The Transition Metals the Lanthanides and the Antinides































- Slides: 55

The Transition Metals, the Lanthanides and the Antinides

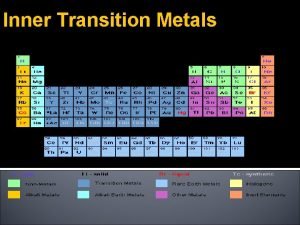
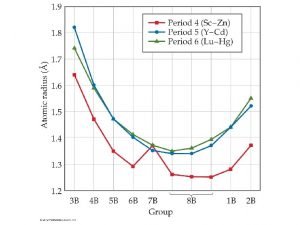
Transition Elements 3 The Metals in the Middle • Groups 3 -12 are called the transition elements. • All of them are metals. • Across any period from Group 3 through 12, the properties of the elements change less noticeably than they do across a period of representative elements. • Most transition elements are found combined with other elements in ores.

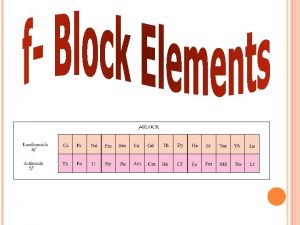
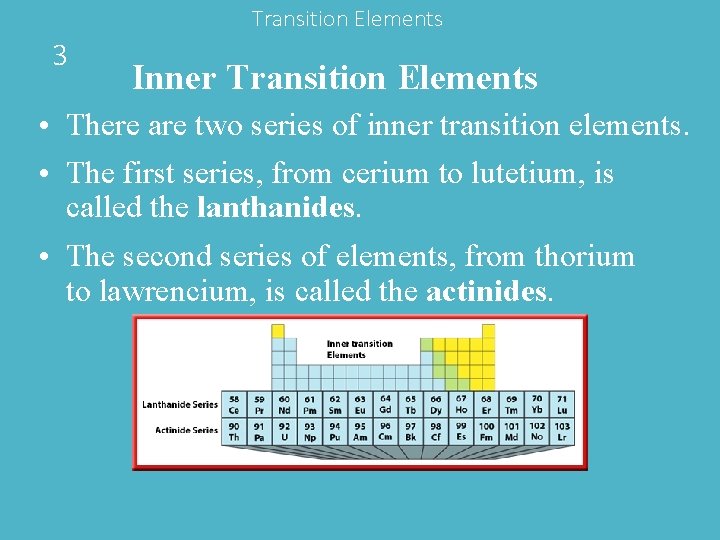
Transition Elements 3 Inner Transition Elements • There are two series of inner transition elements. • The first series, from cerium to lutetium, is called the lanthanides. • The second series of elements, from thorium to lawrencium, is called the actinides.

Transition Elements 3 The Lanthanides • They are soft, malleable, shiny metals with high conductivity. • They are mixed with more common metals to produce alloys, which are a mixture of metal with one other element, usually another metal. • Despite the name rare earth, the lanthanides are not as rare as originally thought. • Cerium makes up 50 percent of an alloy called misch (MIHSH) metal. • Flints in lighters are made from misch metal.

Transition Elements 3 The Actinides • All the actinides are radioactive. • The nuclei of atoms of radioactive elements are unstable and decay to form other elements. • Thorium, protactinium, and uranium are the only actinides that now are found naturally on Earth. • Uranium is found in Earth’s crust because its half-life is long— 4. 5 billion years.

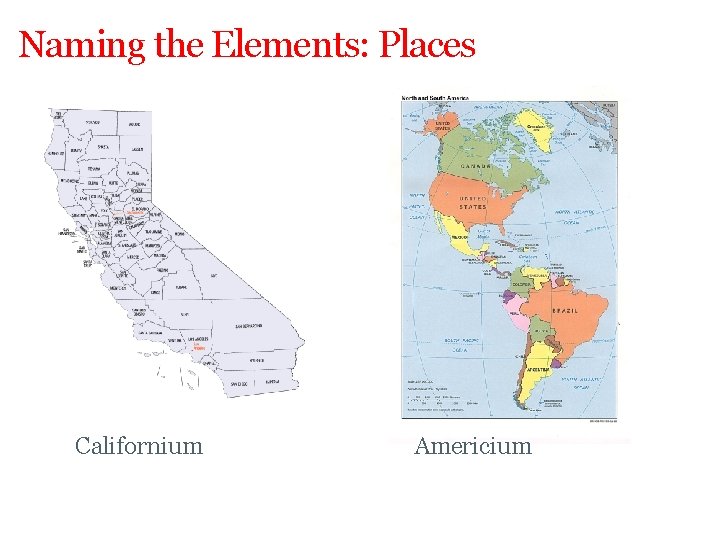
Transition Elements 3 The Actinides • All other actinides are synthetic elements. • Synthetic elements are made in laboratories and nuclear reactors. • Plutonium is used as a fuel in nuclear power plants. • Americium is used in some home smoke detectors. • Californium-252 is used to kill cancer cells.

Synthetic & Radioactive Elements Chemistry

Synthetic Definition? Not of natural origin; prepared or made artificially

Synthetic Elements - Metals • Elements with atomic numbers higher than 92 are sometimes described as synthetic elements because they are not found naturally on Earth. • Instead, elements that follow uranium are made – or synthesized – when nuclear particles are forced to crash into one another. • To make even heavier elements (with atomic numbers above 95), scientists use powerful machines called particle accelerators which move atomic nuclei faster and faster until they reach very high speeds and then crash into each other.

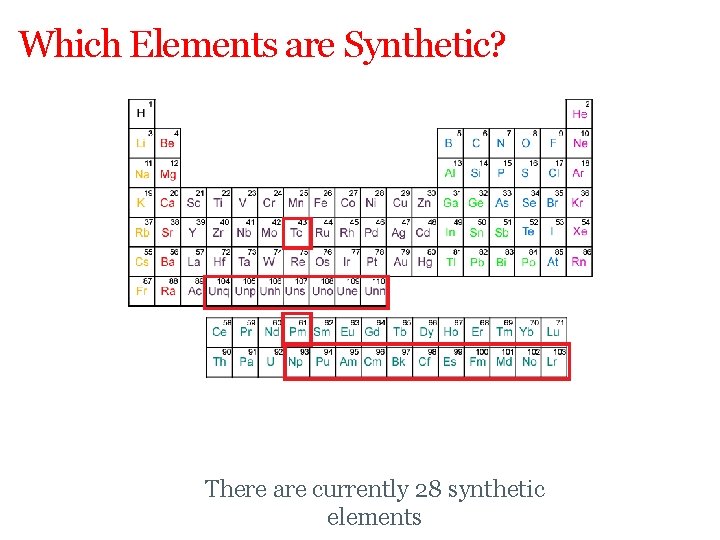
Which Elements are Synthetic? There are currently 28 synthetic elements

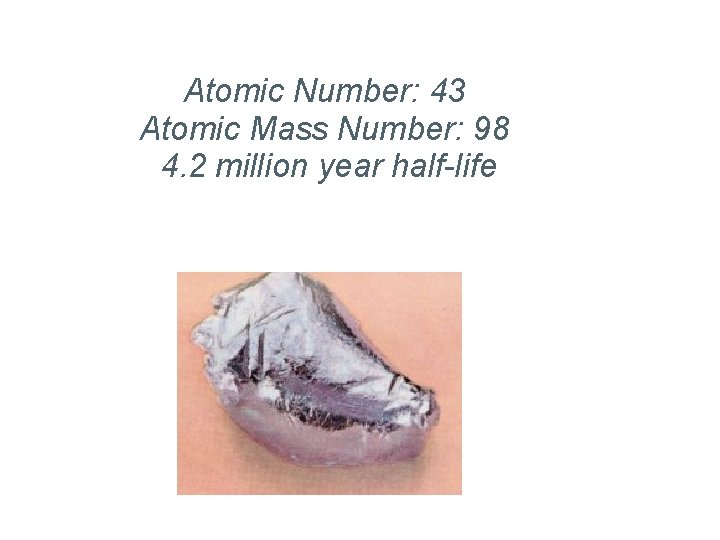
Technetium Atomic Number: 43 Atomic Mass Number: 98 4. 2 million year half-life

Some other Synthetic Elements are. . . Promethium (61) Curium (96) Hassium (108)

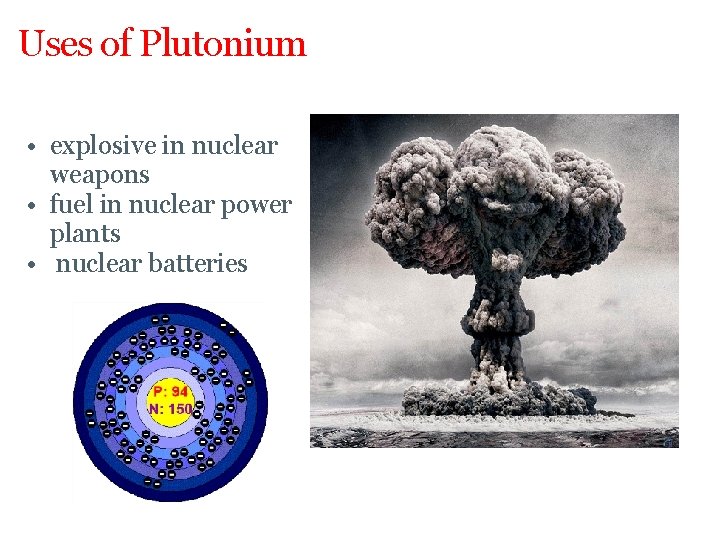
Uses of Plutonium • explosive in nuclear weapons • fuel in nuclear power plants • nuclear batteries

Why Make Synthetic Elements? • Many have no practical use • Made to study properties

Naming the Elements: Places Californium Americium

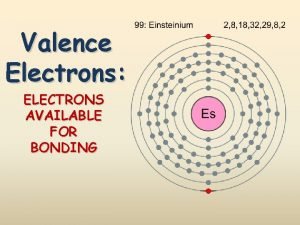
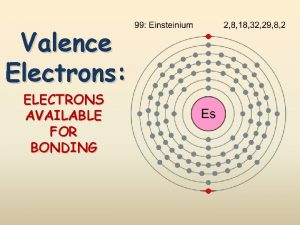
Naming the Elements: Famous Scientists Einsteinium Curium Seaborgium Element names are often controversial: • • International Union of Pure and Applied Chemistry tried to tell team that no elements can be named after living scientists Also can be controversy over who found the element because whoever found it gets to name it

Synthetic Elements are made in a Proton Accelerator




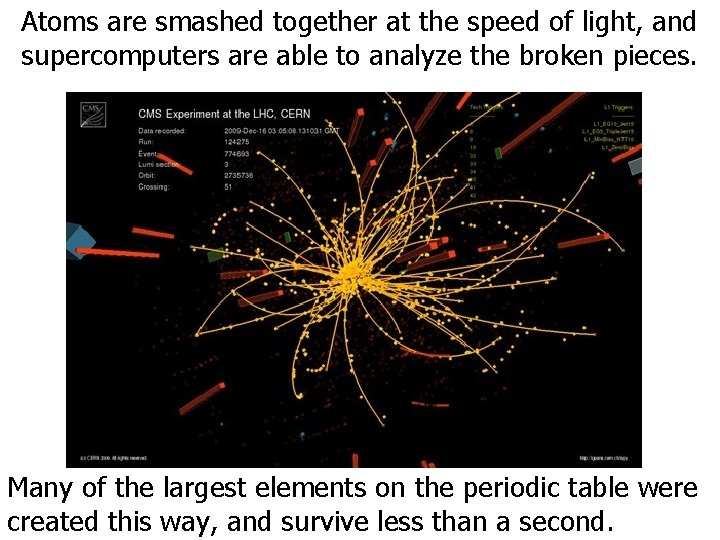
Atoms are smashed together at the speed of light, and supercomputers are able to analyze the broken pieces. Many of the largest elements on the periodic table were created this way, and survive less than a second.

Radioactive Elements Unstable elements whose nucleus breaks down and gives off particles, radiation, and energy

Radiation is invisible energy or particles released by radioactive elements that can damage biological tissue.

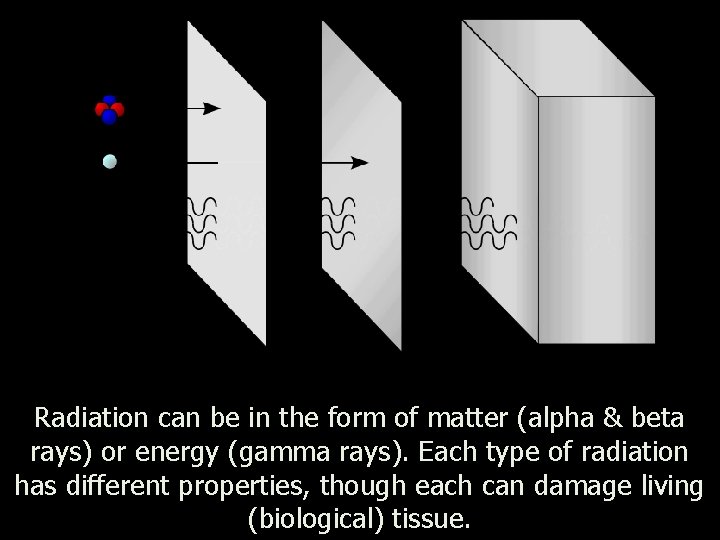
Radiation can be in the form of matter (alpha & beta rays) or energy (gamma rays). Each type of radiation has different properties, though each can damage living (biological) tissue.

What happens when living things are exposed to large amounts of radiation?

Types of Radiation Exposure Acute Short-term exposure – effect on biological tissue depends on the intensity of the radiation Chronic Long-term exposure of a small amount of radiation

Acute Effects Occur after short-term exposure Ex. Bombs, medical doses, industrial accidents, etc Effects include: Cell death Lethal cell DNA mutations Nonlethal cell DNA mutations (cancer) Mutations to reproductive cells resulting in birth defects


Chronic Exposure Long-term exposure, often the result of living or working in an area with low-levels of radiation Ex. Laying out in the sun everyday. Effects include: Lethal and non-Lethal DNA mutation (cancer) Mutations to reproductive cells resulting in birth defects


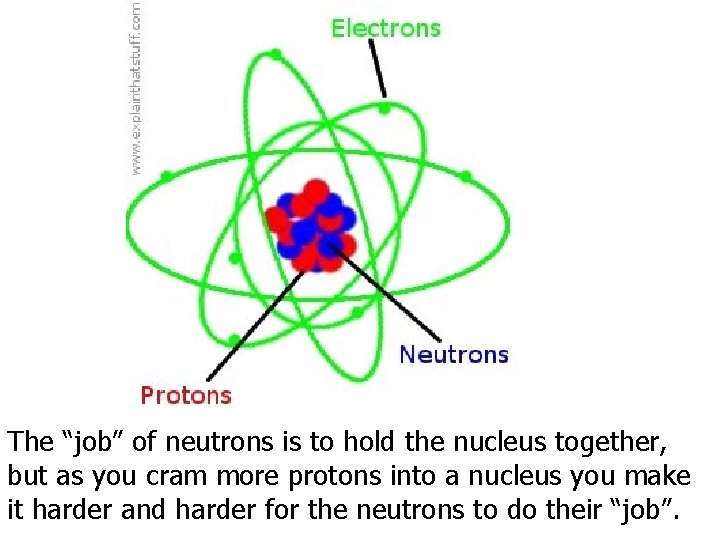
Why are some elements radioactive?

The “job” of neutrons is to hold the nucleus together, but as you cram more protons into a nucleus you make it harder and harder for the neutrons to do their “job”.

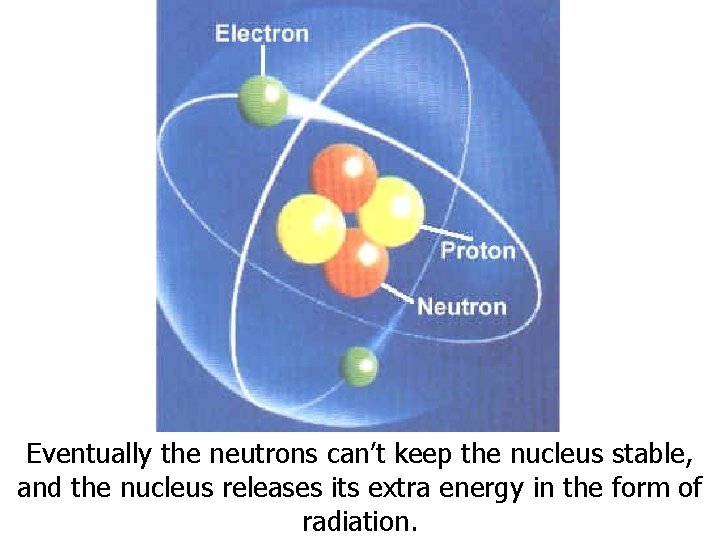
Eventually the neutrons can’t keep the nucleus stable, and the nucleus releases its extra energy in the form of radiation.

So is radiation all bad? ? No, it has a lot of uses!

Seeing in metal stuff


Radiation is either electromagnetic waves or particles of various energy. Irradiation is the process by which something is exposed to radiation. Radiation occurs when an object emits electromagnetic energy. Irradiation occurs when that energy is absorbed by another object.

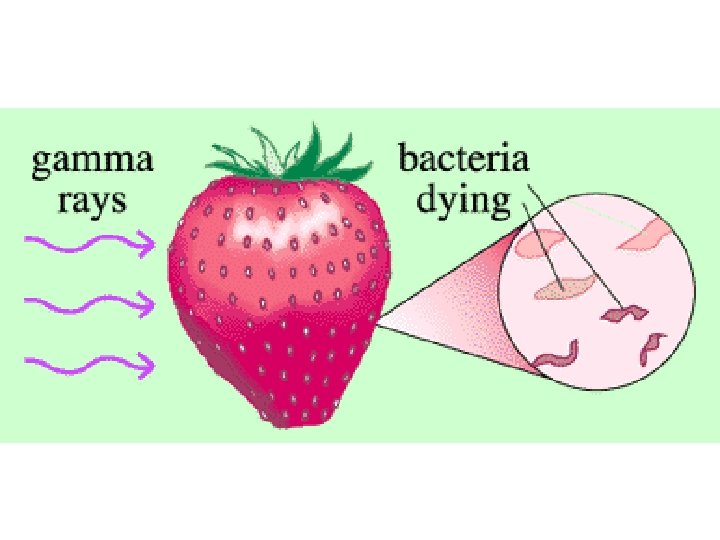
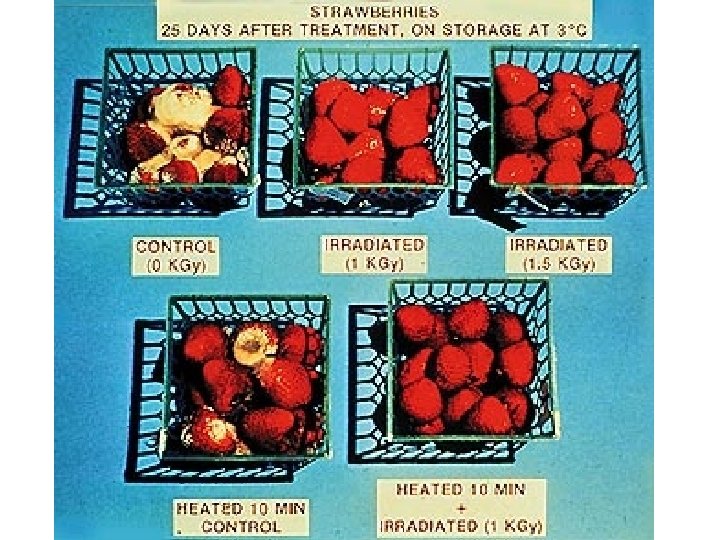
Irradiation This treatment is used to improve food safety by extending product shelf-life (preservation), reducing the risk of foodborne illness, delaying or eliminating sprouting or ripening, by sterilization of foods, and as a means of controlling insects and invasive pests





Nuclear Power

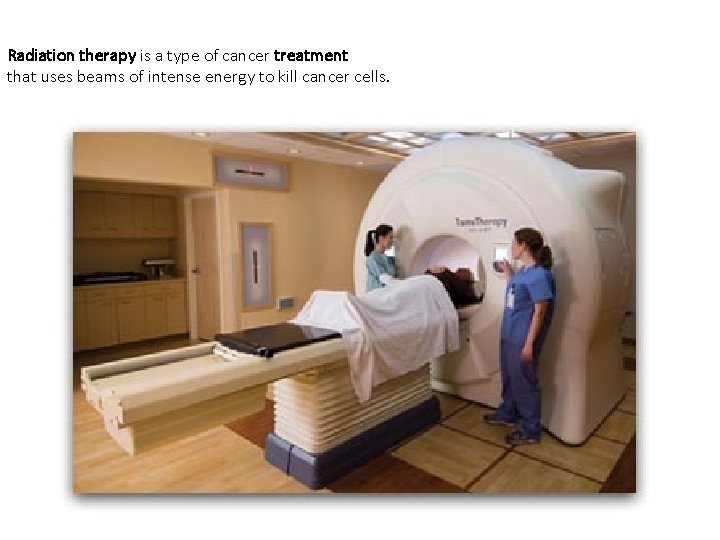
Radiation therapy is a type of cancer treatment that uses beams of intense energy to kill cancer cells.

Nuclear Bombs

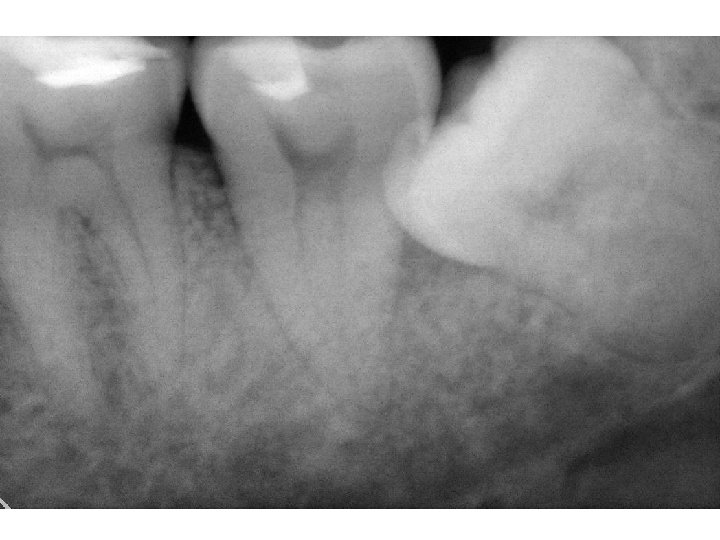
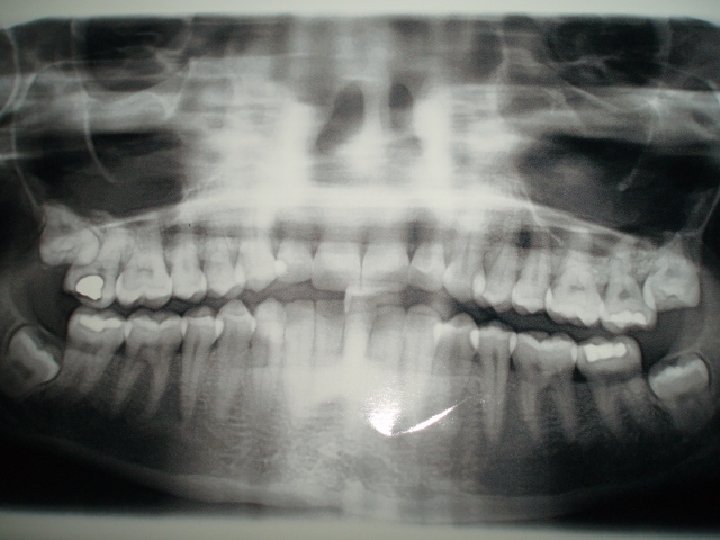
Examining internal structures





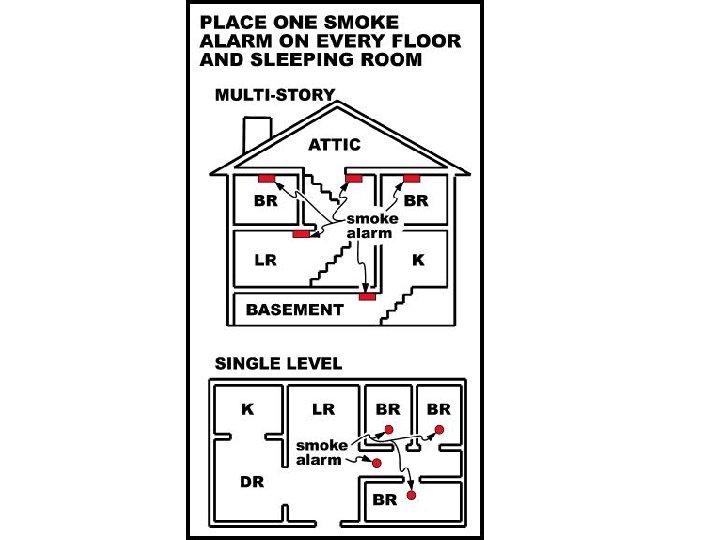
Smoke Detectors If smoke blocks the path of radiation between two points, it goes off



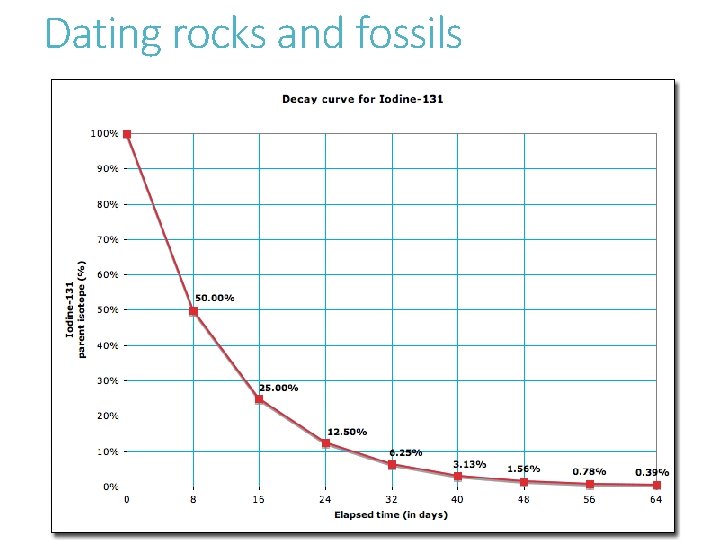
Dating rocks and fossils

The End!
 Antinides
Antinides Lanthanides have poor tendency to form complexes
Lanthanides have poor tendency to form complexes Magnetic properties of lanthanides
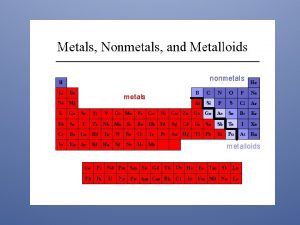
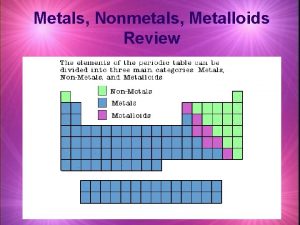
Magnetic properties of lanthanides Metals metalloids and nonmetals periodic table
Metals metalloids and nonmetals periodic table Characteristics of metals
Characteristics of metals Metals and non metals grade 5
Metals and non metals grade 5 Matter and materials (grade 7 worksheets)
Matter and materials (grade 7 worksheets) Non metals examples
Non metals examples Ferrous metals vs non ferrous metals
Ferrous metals vs non ferrous metals Valence electrons in ga
Valence electrons in ga Elements with 7 valence electrons
Elements with 7 valence electrons Transition metals valence electrons
Transition metals valence electrons Inner transition metals
Inner transition metals Vbt of cr(nh3)6 3+
Vbt of cr(nh3)6 3+ A linear complex ion with ligands on the x-axis
A linear complex ion with ligands on the x-axis Inner transition metals definition
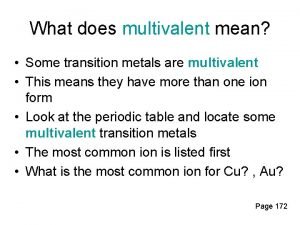
Inner transition metals definition Are all transition metals multivalent
Are all transition metals multivalent Ionization energy transition metals
Ionization energy transition metals Ionic compounds containing transition metals
Ionic compounds containing transition metals Knockhardy publishing
Knockhardy publishing Transition metals display great similarities
Transition metals display great similarities Periodic metals and nonmetals
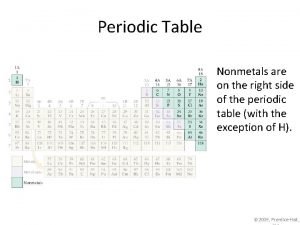
Periodic metals and nonmetals Periodic table separating metals and nonmetals
Periodic table separating metals and nonmetals What separates metals from nonmetals
What separates metals from nonmetals Where are the non metals on periodic table
Where are the non metals on periodic table The physical properties of metals include luster and
The physical properties of metals include luster and Metals nonmetals and metalloids answer key
Metals nonmetals and metalloids answer key Compare metals nonmetals and metalloids
Compare metals nonmetals and metalloids Metals nonmetals and metalloids difference
Metals nonmetals and metalloids difference Is boron shiny or dull
Is boron shiny or dull Difference between metal oxides and non metal oxides
Difference between metal oxides and non metal oxides Bronze vs brass
Bronze vs brass Metals nonmetals and semimetals
Metals nonmetals and semimetals Uses of non metals
Uses of non metals Elements and their properties chapter 17
Elements and their properties chapter 17 Optical properties of metals and nonmetals
Optical properties of metals and nonmetals Reactivity for alkali metals
Reactivity for alkali metals Section 4 metallic bonds and the properties of metals
Section 4 metallic bonds and the properties of metals Section 4 metallic bonds and the properties of metals
Section 4 metallic bonds and the properties of metals Multivalent compounds worksheet
Multivalent compounds worksheet Chapter 7 ionic compounds and metals
Chapter 7 ionic compounds and metals Chapter 7 ionic compounds and metals assessment answer key
Chapter 7 ionic compounds and metals assessment answer key Brittle nonmetals
Brittle nonmetals Periodic table divided in metals nonmetals and metalloids
Periodic table divided in metals nonmetals and metalloids Chapter 4 metals and nonmetals
Chapter 4 metals and nonmetals Poem about metals nonmetals and metalloids
Poem about metals nonmetals and metalloids What is a nonmetal element
What is a nonmetal element Extrusion and drawing difference
Extrusion and drawing difference Brass ionic or covalent
Brass ionic or covalent Ionic compounds properties
Ionic compounds properties Chapter 17 overview elements and their properties
Chapter 17 overview elements and their properties 7 ionic and metallic bonding practice problems
7 ionic and metallic bonding practice problems Anthanides
Anthanides Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể