Metals Ferrous Metals Nonferrous Metals Alloys Useful Terms








- Slides: 14

Metals • Ferrous Metals • Non-ferrous Metals • Alloys

Useful Terms for metals • Ferrous Metals • Any metal that contains iron is a ferrous metal. • Non ferrous metals • This is any metal that does not contain iron. • An alloy • This is a mixture of two or more metals. • The charge • These are the materials that are put into the furnace to produce the metal. • The operation (smelting) • This is how the furnace works how it makes the metal. • The products • These are the materials that come out of the furnace.

Ferrous Metals All metals that contain Iron (Fe) are ferrous metals

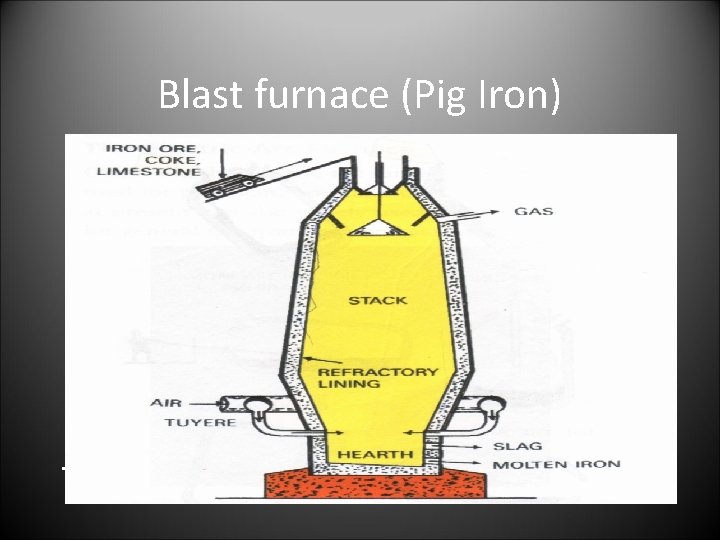
Blast furnace (Pig Iron) • The blast Furnace.

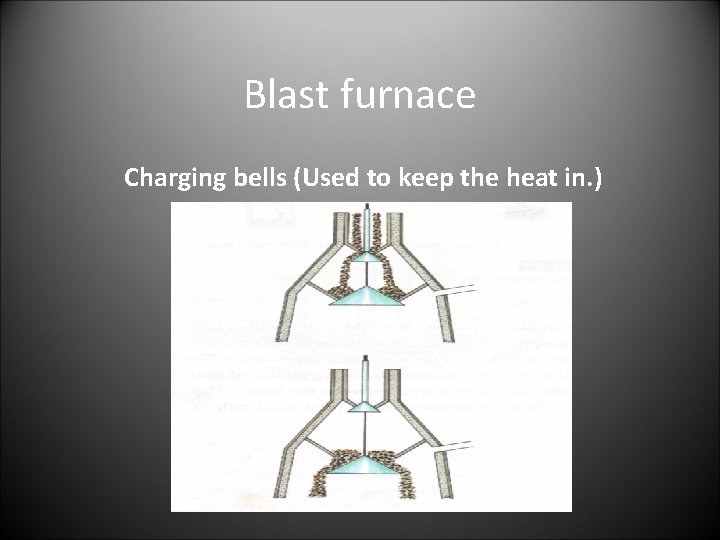
Blast furnace Charging bells (Used to keep the heat in. )

Blast Furnace • The Charge • iron ore, coke, limestone • Operations • the charge is loaded into the furnace using the charging bells. The coke burns when hot air is blown in through the tuyere “the blast” and the impurities mix with the limestone to give slag. The slag is taken off leaving molten iron to be tapped off. • The Products • molten iron, Slag , waste gasses

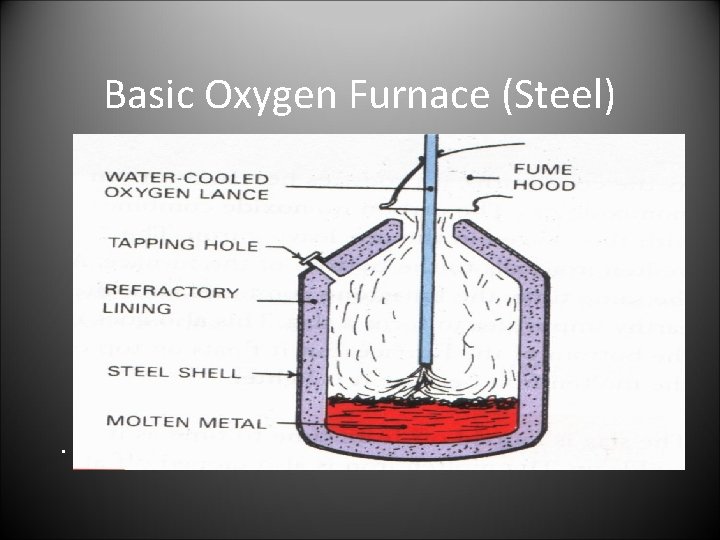
Basic Oxygen Furnace (Steel) • The basic Oxygen Furnace.

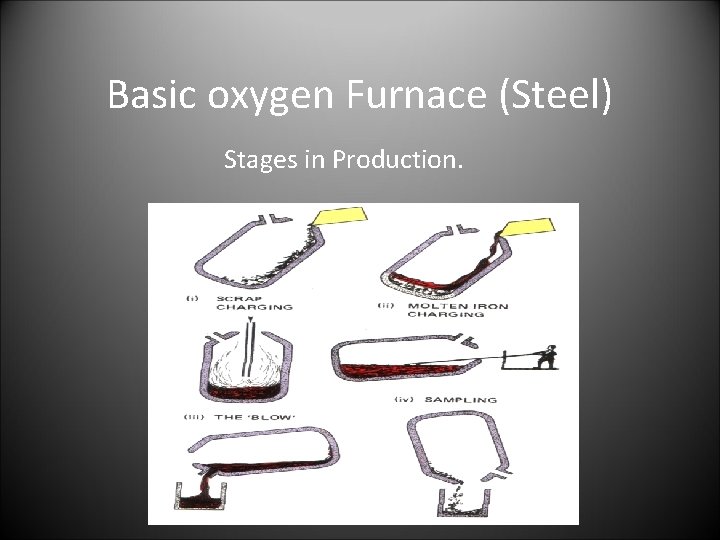
Basic oxygen Furnace (Steel) Stages in Production.

Basic oxygen Furnace. • The charge: • • Molten Iron and scrap steel. (This is where the Carbon for the steel originates. ) limestone • The operation: • • The furnace is charged firstly with scrap steel. Molten iron from the blast furnace is then charged. The oxygen lance is then lowered in and oxygen is blown in causing the impurities and extra carbon to mix with the limestone making slag. The lance is kept cool with water. The steel is sampled to check for the right amount of carbon. The furnace is tilted and the molten steel poured out through the tapping hole. The slag is then emptied out the top. • The products • • • molten steel slag waste gasses

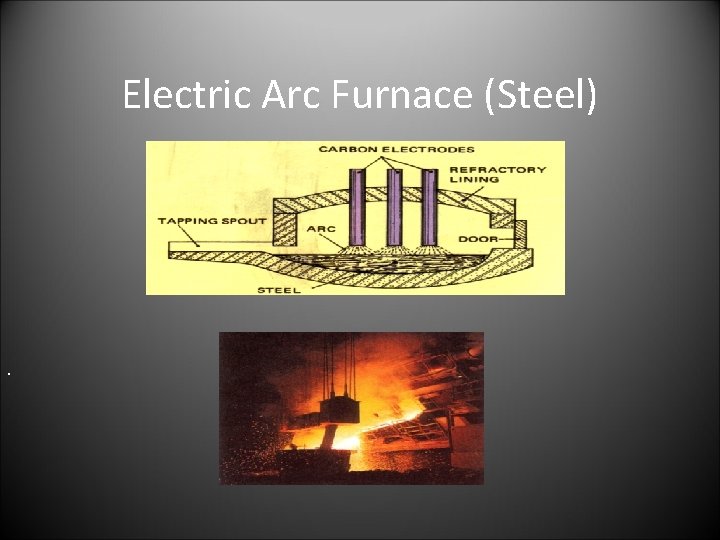
Electric Arc Furnace (Steel) .

The Electric Arc. • The charge: • Molten Iron and • scrap steel • limestone • The operation: • the carbon rods and roof are lifted off. The furnace is charged. The rods are lowered an arc is made between the charge and the rods producing heat. The steel is sampled. The furnace is on rollers and is tilted for slagging and then tapping. • The products • High Quality molten steel • slag

Non Ferrous Metals. • Aluminium (Al) This is silver in colour, it is very strong but light and is malleable and ductile. It is a good conductor of heat and electricity. An oxide forms on the outside preventing corrosion. It is used for aircraft bodies, drinks cans, high tension wires. • Copper (Cu) This is reddish brown in colour and is malleable and ductile. It is a good conductor of heat and electricity. It turns green as it corrodes. It is used in electrical wiring, heating pipes and for roofing.

Non Ferrous Metals. • Lead (Pb) This is a very heavy metal, it is a dull grey colour and is flexible at room temperature. It is poisonous if handled to often. It is used for making batteries and also for roofing. • Zinc (Zn) This is a grey colour. It does not corrode easily and so it is used for galvanizing (coating steel) to stop rusting. • Tin (Sn) This is a silvery white metal, it is weak and generally combined with other metals.

Alloys. An alloy: is a mixture of two or more metals. Steel is one example. • Brass (Cu. Zn) This is a combination of copper and zinc. It is a gold colour and does not rust easily. It is used to make hinges, screws, outside taps and musical instruments. • Bronze (Cu. Sn) This is a combination of copper and tin. It is a dark green colour and is easily cast making it ideal for statues. • Soft solder (Pb. Sn) This is a combination of lead and tin. It has a very low melting point and so it can be used to join electronic components.