Guidelines for Clinical Trials in Uganda Jasper OgwalOkeng





















- Slides: 23

Guidelines for Clinical Trials in Uganda Jasper Ogwal-Okeng Gulu University Research Workshop 3 rd-6 th March 08

Clinical Trials �Evaluation of a Product for Clinical Effects �Safety and Effectiveness �Product can be �Drugs �Vaccines

Phases of Clinical Trials �Phase I: Healthy volunteers �Phase IIa: Early phase II �Phase IIb: Late phase II �Phase III: RCT, blinded �Phase IV: Pharmacovigilance

Phase I �Follows successful pharmacological and toxicological studies in animals �Healthy volunteers �Safety and pharmacokinetic data obtained (not efficacy-since no symptoms) �Start with 1/5 th or 1/10 th maximum tolerated dose in the most sensitive animal species �Placebo and double-blinded

Phase II Studies �First administered to patients �Phase II a (Early phase II) �Potential benefits and side effects �Establish dose range for phase IIb �Phase IIb (Late phase II) �Establish efficacy in specific disease �Compare efficacy and side effects with other drugs for same conditions

Phase III studies �Randomized, controlled, double-blind ed �Sufficient sample size for statistical evaluation of efficacy and safety. �Successful phase III trial leads to New Drug Submission (NDS) �NDS requests permission to market new drug

Phase IV �After drug obtained marketing license �Monitored for �Rare side effects �Chronic toxicity e. g. cancer after many people- years of use �Previously unknown interactions �Potential new therapeutic use �Dose modifications

Guidelines for Clinical Ttrials in Uganda �All medicines used in Uganda should be registered with National Drug Authority (NDA) �Written approval of NDA needed for clinical of drugs (registered/unregistered) used in Uganda �Guidelines gives procedures of application for clinical Trials

APPLICATION SUBMISSION, REVIEW AND EVALUATION �Procedures for submission �Procedures for Review and Approval �Institutional Review Boards �Amendments to Trial Protocol �Inspection/Audit by NDA �Reports and Final Review

Procedure for Application submission �To Executive Secretary/Registrar NDA �Fee and structure �Clinical Trial application form �Documents accompanying application form (Appendices 1 -17)

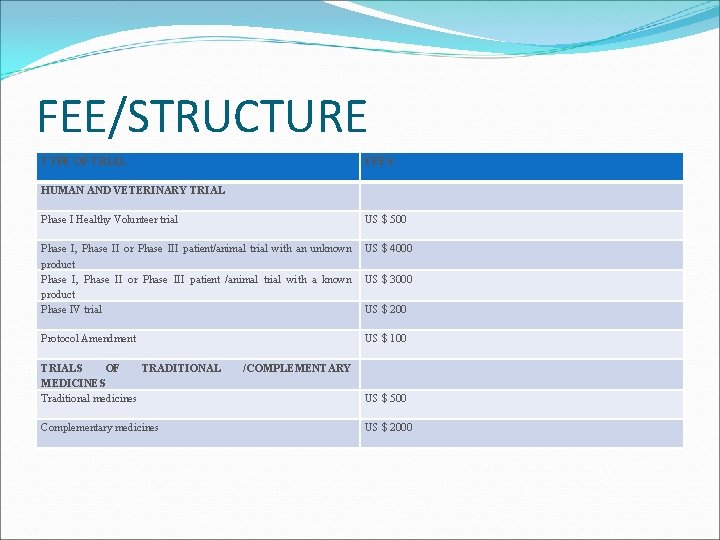
FEE/STRUCTURE TYPE OF TRIAL FEES HUMAN AND VETERINARY TRIAL Phase I Healthy Volunteer trial US $ 500 Phase I, Phase II or Phase III patient/animal trial with an unknown product Phase I, Phase II or Phase III patient /animal trial with a known product Phase IV trial US $ 4000 Protocol Amendment US $ 100 TRIALS OF TRADITIONAL MEDICINES Traditional medicines Complementary medicines US $ 3000 US $ 200 /COMPLEMENTARY US $ 500 US $ 2000

Review and Approval of Applications �Completeness-form, document and fee �Application reference number �Supplementary Data and updates �Expert Review (appointed by NDA) �Approval by NDA’s Clinical Trials Committee (CTC) �Approval communicated in writing �Post Trial Review

Institutional Review Boards �Established in the institution where research is done �Ensures safety, integrity and human rights issues �CTC of NDA oversees all IRBs �NDA approves application after IRB and NCST approvals

Amendments to Trial Protocol �May be partial or complete. �Urgent-Change and inform IRB, NCST, NDA �Otherwise resubmit to NDA and wait decision

Inspection/Audit by NDA �To verify monitoring and audit of protocol �Facilities �Research staff �Compliance with protocol �Serious Adverse Events being reported

Reports and Final Review �Reporting of Serious Adverse Events �Interim and Final Trial Reports �Dissemination and Publication �Archiving

CLINICAL TRIAL LICENSE (CTL) �Approval for importation/manufacture of CT commodities given after CT approval �Products that require CTL �Procedures for Application for CTL �Conditions for CTL �Importation and Release of Investigational Product (IP) �Documentation for IP Release

Products that Require CTL �Unregistered products, including placebos �Registered �Used/assembled in different form from approved form �Used for unapproved indication �Used to gain further information about approved use �Local product manufactured for Clinical Trial

Application Procedures for CTL �Who to apply: �Principal Investigator �Sponsor (authorized person from a pharmaceutical company)

Attachments �Format for Clinical Trial Protocol �Investigator’s brochure �Guide for labeling clinical trial medicines �Letter of authorization �Clinical Trial application form �Format for clinical trial reports �Declaration by Investigators �Check list for required documentation

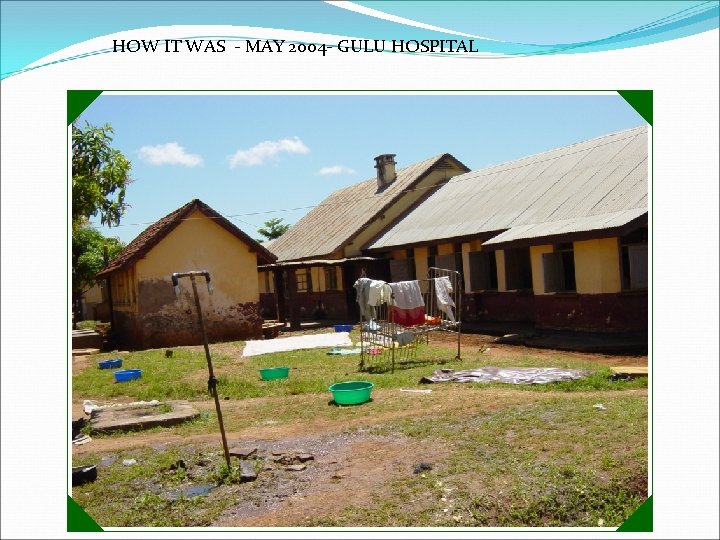
HOW IT WAS - MAY 2004 - GULU HOSPITAL

HOW IT IS – OCTOBER 2004 - FACULTY OF MEDICINE

Good Luck
 Jasper clinical research
Jasper clinical research Prs clinical trial
Prs clinical trial Lej hub customs dhl
Lej hub customs dhl Phs human subjects and clinical trials information
Phs human subjects and clinical trials information Audits and inspections of clinical trials
Audits and inspections of clinical trials Clinical research statistician
Clinical research statistician Clinical trials.gov login
Clinical trials.gov login Mpn clinical trials
Mpn clinical trials Nida clinical trial network
Nida clinical trial network Clinical trials quality by design
Clinical trials quality by design Clinical hysteria salem witch trials
Clinical hysteria salem witch trials Mrc clinical trials unit
Mrc clinical trials unit Ivr iwr studies
Ivr iwr studies Prs registration
Prs registration Site initiation visit in clinical trials ppt
Site initiation visit in clinical trials ppt Mpn clinical trials
Mpn clinical trials Readyset ohsu
Readyset ohsu Clinical trials
Clinical trials York trials unit
York trials unit Clinical trials
Clinical trials Clinical trial api
Clinical trial api Bedside clinical guidelines partnership
Bedside clinical guidelines partnership Easl 2018 decompensated cirrhosis
Easl 2018 decompensated cirrhosis Gerd clinical practice guidelines
Gerd clinical practice guidelines