The Role of the Statistician in Clinical Research


























- Slides: 48

The Role of the Statistician in Clinical Research Teams Elizabeth Garrett-Mayer, Ph. D. Division of Biostatistics Department of Oncology

Statistics • Statistics is the art/science of summarizing data • Better yet…summarizing data so that non-statisticians can understand it • Clinical investigations usually involve collecting a lot of data. • But, at the end of your trial, what you really want is a “punch-line: ” – Did the new treatment work? – Are the two groups being compared the same or different? – Is the new method more precise than the old method? • Statistical inference is the answer!

Do you need a statistician as part of your clinical research team? • YES! • Simplest reasons: s/he will help to optimize – Design – Analysis – Interpretation of results – Conclusions

What if I already know how to calculate sample size and perform a t-test? • • • Statisticians might know a better approach Trained more formally in design options More “bang for your buck” Tend to be less biased Adds credibility to your application Use resources that are available to you

Different Roles • Very collaborative – – Active co-investigator Helps develop aims and design Brought in early in planning Continues to input throughout trial planning and while study continues • Consultants – Inactive co-investigator – Often not brought in until either • You need a sample size calculation several days before submission • Trial has been criticized/rejected for lack of statistical input • You’ve finally collected all of the data and don’t know what to do next. – Only involved sparsely for planning or for analysis.

Find a statistician early • Your trial can only benefit from inclusion of a statistician • Statisticians cannot rescue a poorly designed trial after the trial has begun. • “Statistical adjustment” in analysis plan does not always work. • Ignorance is not bliss: – Some clinical investigators are trained in statistics – But usually not all aspects! – Despite inclination to choose a particular design or analysis method, there might be better ways.

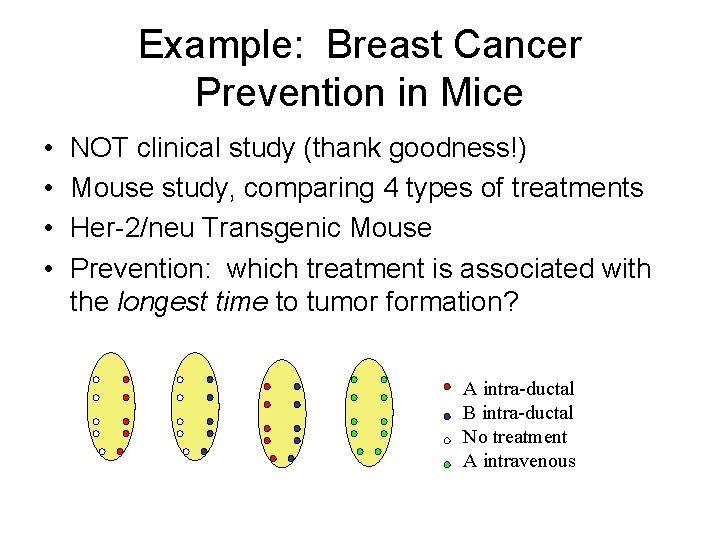
Example: Breast Cancer Prevention in Mice • • NOT clinical study (thank goodness!) Mouse study, comparing 4 types of treatments Her-2/neu Transgenic Mouse Prevention: which treatment is associated with the longest time to tumor formation? A intra-ductal B intra-ductal No treatment A intravenous

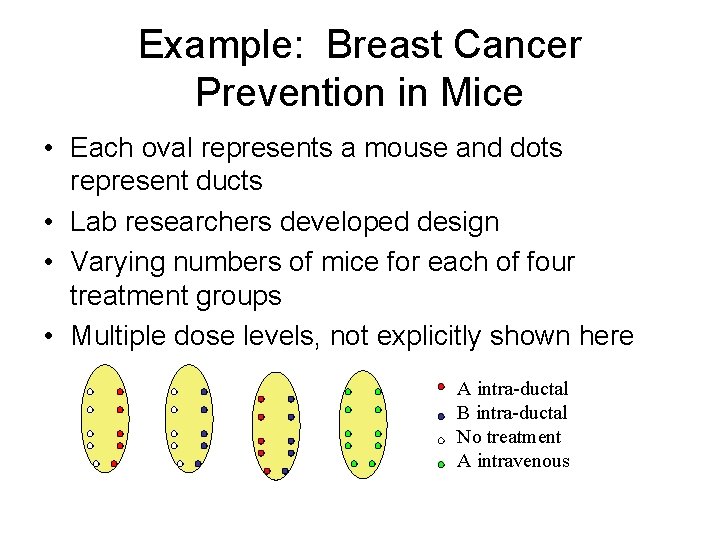
Example: Breast Cancer Prevention in Mice • Each oval represents a mouse and dots represent ducts • Lab researchers developed design • Varying numbers of mice for each of four treatment groups • Multiple dose levels, not explicitly shown here A intra-ductal B intra-ductal No treatment A intravenous

How to analyze? • Researchers had created their own survival curves. • Wanted “p-values” • Some design issues: – What is the unit of analysis? – How do we handle the imbalance? – Treatment B has no “control” side

Our approach • Statistical adjustments used • Treat “side of mouse” as unit of analysis – Cannot use mouse: multiple treatments per mouse in many cases. Too many categories (once dose was accounted for) – Cannot use duct: hard to model the “dependence” within side and within mice. – Still need to include dependence within mice. • Need to adjust for treatment on “other side” – Problems due to imbalance of doses – Could only adjust for “active” versus “inactive” treatment on other side.

Our approach • • • We “rescued” this study! Not optimal, but “best” given the design Adjustments might not be appropriate We “modeled” the data—we made assumptions We could not implement all the adjustments we would have liked to. • Better approach? – Start with a good design! – We would have suggested balance – Would NOT have given multiple treatments within mice.

Statisticians: Specific Responsibilities • Design – Choose most efficient design – Consider all aims of the study – Particular designs that might be useful • Cross-over • Pre-post • Factorial – Sample size considerations – Interim monitoring plan

Statisticians: Specific Responsibilities • Assistance in endpoint selection – Subjective vs. objective – Measurement issues • Is there measurement error that should be considered? • What if you are measuring pain? QOL? – Multiple endpoints (e. g. safety AND efficacy) – Patient benefit versus biologic/PK endpoint – Primary versus secondary – Continuous versus categorical outcomes

Statisticians: Specific Responsibilities • Analysis Plan – Statistical method for EACH aim – Account for type I and type II errors – Stratifications or adjustments are included if necessary – Simpler is often better – Loss to follow-up: missing data?

Sample Size and Power • The most common reason we get contacted • Sample size is contingent on design, analysis plan, and outcome • With the wrong sample size, you will either – Not be able to make conclusions because the study is “underpowered” – Waste time and money because your study is larger than it needed to be to answer the question of interest • And, with wrong sample size, you might have problems interpreting your result: – Did I not find a significant result because the treatment does not work, or because my sample size is too small? – Did the treatment REALLY work, or is the effect I saw too small to warrant further consideration of this treatment? – This is an issue of CLINICAL versus STATISTICAL signficance

Sample Size and Power • Sample size ALWAYS requires the investigator to make some assumptions – How much better do you expect the experimental therapy group to perform than the standard therapy groups? – How much variability do we expect in measurements? – What would be a clinically relevant improvement? • The statistician CANNOT tell you what these numbers should be (unless you provide data) • It is the responsibility of the clinical investigator to define these parameters

Sample Size and Power • Review of power – Power = The probability of concluding that the new treatment is effective if it truly is effective – Type I error = The probability of concluding that the new treatment is effective if it truly is NOT effective – (Type I error = alpha level of the test) – (Type II error = 1 – power) • When your study is too small, it is hard to conclude that your treatment is effective

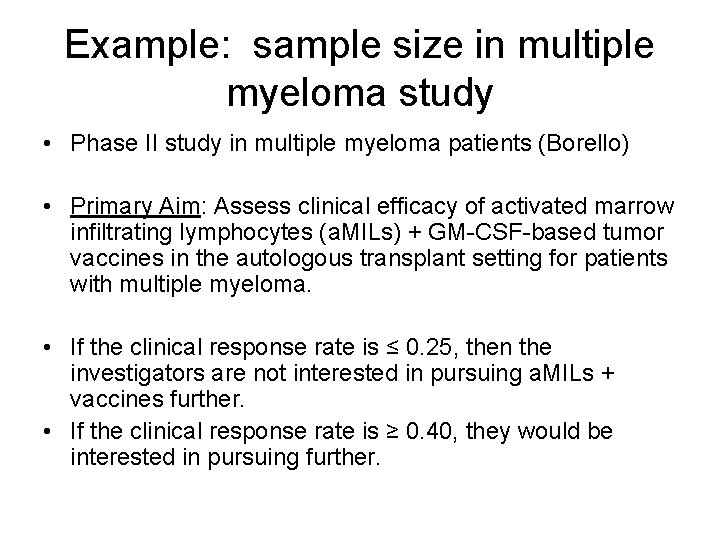
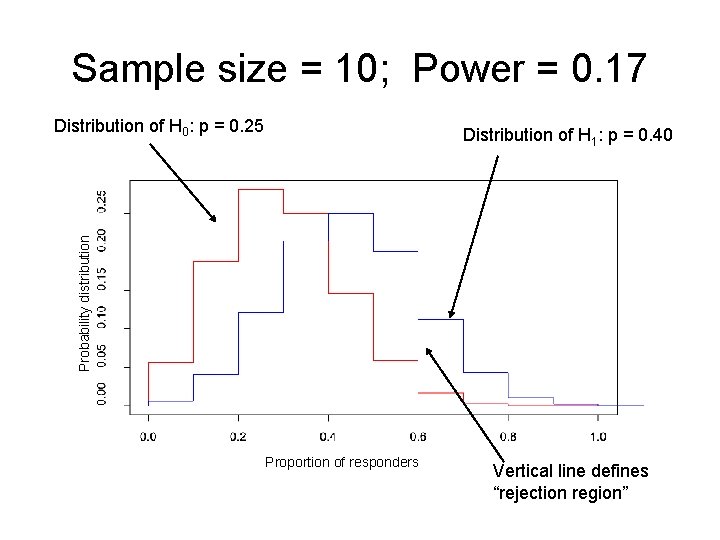
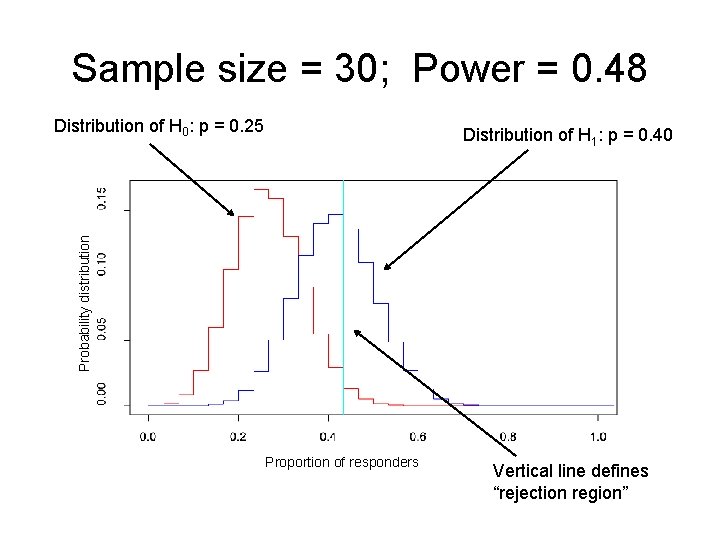
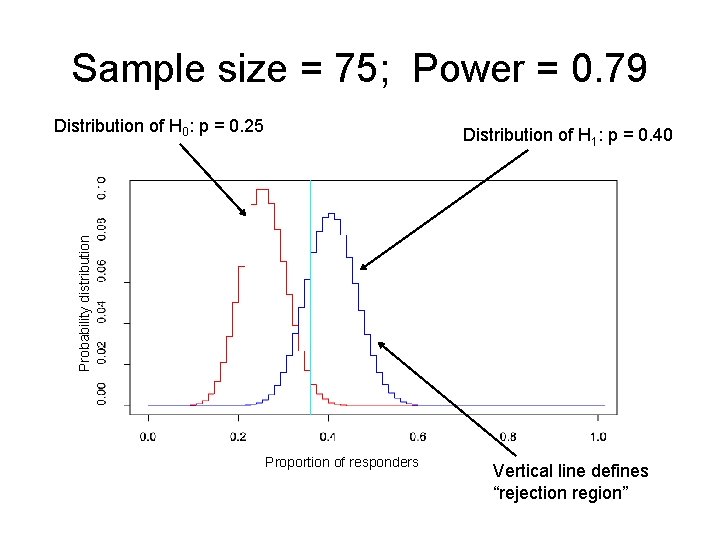
Example: sample size in multiple myeloma study • Phase II study in multiple myeloma patients (Borello) • Primary Aim: Assess clinical efficacy of activated marrow infiltrating lymphocytes (a. MILs) + GM-CSF-based tumor vaccines in the autologous transplant setting for patients with multiple myeloma. • If the clinical response rate is ≤ 0. 25, then the investigators are not interested in pursuing a. MILs + vaccines further. • If the clinical response rate is ≥ 0. 40, they would be interested in pursuing further.

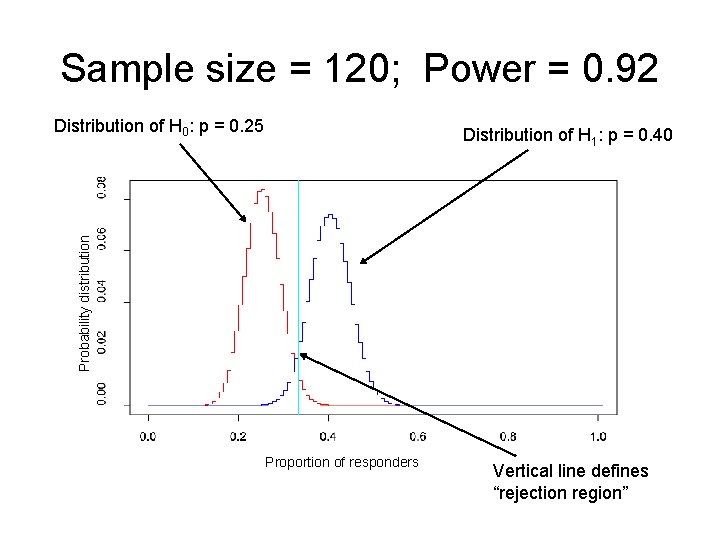
Example: sample size in multiple myeloma study • H 0: p = 0. 25 • H 1: p = 0. 40 • We want to know what sample size we need to have large power and small type I error. – If the treatment DOES work, then we want to have a high probability of concluding that H 1 is “true. ” – If the treatment DOES NOT work, then we want a low probability of concluding that H 1 is “true. ”

Sample size = 10; Power = 0. 17 Distribution of H 0: p = 0. 25 Probability distribution Distribution of H 1: p = 0. 40 Proportion of responders Vertical line defines “rejection region”

Sample size = 30; Power = 0. 48 Distribution of H 0: p = 0. 25 Probability distribution Distribution of H 1: p = 0. 40 Proportion of responders Vertical line defines “rejection region”

Sample size = 75; Power = 0. 79 Distribution of H 0: p = 0. 25 Probability distribution Distribution of H 1: p = 0. 40 Proportion of responders Vertical line defines “rejection region”

Sample size = 120; Power = 0. 92 Distribution of H 0: p = 0. 25 Probability distribution Distribution of H 1: p = 0. 40 Proportion of responders Vertical line defines “rejection region”

Not always so easy • More complex designs require more complex calculations • Usually also require more assumptions • Examples: – Longitudinal studies – Cross-over studies – Correlation of outcomes • Often, “simulations” are required to get a sample size estimate.

Most common problems seen in study proposals when a statistician is not involved • Outcomes are not clearly defined • There is not an analysis plan for secondary aims of the study • Sample size calculation is too simplistic or absent • Assumptions of statistical methods are not appropriate

Examples: trials with additional statistical needs • Major clinical trials (e. g. Phase III studies) • Continual reassessment method (CRM) studies) • Longitudinal studies • Study of natural history of disease/disorder • Studies with ‘non-random’ missing data

Major trials • Major trials usually are monitored periodically for safety and ethical concerns. • Monitoring board: Data (Safety and) Monitoring Committee (DMC or DSMC) • In these, trials, ideally you would have three statisticians (Pocock, 2004, Statistics in Medicine) – Study statistician – DMC statistician – Independent statistician • Why? – Interim analyses require “unbiased” analysis and interpretation of study data. • Industry- versus investigator-initiated trials …differences?

Study Statistician • Overall statistical responsibility • Actively engaged in design, conduct, final analysis • Not involved in interim analyses • Want them to remain ‘blinded’ until the study is complete

DMC Statistician • Experienced trialist • Evaluate interim results • Decide (along with rest of DMC) whether trial continues • No conflict of interest

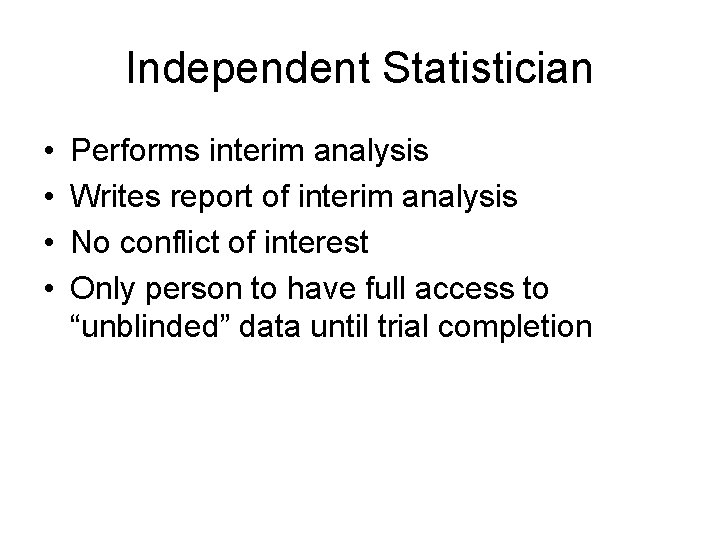
Independent Statistician • • Performs interim analysis Writes report of interim analysis No conflict of interest Only person to have full access to “unblinded” data until trial completion

High-maintenance trials • Some trials require statistical decision-making during the trial • Simon “two-stage” design: – Stage 1: Treat about half the patients. – Stage 2: If efficacy at stage 1 meets some standard, then enroll the remainder of patients • “Adaptive” and “Sequential” trials: final sample size is determined somewhere in the middle of the trial • Continual Reassessment Method…

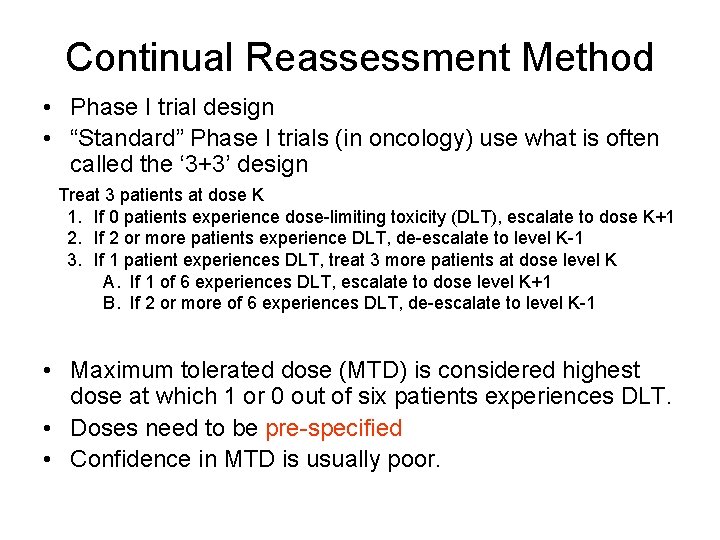
Continual Reassessment Method • Phase I trial design • “Standard” Phase I trials (in oncology) use what is often called the ‘ 3+3’ design Treat 3 patients at dose K 1. If 0 patients experience dose-limiting toxicity (DLT), escalate to dose K+1 2. If 2 or more patients experience DLT, de-escalate to level K-1 3. If 1 patient experiences DLT, treat 3 more patients at dose level K A. If 1 of 6 experiences DLT, escalate to dose level K+1 B. If 2 or more of 6 experiences DLT, de-escalate to level K-1 • Maximum tolerated dose (MTD) is considered highest dose at which 1 or 0 out of six patients experiences DLT. • Doses need to be pre-specified • Confidence in MTD is usually poor.

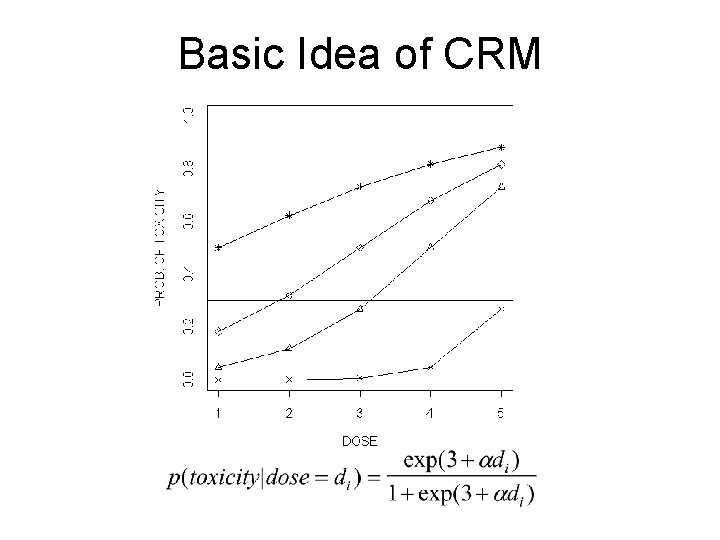
Continual Reassessment Method • Allows statistical modeling of optimal dose: dose -response relationship is assumed to behave in a certain way • Can be based on “safety” or “efficacy” outcome (or both). • Design searches for best dose given a desired toxicity or efficacy level and does so in an efficient way. • This design REALLY requires a statistician throughout the trial. • Example: Phase I/II trial of Samarium 153 in High Risk Osteogenic Sarcoma (Schwartz)

CRM history in brief • Originally devised by O’Quigley, Pepe and Fisher (1990) where dose for next patient was determined based on responses of patients previously treated in the trial • Due to safety concerns, several authors developed variants – – Modified CRM (Goodman et al. 1995) Extended CRM [2 stage] (Moller, 1995) Restricted CRM (Moller, 1995) and others….

Basic Idea of CRM

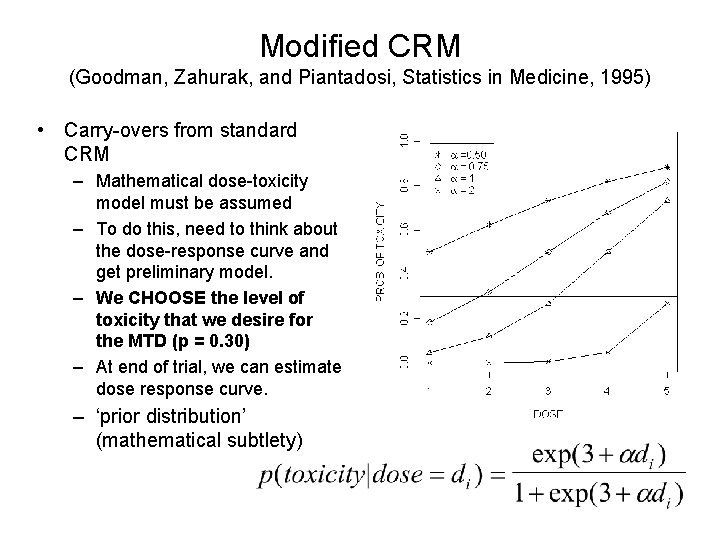
Modified CRM (Goodman, Zahurak, and Piantadosi, Statistics in Medicine, 1995) • Carry-overs from standard CRM – Mathematical dose-toxicity model must be assumed – To do this, need to think about the dose-response curve and get preliminary model. – We CHOOSE the level of toxicity that we desire for the MTD (p = 0. 30) – At end of trial, we can estimate dose response curve. – ‘prior distribution’ (mathematical subtlety)

Modified CRM by Goodman, Zahurak, and Piantadosi (Statistics in Medicine, 1995) • Modifications by Goodman et al. – Use ‘standard’ dose escalation model until first toxicity is observed: • Choose cohort sizes of 1, 2, or 3 • Use standard ‘ 3+3’ design (or, in this case, ‘ 2+2’) – Upon first toxicity, fit the dose-response model using observed data • Estimate • Find dose that is closest to toxicity of 0. 3. – Does not allow escalation to increase by more than one dose level. – De-escalation can occur by more than one dose level. – Dose levels are discrete: need to round to closest level

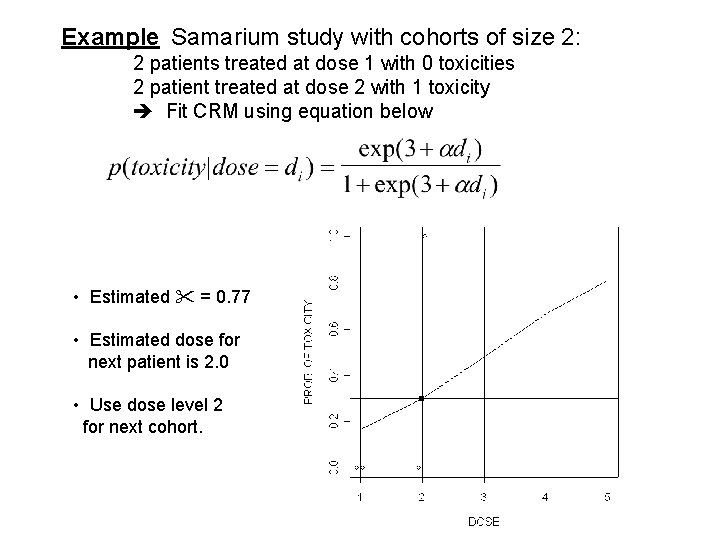
Example Samarium study with cohorts of size 2: 2 patients treated at dose 1 with 0 toxicities 2 patient treated at dose 2 with 1 toxicity Fit CRM using equation below • Estimated = 0. 77 • Estimated dose for next patient is 2. 0 • Use dose level 2 for next cohort.

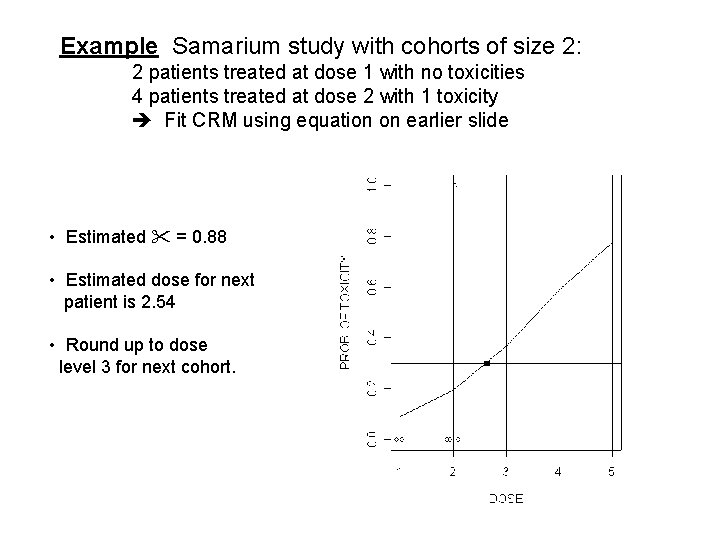
Example Samarium study with cohorts of size 2: 2 patients treated at dose 1 with no toxicities 4 patients treated at dose 2 with 1 toxicity Fit CRM using equation on earlier slide • Estimated = 0. 88 • Estimated dose for next patient is 2. 54 • Round up to dose level 3 for next cohort.

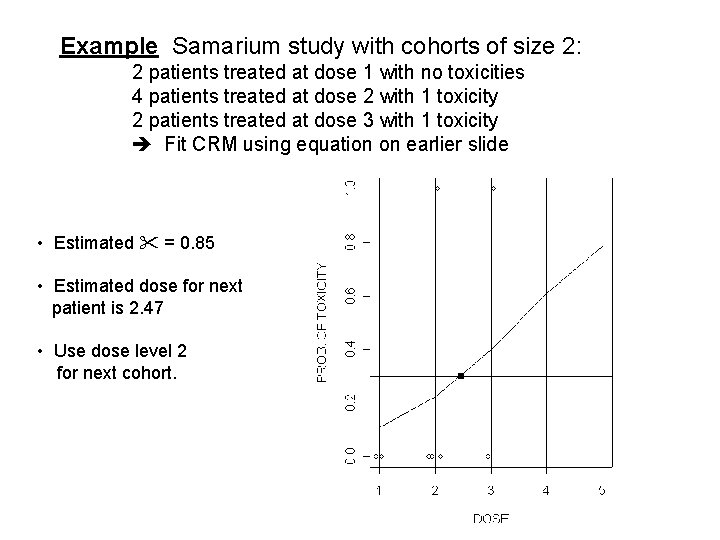
Example Samarium study with cohorts of size 2: 2 patients treated at dose 1 with no toxicities 4 patients treated at dose 2 with 1 toxicity 2 patients treated at dose 3 with 1 toxicity Fit CRM using equation on earlier slide • Estimated = 0. 85 • Estimated dose for next patient is 2. 47 • Use dose level 2 for next cohort.

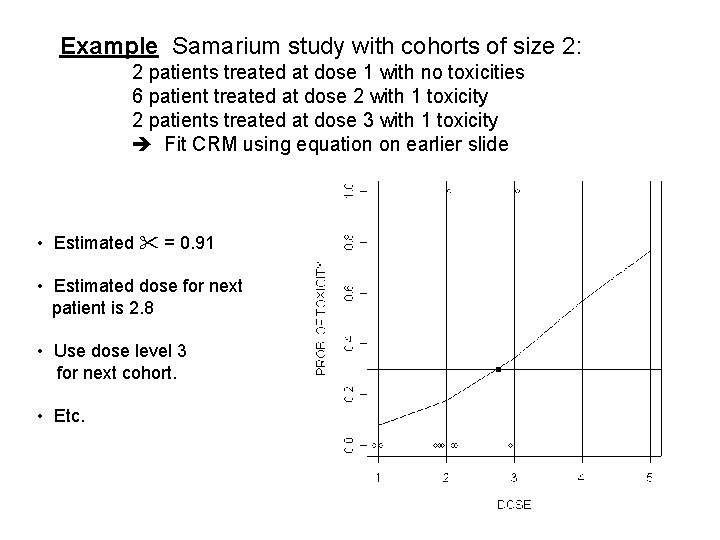
Example Samarium study with cohorts of size 2: 2 patients treated at dose 1 with no toxicities 6 patient treated at dose 2 with 1 toxicity 2 patients treated at dose 3 with 1 toxicity Fit CRM using equation on earlier slide • Estimated = 0. 91 • Estimated dose for next patient is 2. 8 • Use dose level 3 for next cohort. • Etc.

Longitudinal Studies • Multiple observations per individual over time • Sample size calculations are HARD • But, analysis is also complex • Standard assumption of “basic” statistical methods and models is that observations are independent • With longitudinal data, we have “correlated” measures within individuals

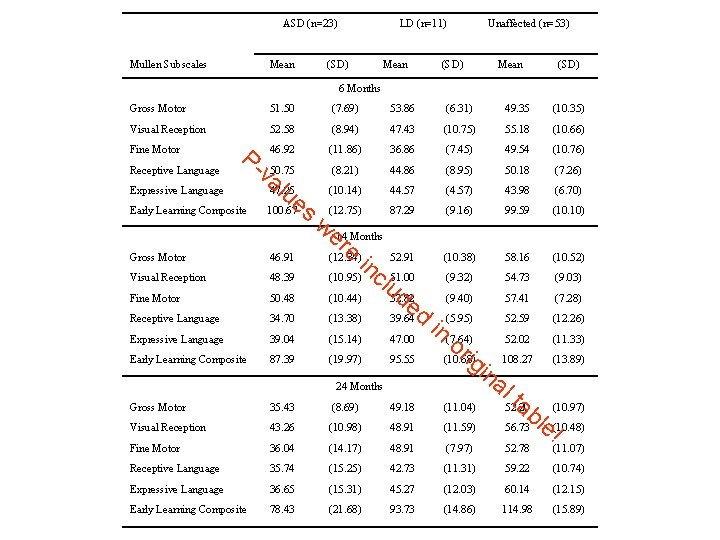
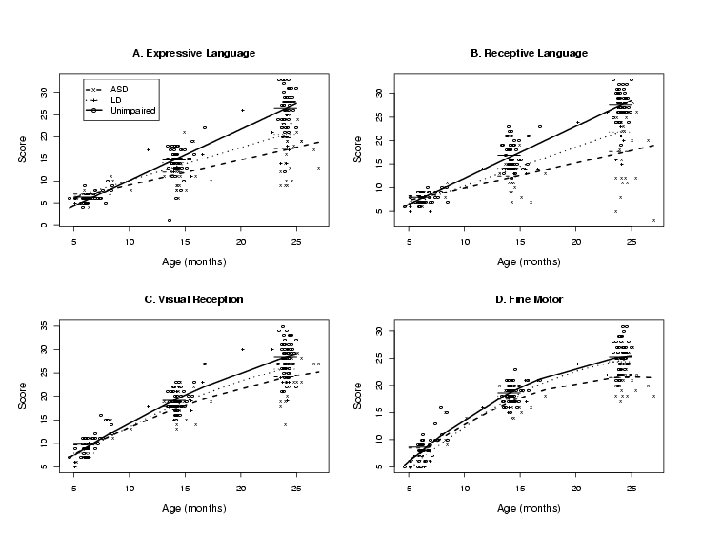
Study of Autism in Young Children (Landa) • Autism usually diagnosed at age 3. • But, there is evidence that there are earlier symptoms that are indicative of autism • Children at high risk of autism (kids with older autism siblings), and “controls” were observed at 6 months, 14 months, and 24 months for symptoms (Mullen) • Prospective study • Children were diagnosed at 36 months into three groups. – ASD (autism-spectrum disorder) – LD (learning disabled) – Unaffected • Earlier symptoms were compared to see if certain symptoms could predict diagnosis.

ASD (n=23) Mullen Subscales Mean LD (n=11) (SD) Mean (SD) Unaffected (n=53) Mean (SD) 6 Months Gross Motor 51. 50 (7. 69) 53. 86 (6. 31) 49. 35 (10. 35) Visual Reception 52. 58 (8. 94) 47. 43 (10. 75) 55. 18 (10. 66) 46. 92 (11. 86) 36. 86 (7. 45) 49. 54 (10. 76) (8. 21) 44. 86 (8. 95) 50. 18 (7. 26) (10. 14) 44. 57 (4. 57) 43. 98 (6. 70) (12. 75) 87. 29 (9. 16) 99. 59 (10. 10) (10. 38) 58. 16 (10. 52) (9. 32) 54. 73 (9. 03) (9. 40) 57. 41 (7. 28) Fine Motor Receptive Language P- Expressive Language Early Learning Composite va 50. 75 lu 47. 25 e 100. 67 s we 14 Months re (12. 34) in 52. 91 (10. 95) c 51. 00 lu de (10. 44) 52. 82 (13. 38) 39. 64 d Gross Motor 46. 91 Visual Reception 48. 39 Fine Motor 50. 48 Receptive Language 34. 70 Expressive Language 39. 04 (15. 14) 47. 00 Early Learning Composite 87. 39 (19. 97) 95. 55 24 Months Gross Motor 35. 43 (8. 69) 49. 18 Visual Reception 43. 26 (10. 98) 48. 91 Fine Motor 36. 04 (14. 17) 48. 91 Receptive Language 35. 74 (15. 25) Expressive Language 36. 65 Early Learning Composite 78. 43 52. 59 (12. 26) in (5. 95) (7. 64) 52. 02 (11. 33) or ig 108. 27 (13. 89) (10. 68) in al ta (10. 97) (11. 04) 52. 20 b (11. 59) 56. 73 le (10. 48) ! (7. 97) 52. 78 (11. 07) 42. 73 (11. 31) 59. 22 (10. 74) (15. 31) 45. 27 (12. 03) 60. 14 (12. 15) (21. 68) 93. 73 (14. 86) 114. 98 (15. 89)

Statistical modeling can help! • Previous table was hard to make conclusions from • Each time point was analyzed separately, and within time points, groups were compared. • Use some reasonable assumptions to help interpretation – Kids have “growth trajectories” that are continuous and smooth – Observations from within the same child are correlated.


Conclusions are much easier • The models that were used to make the graphs as somewhat complicated • But, they are “behind the scenes” • The important information is presented clearly and succintly • ANOVA approach does not “summarize” data

Concluding Remarks • Get your statistician involved as soon as you begin to plan your study • Things statisticians do not like: – Being contacted several days before grant/protocol/proposal is due – Rewriting inappropriate statistical sections – Analyzing data that has arisen from a poorly designed trial • Statisticians have a lot to add – “fresh” perspective on your study – Study will be more efficient!
 Role of statistician in clinical trials
Role of statistician in clinical trials Industrial statistician
Industrial statistician A statistician read that at least 77
A statistician read that at least 77 Independent statistician
Independent statistician How are they connected?
How are they connected? A statistician read that at least 77
A statistician read that at least 77 Azure worker role
Azure worker role Rollenmodell
Rollenmodell Statuses and their related roles determine
Statuses and their related roles determine Society of clinical research associates
Society of clinical research associates Dr charlotte lemech
Dr charlotte lemech Research design in clinical psychology
Research design in clinical psychology Pi clinical research consultancy
Pi clinical research consultancy Good documentation practices in clinical research
Good documentation practices in clinical research Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Kavi institute of clinical research
Kavi institute of clinical research Academic cro
Academic cro Translating research findings to clinical nursing practice
Translating research findings to clinical nursing practice Jasper clinical research
Jasper clinical research Asbmt clinical research training course
Asbmt clinical research training course Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Clinical research support services
Clinical research support services Clinical research definition
Clinical research definition Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Questra clinical research
Questra clinical research Mrc senior clinical fellowship
Mrc senior clinical fellowship Foundations of clinical research applications to practice
Foundations of clinical research applications to practice Ivr iwr studies
Ivr iwr studies Diagnostic role of marketing research
Diagnostic role of marketing research Role of research in marketing
Role of research in marketing Role of community health
Role of community health Quantitative vs qualitative
Quantitative vs qualitative Role of marketing research
Role of marketing research Role of literature review in research
Role of literature review in research Role of business research
Role of business research Role of media research
Role of media research Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Tư thế worm breton là gì
Tư thế worm breton là gì Alleluia hat len nguoi oi
Alleluia hat len nguoi oi Môn thể thao bắt đầu bằng chữ đua
Môn thể thao bắt đầu bằng chữ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới