Metals Chapter 3 Lesson 2 Metals Metals have











- Slides: 13

Metals Chapter 3 Lesson 2

Metals • Metals have luster: the ability to reflect light • Metals are ductile: the ability to be pulled into wires • Metals are malleable: the ability of a substance to be hammered or rolled into sheet

Metals • Metals generally have a great density, strength, boiling point, and melting point than other elements. • All metals are solids at room temperature except for mercury, which is a liquid.

Alkali Metals • Group 1 on the periodic table • One valence electron – Valence electrons are the electrons in the outermost shell of the atom • Soft, white, shiny metals • Lowest density of all metals.

Alkali Metals • Extremely reactive, not found uncombined in nature • Samples must be stored in oil or kerosene to keep them from combining explosively with water or oxygen in the air

Uses for Alkali Metals • Lithium is used in batteries • Na. Cl is table salt • Sodium and potassium are dietary requirements

Alkaline Earth Metals • Group 2 on the periodic table • Two valence electrons • Very reactive, never found in nature as uncombined elements • Soft and silvery

Uses for Alkaline Earth Metals • Beryllium is found in emeralds and aquamarine • Magnesium is found in chlorophyll

The Metals in the Middle • The transition metals are made up of groups 3 through 12. • Most transition metals are found combined with other elements, some are found alone.

Uses of Transition Metals • Make good building materials • Chromium is used for brightly colored paints. • Mercury was used in thermometers and barometers. • Tungsten has the highest melting point of any element.

Inner Transition Elements • Lanthanides: elements 58 to 71 – Lanthanide series elements are used to make strong magnets.

• Actinides: elements 90 to 103 – All of these elements are radioactive. – Thorium, protactinium and uranium are found on Earth. All others are synthetic. – Americium is used in smoke detectors. – Plutonium is used as fuel in nuclear power plants.

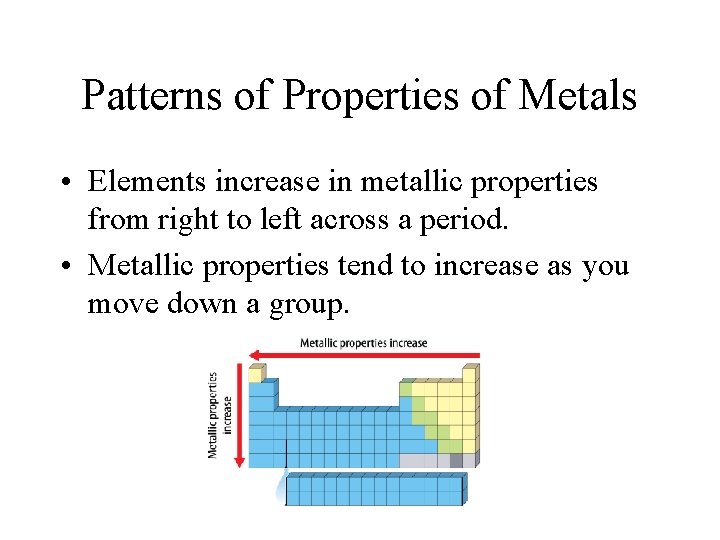
Patterns of Properties of Metals • Elements increase in metallic properties from right to left across a period. • Metallic properties tend to increase as you move down a group.