harmonization (ICH): implemented in 1997, developed GCP guidelines that are in use today

Good Clinical Practice at the Research Site Guiding principles of clinical GCP Delegation log and other documentation Investigational Research product ethics boards

Informed Consent Process GCP for informed consent Environment and interpersonal factors that affect receiving consent Issues affecting information and understanding such as age, culture, learning style and language Did an exercise on ICH guidelines for GCP of Informed Consent (Elements A-T) that was particularly helpful Tip sheet on drafting Informed Consent in my workbook is also a useful tool

Safety for the Research Subject Safety reporting Adverse Events (AEs) § Any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product which does not necessarily have to have a causal relationship with this treatment Serious Adverse Events (SAEs) Exercise determining if AE/SAE related to study participation

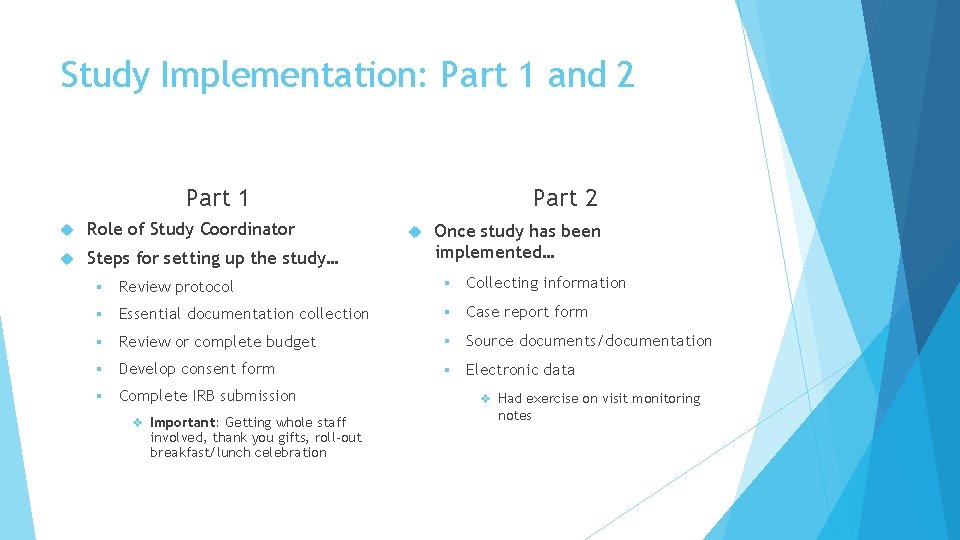
Study Implementation: Part 1 and 2 Part 1 Role of Study Coordinator Steps for setting up the study… Part 2 Once study has been implemented… § Review protocol § Collecting information § Essential documentation collection § Case report form § Review or complete budget § Source documents/documentation § Develop consent form § Electronic data § Complete IRB submission v Important: Getting whole staff involved, thank you gifts, roll-out breakfast/lunch celebration v Had exercise on visit monitoring notes

Monitoring Visits The role of site/study monitor How to have a successful site visit Fun skit on everything that can/could go wrong at a monitoring visit

Audits and Inspections The purpose of an audit/inspection is to evaluate the conduct of the trial and assess compliance with protocols, SOPs, GCP, and the applicable regulatory requirements Quality management systems Quality assurance § Compliance § Documentation § Transparency § Ethical conduct How to prepare for site audits and inspections

Continuous Quality Improvement “All improvement involves change, not all changes are improvement” Proactively assessing performance of the study team How to develop a Corrective Action/Preventive Action Plan (CAPA) The importance of Standard Operating Procedures (SOPs)

What else did I learn? Question to my fellow attendees: Do you charge PI salaries on your studies/trials? Answer was a resounding YES! They work in the negotiating stage to be sure salaries are covered appropriately, and begin charging salaries to accounts right away. Question to presenters/SOCRA organizers: As a newcomer to clinical trial research, what workshops would you recommend? The annual meeting does pre-conference workshops for beginners, that cover GCP, budgeting/finances, IRB and more. Would recommend that over taking additional workshops for newcomers. Question to presenter: We are in the starting phasing of proposing a clinical trial unit at our hospital, do you have resources available in that regard? Email us at anytime, and we can send you an outline/checklist of what you’ll need to get your program started

And finally… The workshop was great and glad I attended, but would definitely be interested in attending the beginner pre-conference workshops in the future. Would recommend membership to anyone working in clinical trials San Diego is a beautiful city with culture, history, and education…and 72 degree weather in November! Many thanks to the DOM Admin Professional Development grant, and the support of my Section, especially Administrative Director, Jen Fosbroke and Section Chief, Alan Farwell, for this opportunity
 What is socra
What is socra Hot site cold site warm site disaster recovery
Hot site cold site warm site disaster recovery What happens at this point?
What happens at this point? Society of clinical data management
Society of clinical data management Dr friedman cushing's
Dr friedman cushing's Canadian society of clinical perfusion
Canadian society of clinical perfusion Gertler econ
Gertler econ Site initiation visit
Site initiation visit Fortune society scattered site housing
Fortune society scattered site housing Scientia clinical research
Scientia clinical research Research design in clinical psychology
Research design in clinical psychology Pi clinical research consultancy
Pi clinical research consultancy Good documentation practices in clinical research
Good documentation practices in clinical research Clinical research statistician
Clinical research statistician Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Kavi kenya
Kavi kenya Academic research organization
Academic research organization Translating research findings to clinical nursing practice
Translating research findings to clinical nursing practice Jasper clinical research
Jasper clinical research