Clinical Research Contract Research and Academic Research Organizations

















- Slides: 20

Clinical Research: Contract Research and Academic Research Organizations Laura Mauri, MD, MSc Brigham and Women’s Hospital Harvard Medical School Harvard Clinical Research Institute

Laura Mauri, MD § Consulting Fees: § Cordis Corporation § Abbott § Medtronic §Research support to Harvard Clinical Research Institute from: §Abbott, Boston Scientific Corporation, Cordis Corporation, Medtronic, Inc. , Bristol-Myers Squibb/Sanofi-Aventis Pharmaceuticals Partnership, Eli Lilly and Company and Daiichi Sankyo Company Limited

CROs and AROs • Contract research organization (CRO) and what services they provide • What is the difference between CRO and ARO (Academic research organization) • What are the relative strengths or limitations of CROs and AROs

CROs and AROs • Both CROs and AROs provide some component of research execution as a service – Not all clinical trial CROs or AROs provide all the services required for a clinical trial

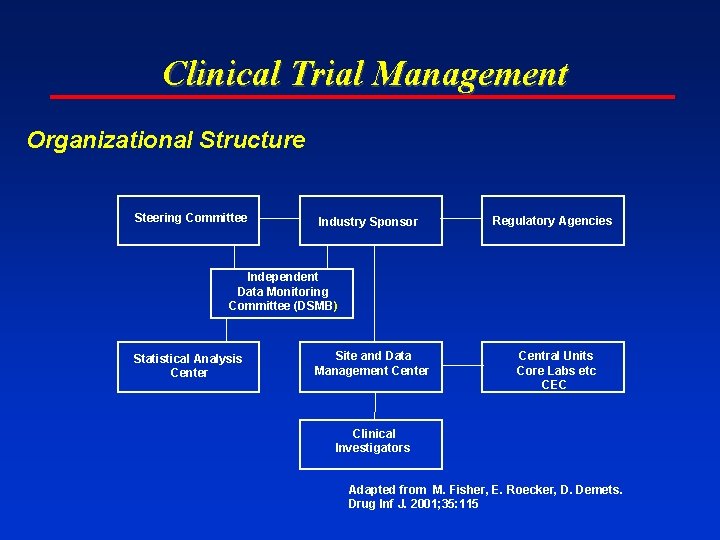
Clinical Trial Management Organizational Structure Steering Committee Industry Sponsor Regulatory Agencies Independent Data Monitoring Committee (DSMB) Statistical Analysis Center Site and Data Management Center Central Units Core Labs etc CEC Clinical Investigators Adapted from M. Fisher, E. Roecker, D. Demets. Drug Inf J. 2001; 35: 115

CROs and AROs • Both CROs and AROs provide some component of research execution as a service Trial Design Project Management Site Management Data Management Safety, Regulatory oversight Management of Clinical Events Committee, DSMB Statistical Analysis and Reporting Publications

CROs and AROs Tasks required of data management – Build validated database, compliant with standards • Secure and verifiable data entry – Procedures for treatment assignment/randomization – Timely data collection from external sources, quality checking, monitoring – Data cleaning, export for event adjudication, safety reporting, and analysis

CROs and AROs • CROs and AROs differ in their stakeholders Academic research organizations stakeholders include: – Clinical investigators – University – Academic medical centers

CROs and AROs • CROs and AROs differ in their mission Beyond providing the service of clinical trial services, academic research organizations generally intend to: – provide clinical context and strategy – provide scientific objectivity – advance medical knowledge and innovation – disseminate results

CROs and AROs • Research functions generally unique to AROs include Trial strategy (clinical and statistical) Principal investigator leadership Clinical coordinating center role

Academic Research Organization Capabilities and Services Unique ARO “Value-Added” Services • Scientific leadership input into trial design and data management plan • Ongoing scientific and clinical management • Establish and manage Clinical Event Committees • Establish and manage independent Data and Safety Monitoring Boards

Academic Research Organization Capabilities and Services Unique ARO “Value-Added” Services • Clinical trial strategy consulting from clinical and statistical experts • Scientific publications (interpretation and early dissemination of results) • Ability to design investigation that spans the results of one individual study or sponsor – extend results beyond those of an individual product evaluation

Academic Research Organization Advantages • Integrated involvement of clinical research operations and scientific leadership • Independence of experts available for trial design, medical management and analysis of results

Academic Research Organization Disadvantages • Sponsor may relinquish some control – Autonomy of academic authors for interpretation and publication – Access of academic authors to dataset

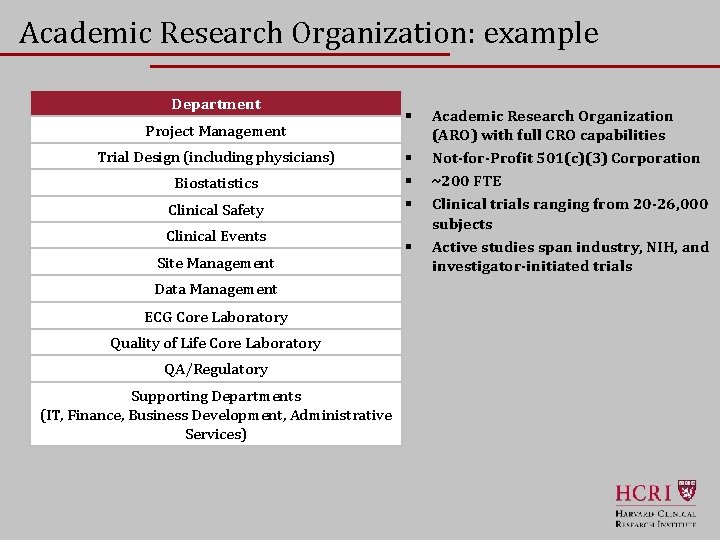
Academic Research Organization: example Department Project Management Trial Design (including physicians) Biostatistics Clinical Safety Clinical Events Site Management § § § Academic Research Organization (ARO) with full CRO capabilities Not-for-Profit 501(c)(3) Corporation ~200 FTE Clinical trials ranging from 20 -26, 000 subjects Active studies span industry, NIH, and investigator-initiated trials Data Management ECG Core Laboratory Quality of Life Core Laboratory QA/Regulatory Supporting Departments (IT, Finance, Business Development, Administrative Services) 16

March 2008 Statement of FDA Principles following DCRIFDA Critical Path Think Tank Meeting on need for DAPT Trial: • A need for a large, pragmatic public health trial exploring the benefit of extending thienopyridine treatment beyond one year in patients treated with DES needs to be done expeditiously • FDA expects that the results of the study will change clinical practice and provide valuable new information in product labeling for DES. Bram Zuckerman TCT 2008

A multistakeholder opportunity • The FDA request resulted in a unique public-private collaboration among 4 manufacturers of DES and current manufacturers of thienopyridine/antiplatelet medications • Adva. Med facilitated a proposal process from academic CROs along the parameters of a basic trial specifications from FDA and industry • 2008 Harvard Clinical Research Institute IDE approved by FDA • 2011 Enrollment +26, 000 subjects worldwide completed • 2012 (Feb) ~9000 randomized • Results expected 2014 • HCRI and the principal investigators are responsible for the independent analysis of trial data, and dissemination of results 18

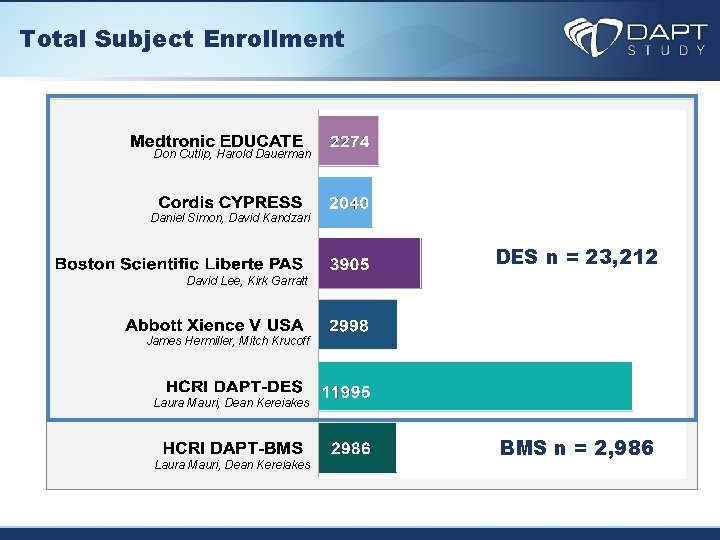
Total Subject Enrollment Don Cutlip, Harold Dauerman Daniel Simon, David Kandzari DES n = 23, 212 David Lee, Kirk Garratt James Hermiller, Mitch Krucoff Laura Mauri, Dean Kereiakes BMS n = 2, 986

A multistakeholder opportunity • Largest randomized trial regarding coronary stents • First randomized trial in cardiology supported by multiple different companies • The DAPT Study has brought together diverse stakeholders • FDA • Academia • Industry (4 stent and 4 drug companies) • Without the context of ARO coordination and leadership, such a multistakeholder effort would not be possible

CROs and AROs • CROs and AROs each may provide sponsors or investigators clinical trial services – While independent statistical analysis may be performed by either type of organization – AROs differ in the central mission of coordinating clinical leadership throughout trial execution, providing scientific objectivity, interpretation, and dissemination of results
 Clinical research organization structure
Clinical research organization structure Contract assets and contract liabilities
Contract assets and contract liabilities Contingent contract and wagering agreement
Contingent contract and wagering agreement Demanding ethical and socially responsible behavior
Demanding ethical and socially responsible behavior International union of forest research organizations
International union of forest research organizations Academic writing and research skills
Academic writing and research skills Power and politics in organizations
Power and politics in organizations Voluntary health and welfare organization examples
Voluntary health and welfare organization examples Floral shops bookstores and farms are examples of
Floral shops bookstores and farms are examples of Formal groups fulfill both and functions in organizations.
Formal groups fulfill both and functions in organizations. Persuasive communication
Persuasive communication Power politics and conflict in organizations
Power politics and conflict in organizations Perceiving ourselves and others in organizations
Perceiving ourselves and others in organizations Introduction to management and organization
Introduction to management and organization Csusm clubs and organizations
Csusm clubs and organizations Power, politics and conflict in organizations
Power, politics and conflict in organizations Physical property of ammonia
Physical property of ammonia Inventing and reinventing organizations
Inventing and reinventing organizations Information systems, organizations, and strategy
Information systems, organizations, and strategy Information systems, organizations, and strategy
Information systems, organizations, and strategy Information systems, organizations, and strategy
Information systems, organizations, and strategy







































