An integrated approach to successful e Clinical trials




















- Slides: 34

An integrated approach to successful e. Clinical trials CCRA Seminar: Standards in Electronic Data Capture London 1 November 2005 Dr Bill Byrom Product Strategy Director, Clin. Phone Group Ltd.

Presentation contents o Common application of IVR/IWR in today’s clinical trials o Why integrate EDC and IVR? o Integration overview o Case study: Procter & Gamble Pharmaceuticals o Demonstration movie o Conclusions

Common IVR / IWR application Typical application areas in today’s clinical trials

IVR/IWR application o Secure telephone / web access for the performance of various site-based, sponsor-based, or patient-based activities. – Randomization – Emergency Code Break – Medication Dispensing and Supply Chain Management – Electronic Patient-Reported Outcomes (e. PRO) – Patient Pre-qualification and Recruitment

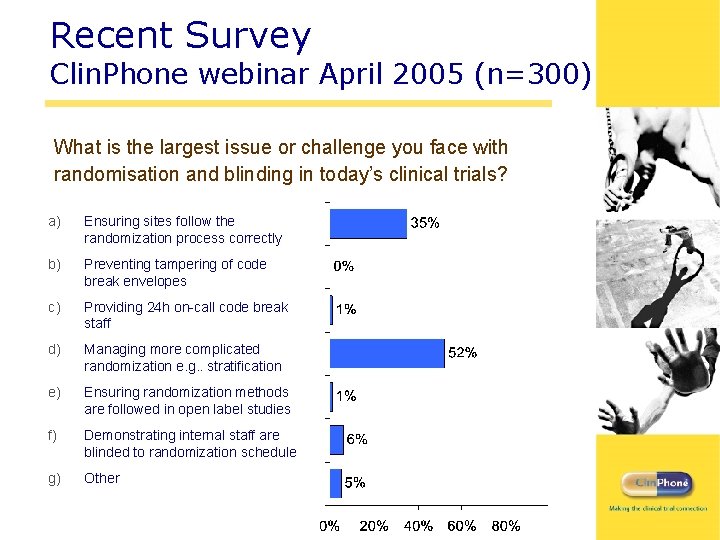
Recent Survey Clin. Phone webinar April 2005 (n=300) What is the largest issue or challenge you face with randomisation and blinding in today’s clinical trials? a) Ensuring sites follow the randomization process correctly b) Preventing tampering of code break envelopes c) Providing 24 h on-call code break staff d) Managing more complicated randomization e. g. . stratification e) Ensuring randomization methods are followed in open label studies f) Demonstrating internal staff are blinded to randomization schedule g) Other

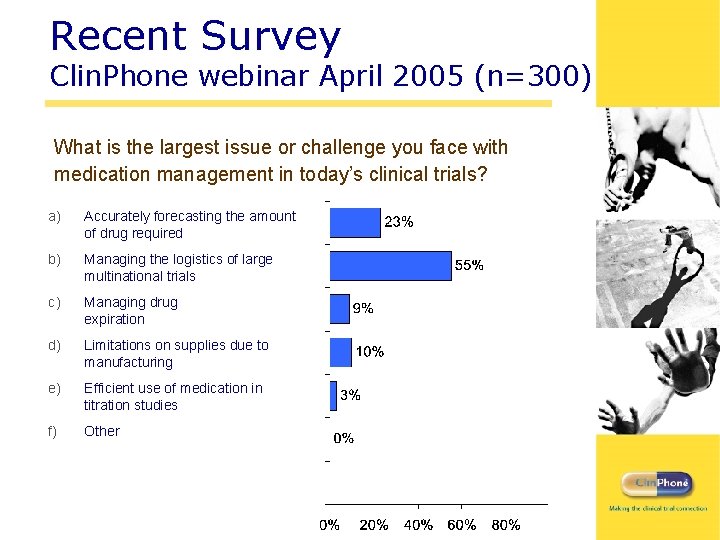
Recent Survey Clin. Phone webinar April 2005 (n=300) What is the largest issue or challenge you face with medication management in today’s clinical trials? a) Accurately forecasting the amount of drug required b) Managing the logistics of large multinational trials c) Managing drug expiration d) Limitations on supplies due to manufacturing e) Efficient use of medication in titration studies f) Other

Medication labelling/dispensing IVR / IWR approach q Unique Medication Numbering – Can be applied to ‘Kit’ of supplies – Can be applied to the Individual Dispensing Unit q Any unit can go to any patient (randomised to same treatment group) q Any unit can be used for any treatment period q Using the smallest dispensing unit gives the most flexibility q Pooling medication across studies possible

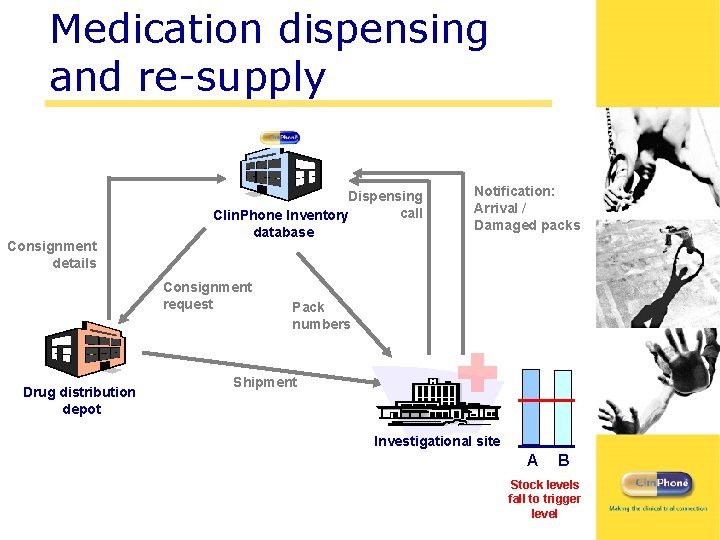
Medication dispensing and re-supply Consignment details Dispensing call Clin. Phone Inventory database Consignment request Drug distribution depot Notification: Arrival / Damaged packs Pack numbers Shipment Investigational site A B Stock levels fall to trigger level

Why Integrate? The benefits of e. Clinical integration

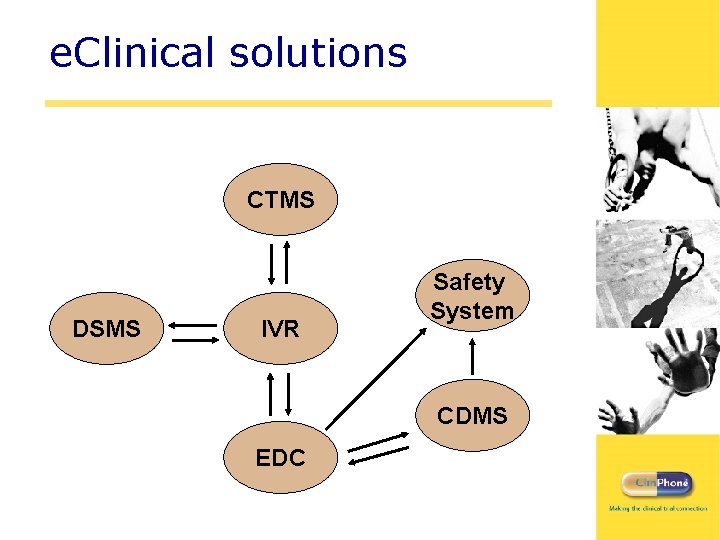
e. Clinical solutions CTMS DSMS IVR Safety System CDMS EDC

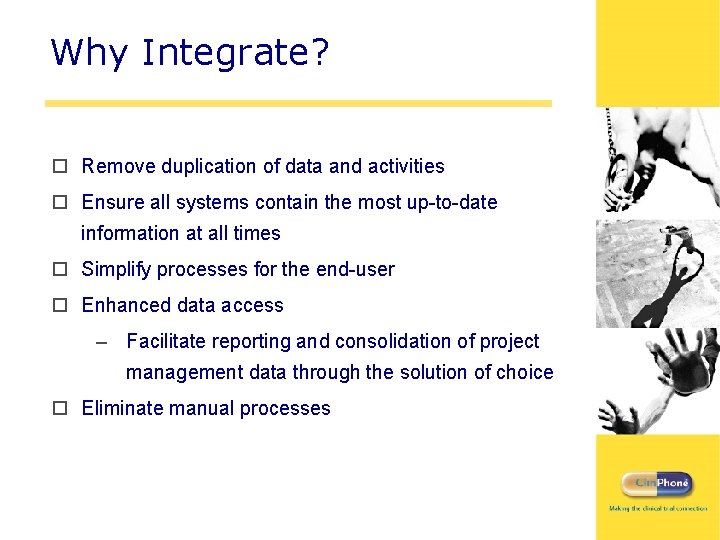
Why Integrate? o Remove duplication of data and activities o Ensure all systems contain the most up-to-date information at all times o Simplify processes for the end-user o Enhanced data access – Facilitate reporting and consolidation of project management data through the solution of choice o Eliminate manual processes

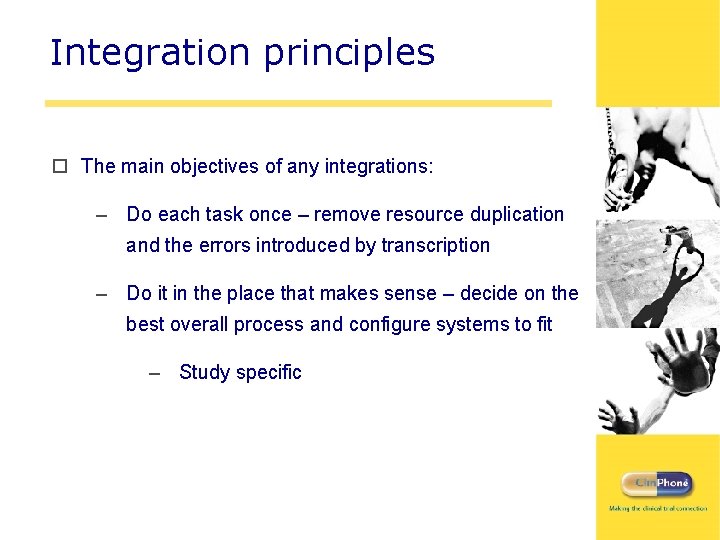
Integration principles o The main objectives of any integrations: – Do each task once – remove resource duplication and the errors introduced by transcription – Do it in the place that makes sense – decide on the best overall process and configure systems to fit – Study specific

The breadth of integration Example 1: patient enrolment CTMS DSMS Drug supply / re-supply request Patient Identifiers, demography and visit frequency data IVR Data for randomisation (eg. stratification variables) Drug Shipment Safety System Randomisation Event Randomisation and Pack Number EDC Patient Enrolment Patient Data CDMS

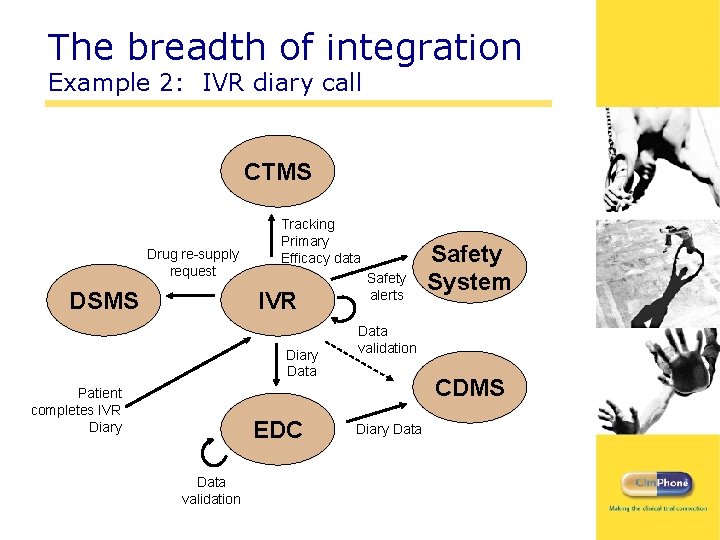
The breadth of integration Example 2: IVR diary call CTMS Drug re-supply request DSMS Tracking Primary Efficacy data IVR Diary Data Patient completes IVR Diary EDC Data validation Safety alerts Safety System Data validation CDMS Diary Data

Common overlap of data Randomization IVR Dispensing e. PRO Screening data Site contact details CTMS EDC

Integration overview How real-time e. Clinical integration is achieved at Clin. Phone

L FTP site L A W E A I F FTP site R XML message E Importer R Database update Transformer I Exporter IVR / IWR database F XML message Event Applicationspecific message format W L L Data interchange overview Application database Applicationspecific Message format Application

IVR-CTMS Integration CTMS DSMS IVR Safety System CDMS EDC

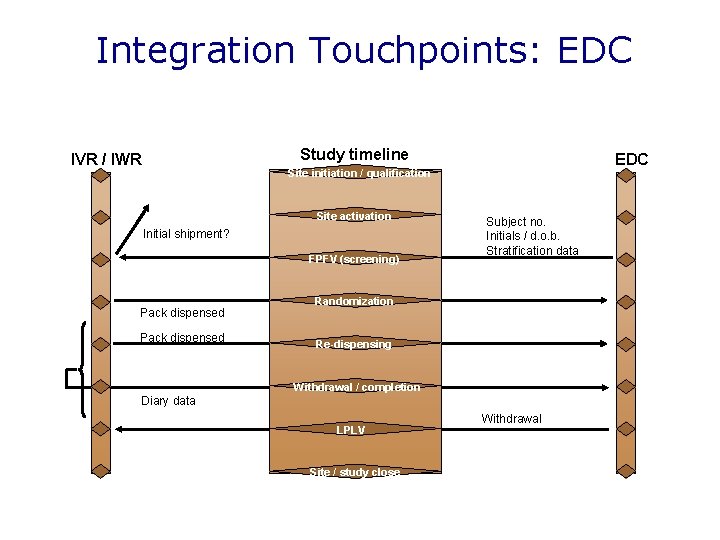
Integration touchpoints: CTMS IVR / IWR Study timeline Site initiation / qualification Site activation Site details Delivery addresses CTMS Patient number ranges Ethics approval Regulatory approval Date first drug shipped Patient tracking data Patient tracking data FSFV (screening) Randomisation Re-dispensing Withdrawal / completion LSLV Site de-activation Site / study close

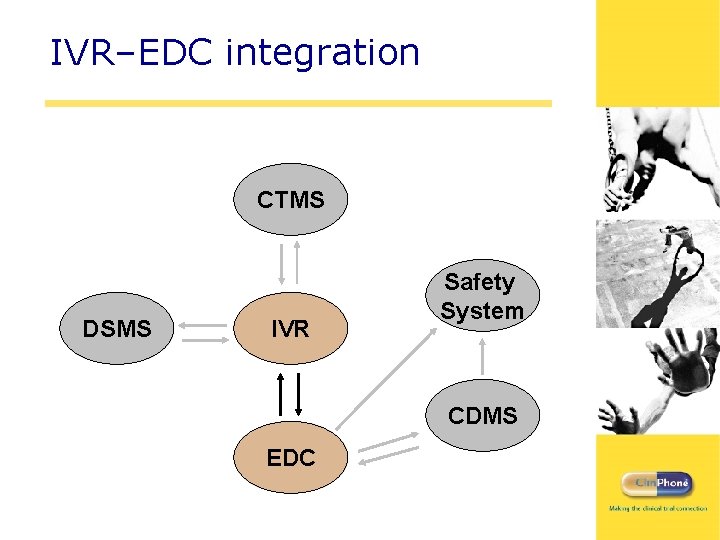
IVR–EDC integration CTMS DSMS IVR Safety System CDMS EDC

Integration Touchpoints: EDC Study timeline IVR / IWR EDC Site initiation / qualification Site activation Initial shipment? FPFV (screening) Pack dispensed Subject no. Initials / d. o. b. Stratification data Randomization Re-dispensing Withdrawal / completion Diary data LPLV Site / study close Withdrawal

Case study IVR-EDC integration Procter and Gamble Pharmaceuticals

P&GP’s Best Practices entry

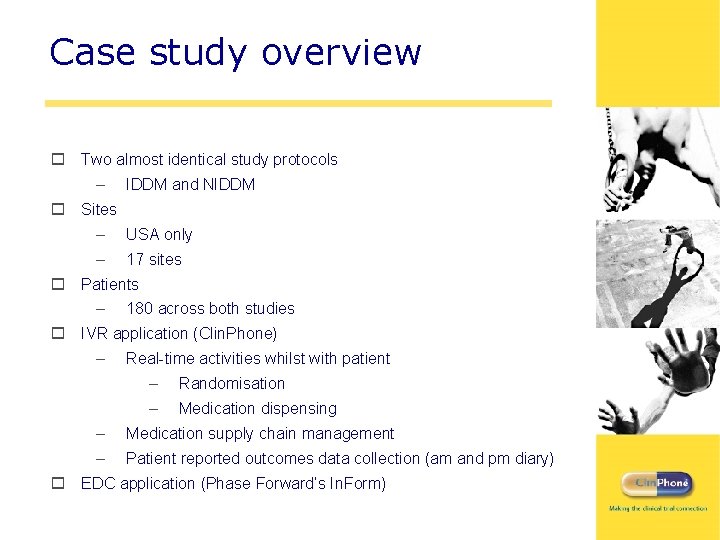
Case study overview o Two almost identical study protocols – IDDM and NIDDM o Sites – USA only – 17 sites o Patients – 180 across both studies o IVR application (Clin. Phone) – Real-time activities whilst with patient – Randomisation – Medication dispensing – Medication supply chain management – Patient reported outcomes data collection (am and pm diary) o EDC application (Phase Forward’s In. Form)

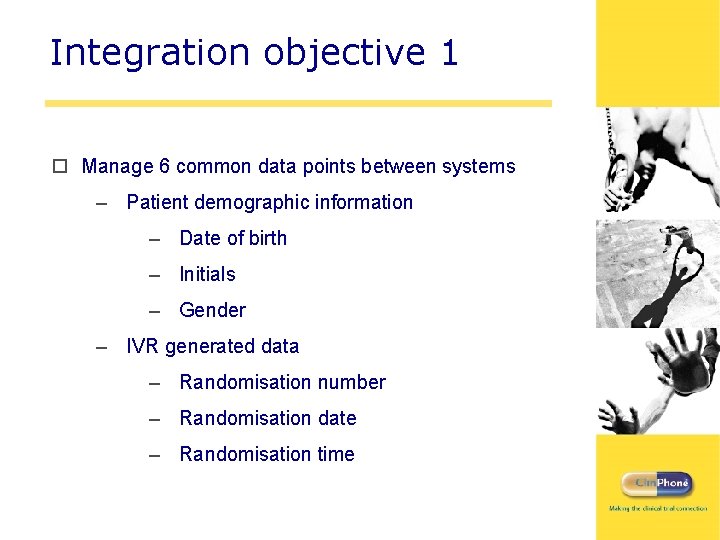
Integration objective 1 o Manage 6 common data points between systems – Patient demographic information – Date of birth – Initials – Gender – IVR generated data – Randomisation number – Randomisation date – Randomisation time

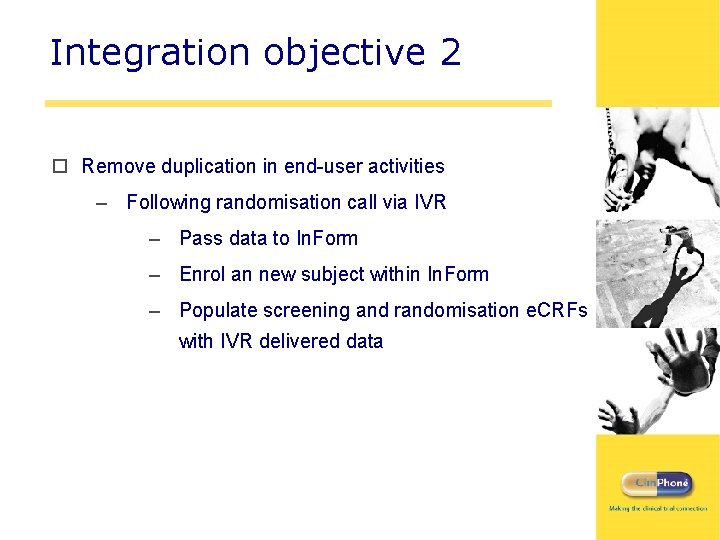
Integration objective 2 o Remove duplication in end-user activities – Following randomisation call via IVR – Pass data to In. Form – Enrol an new subject within In. Form – Populate screening and randomisation e. CRFs with IVR delivered data

Integration objective 3 o Populate e. CRFs with patient diary data in real-time – AM fasting blood glucose reading – PM sum of three daily dosing insulin volumes o Eliminate site data entry of paper diary data o Satisfy regulatory requirements for Investigators to take responsibility for diary data – Monitor diary compliance in real-time – Review and report diary data alongside other clinical data

P&GP key benefits realised o Site coordinators have less data entry to perform o The P&GP data management group do not have to check for discrepancies between common data points in the IVR and EDC systems o Queries from discrepancies are eliminated – Time and financial benefit for Sponsor and site staff o Patient diary compliance proactively monitored o Investigators have immediate access to diary data via EDC – Regulatory requirements – Improvements in patient care and monitoring o More efficient workflow – May improve database lock time

Demonstration movie EDC - IVR integration

Integration example o Screening data entered within EDC application o Randomization performed using IWR o Data received by EDC o Patient diary event sent from IVR / IWR to EDC Play movie

Summary and conclusions

The Future of Clinical Trials o A multitude of e. Clinical technologies are in use today – All are being used in some trials – Some are tightly integrated with others – Others still used in isolation o Integration can provide powerful Sponsor and site enduser benefits o e. Clinical Trials of the future? – Evolution … not Revolution – Increased adoption will lead to increased requirement to integrate – Highly integrated “seamless” solutions

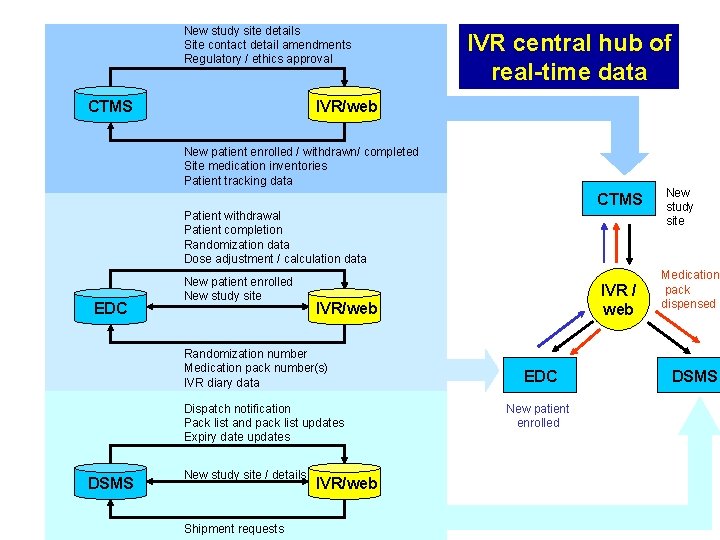
New study site details Site contact detail amendments Regulatory / ethics approval CTMS IVR/web New patient enrolled / withdrawn/ completed Site medication inventories Patient tracking data IVR central hub of real-time data Clinical Trial Management System CTMS Patient withdrawal Patient completion Randomization data Dose adjustment / calculation data EDC New patient enrolled New study site IVR/web Randomization number Medication pack number(s) IVR diary data Dispatch notification Pack list and pack list updates Expiry date updates DSMS New study site / details Shipment requests IVR/web New study site Electronic Data Medication pack IVR / Capture dispensed web System EDC DSMS New patient enrolled Drug Supply Chain Management System

For more information www. clinphone. com info@clinphone. com