The Transition Metals General Properties of Transition Metals

- Slides: 15

The Transition Metals

General Properties of Transition Metals Do Now: What do you know already about the transition metals?

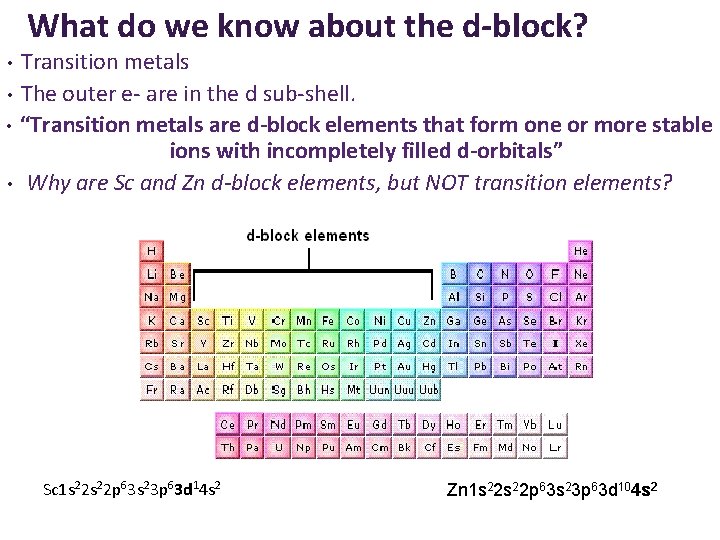
What do we know about the d-block? Transition metals • The outer e- are in the d sub-shell. • “Transition metals are d-block elements that form one or more stable ions with incompletely filled d-orbitals” • Why are Sc and Zn d-block elements, but NOT transition elements? • Sc 1 s 22 p 63 s 23 p 63 d 14 s 2 Zn 1 s 22 p 63 s 23 p 63 d 104 s 2

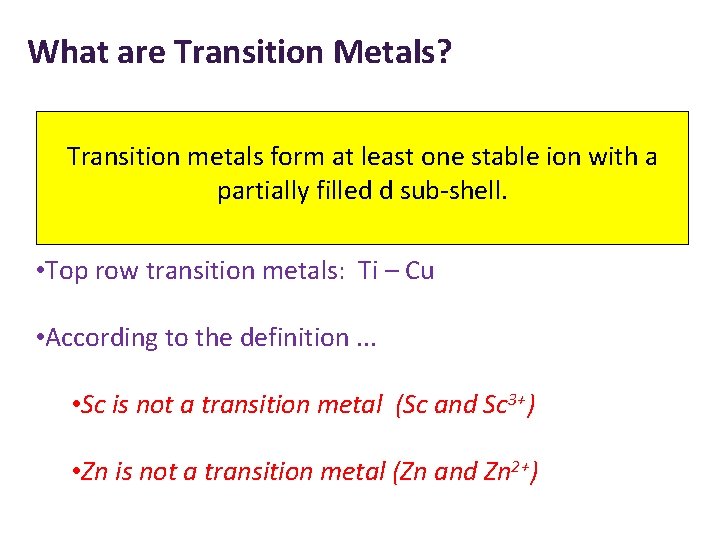
What are Transition Metals? Transition metals form at least one stable ion with a partially filled d sub-shell. • Top row transition metals: Ti – Cu • According to the definition. . . • Sc is not a transition metal (Sc and Sc 3+) • Zn is not a transition metal (Zn and Zn 2+)

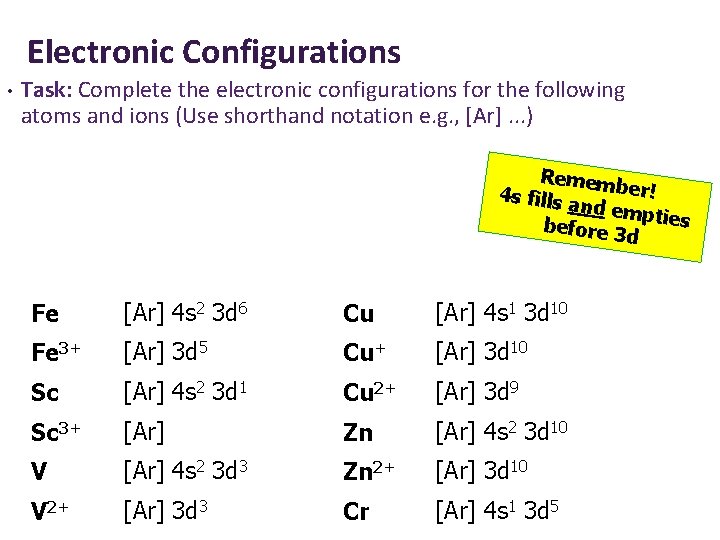
Electronic Configurations • Task: Complete the electronic configurations for the following atoms and ions (Use shorthand notation e. g. , [Ar]. . . ) Remem ber! 4 s fills a nd emp before 3 ties d Fe [Ar] 4 s 2 3 d 6 Cu [Ar] 4 s 1 3 d 10 Fe 3+ [Ar] 3 d 5 Cu+ [Ar] 3 d 10 Sc [Ar] 4 s 2 3 d 1 Cu 2+ [Ar] 3 d 9 Sc 3+ [Ar] Zn [Ar] 4 s 2 3 d 10 V [Ar] 4 s 2 3 d 3 Zn 2+ [Ar] 3 d 10 V 2+ [Ar] 3 d 3 Cr [Ar] 4 s 1 3 d 5

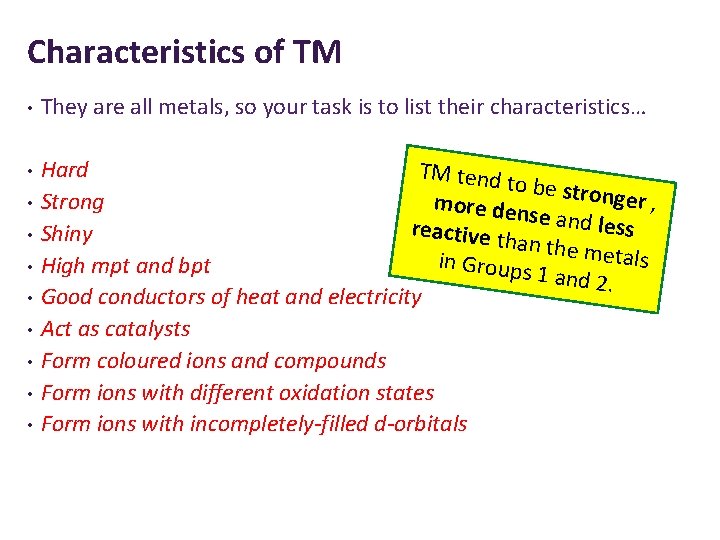
Characteristics of TM • They are all metals, so your task is to list their characteristics… • Hard TM tend to be stro nger , more den Strong se and le ss r eactive th Shiny an the m etals in Groups High mpt and bpt 1 and 2. Good conductors of heat and electricity Act as catalysts Form coloured ions and compounds Form ions with different oxidation states Form ions with incompletely-filled d-orbitals • •

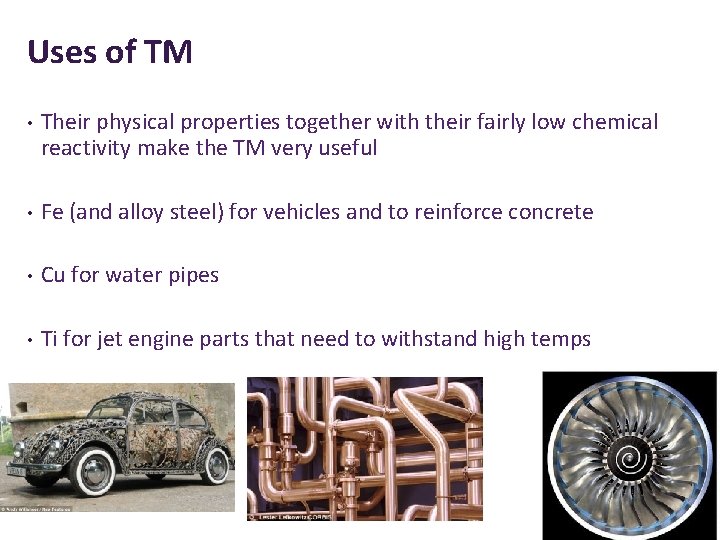
Uses of TM • Their physical properties together with their fairly low chemical reactivity make the TM very useful • Fe (and alloy steel) for vehicles and to reinforce concrete • Cu for water pipes • Ti for jet engine parts that need to withstand high temps

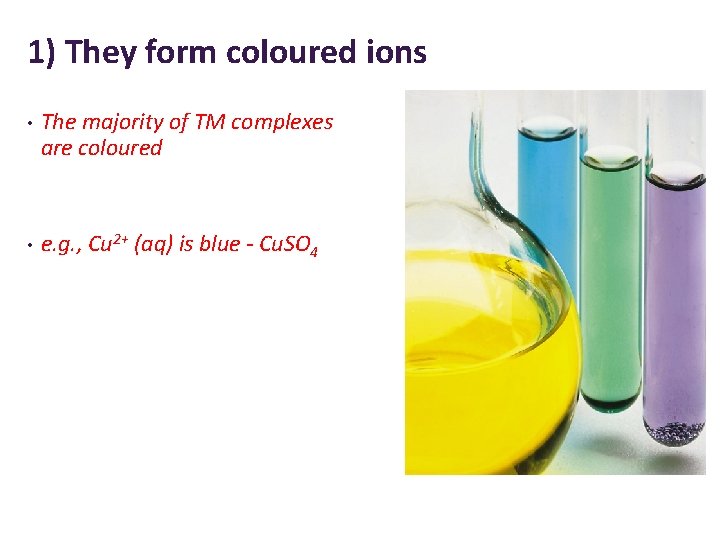
1) They form coloured ions • The majority of TM complexes are coloured • e. g. , Cu 2+ (aq) is blue - Cu. SO 4

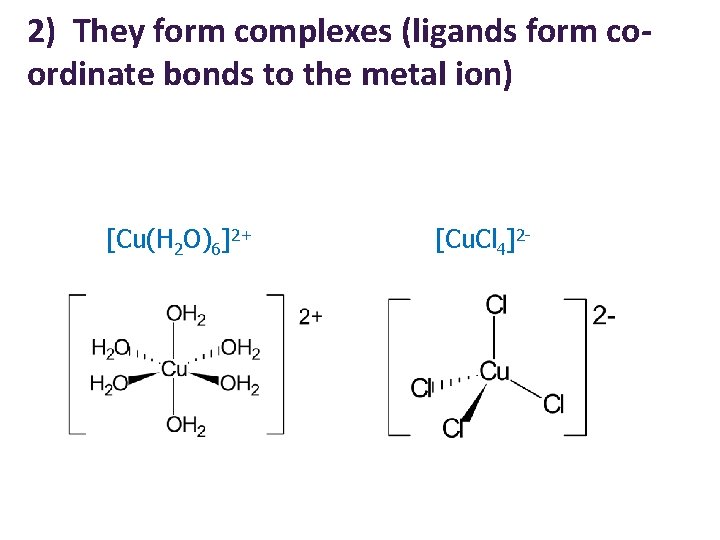
2) They form complexes (ligands form coordinate bonds to the metal ion) [Cu(H 2 O)6]2+ [Cu. Cl 4]2 -

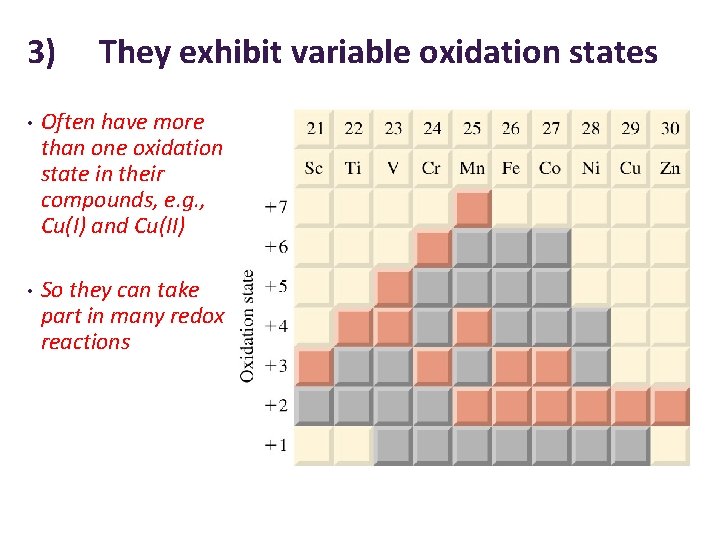
3) They exhibit variable oxidation states • Often have more than one oxidation state in their compounds, e. g. , Cu(I) and Cu(II) • So they can take part in many redox reactions

4) They show catalytic activity Ni Margarine production V 2 O 5 Contact Process making SO 3 for H 2 SO 4 Fe Haber process to make NH 3 Pt, Pd Catalytic converters Mn. O 2 Decomposition of H 2 O 2

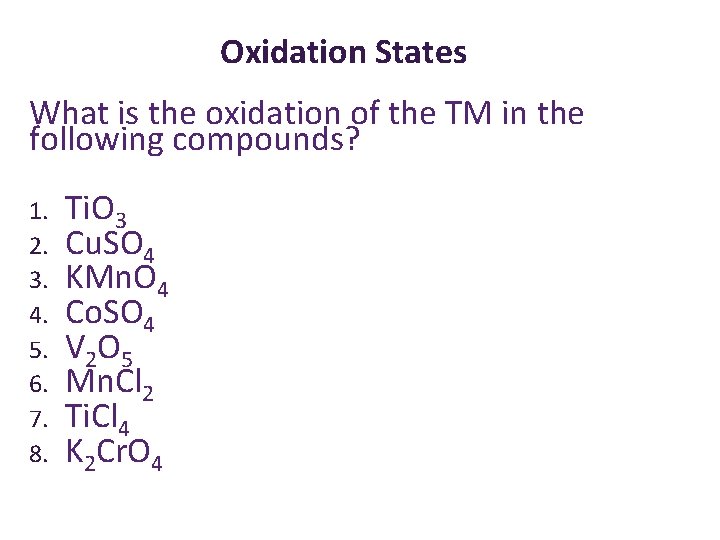
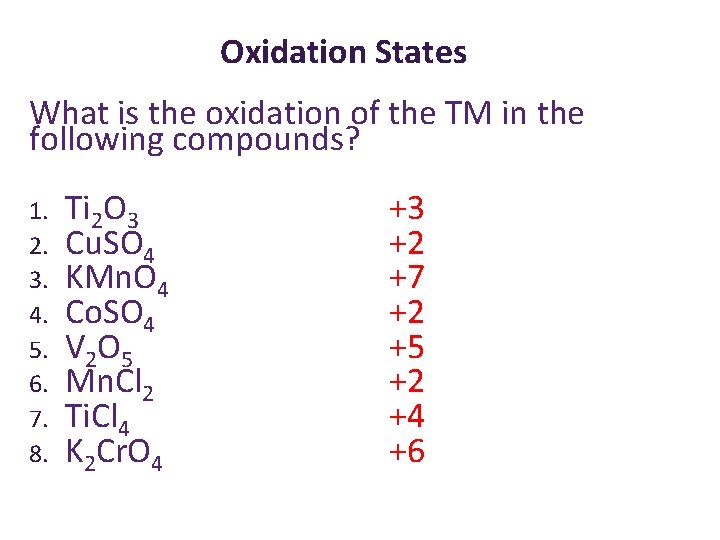
Oxidation States What is the oxidation of the TM in the following compounds? 1. 2. 3. 4. 5. 6. 7. 8. Ti. O 3 Cu. SO 4 KMn. O 4 Co. SO 4 V 2 O 5 Mn. Cl 2 Ti. Cl 4 K 2 Cr. O 4

Oxidation States What is the oxidation of the TM in the following compounds? 1. 2. 3. 4. 5. 6. 7. 8. Ti 2 O 3 Cu. SO 4 KMn. O 4 Co. SO 4 V 2 O 5 Mn. Cl 2 Ti. Cl 4 K 2 Cr. O 4 +3 +2 +7 +2 +5 +2 +4 +6

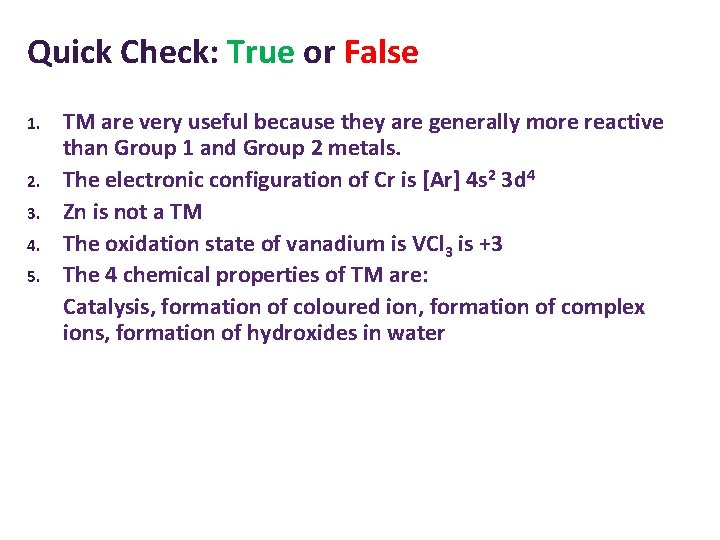
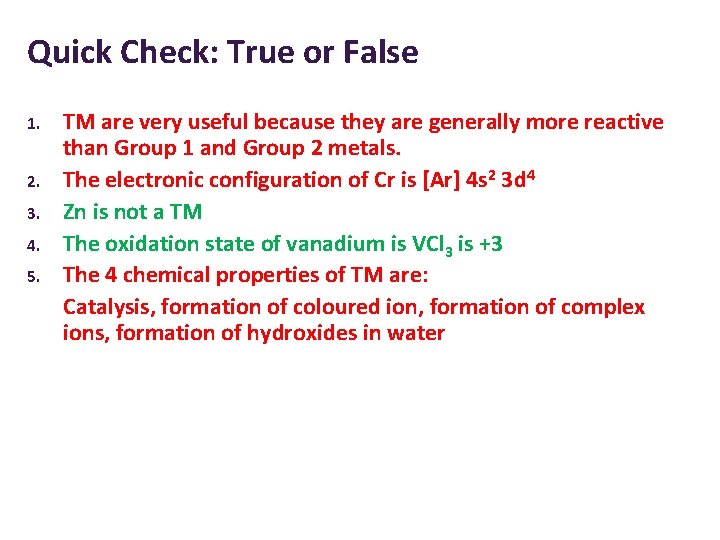
Quick Check: True or False 1. 2. 3. 4. 5. TM are very useful because they are generally more reactive than Group 1 and Group 2 metals. The electronic configuration of Cr is [Ar] 4 s 2 3 d 4 Zn is not a TM The oxidation state of vanadium is VCl 3 is +3 The 4 chemical properties of TM are: Catalysis, formation of coloured ion, formation of complex ions, formation of hydroxides in water

Quick Check: True or False 1. 2. 3. 4. 5. TM are very useful because they are generally more reactive than Group 1 and Group 2 metals. The electronic configuration of Cr is [Ar] 4 s 2 3 d 4 Zn is not a TM The oxidation state of vanadium is VCl 3 is +3 The 4 chemical properties of TM are: Catalysis, formation of coloured ion, formation of complex ions, formation of hydroxides in water
 Nonmetals on the periodic table
Nonmetals on the periodic table Ferrous metals vs non ferrous metals
Ferrous metals vs non ferrous metals Compare metals nonmetals and metalloids
Compare metals nonmetals and metalloids Metals and non metals grade 5
Metals and non metals grade 5 Matter and materials (grade 7 worksheets)
Matter and materials (grade 7 worksheets) Metalloids
Metalloids Group with 6 valence electrons
Group with 6 valence electrons Elements with 7 valence electrons
Elements with 7 valence electrons Transition metals valence electrons
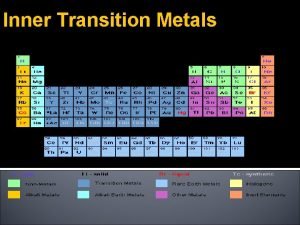
Transition metals valence electrons Inner transition metals
Inner transition metals Spectrochemical series
Spectrochemical series Biological importance of transition metals
Biological importance of transition metals Inner transition metals definition
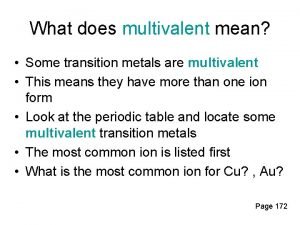
Inner transition metals definition Multivalent metal example
Multivalent metal example Ionization energy for transition metals
Ionization energy for transition metals Ionic compounds containing transition metals
Ionic compounds containing transition metals