Metals Chapter 35 What are Metals Metals are











- Slides: 17

Metals Chapter 35

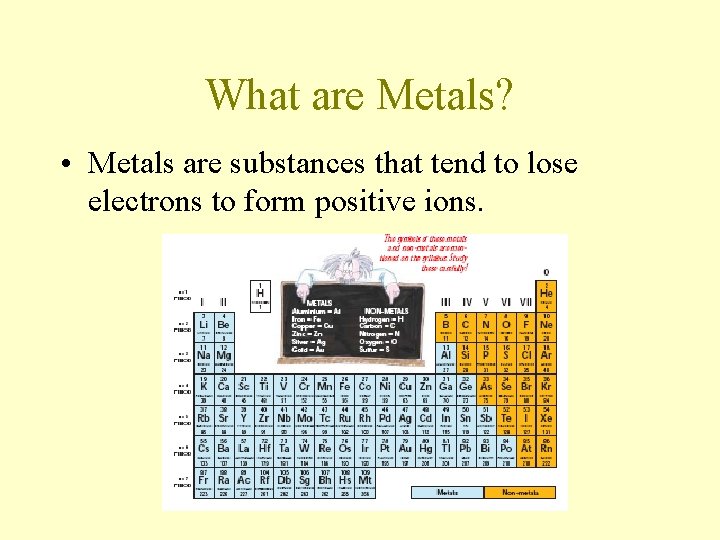
What are Metals? • Metals are substances that tend to lose electrons to form positive ions.

Properties of Metals • Metals are usually strong, hard and shiny. • They have high densities, high melting points and boiling points. • They are good conductors of heat and electricity. • They can be hammered into different shape(malleable) • They can be stretched out into thin wires(ductile).

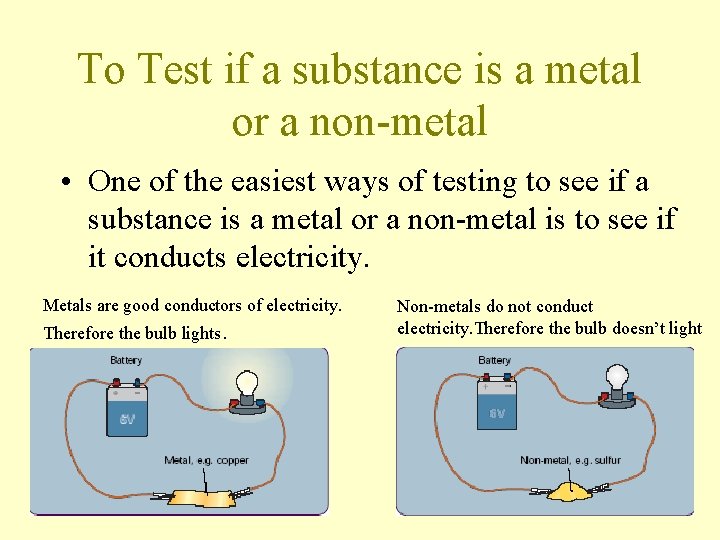
To Test if a substance is a metal or a non-metal • One of the easiest ways of testing to see if a substance is a metal or a non-metal is to see if it conducts electricity. Metals are good conductors of electricity. Therefore the bulb lights. Non-metals do not conduct electricity. Therefore the bulb doesn’t light

Corrosion of Metals • Corrosion is any undesired process in which a metal is converted to one of its compounds. • When corrosion occurs the metal reacts with water, oxygen or other chemicals to form an oxide or some other compound. • The corrosion of Iron or Steel is called rusting.

Rusting • The corrosion of Iron or steel is called rusting. • Rusting takes place in the presence of Water and Oxygen.

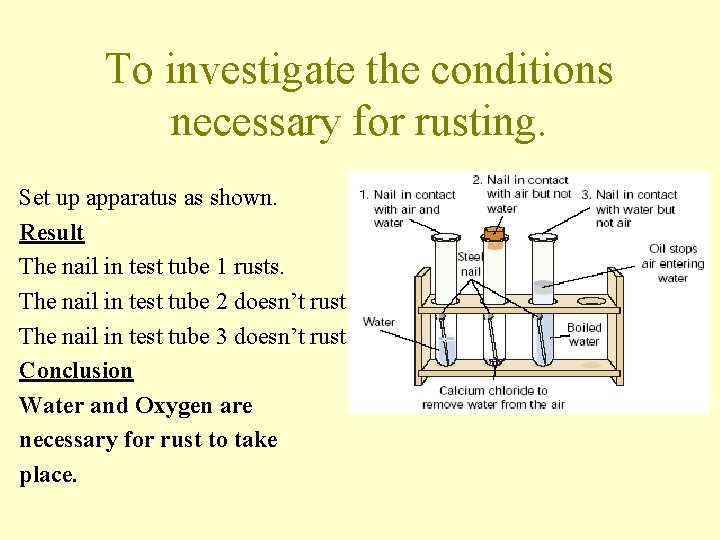
To investigate the conditions necessary for rusting. Set up apparatus as shown. Result The nail in test tube 1 rusts. The nail in test tube 2 doesn’t rust. The nail in test tube 3 doesn’t rust Conclusion Water and Oxygen are necessary for rust to take place.

Prevention of Corrosion(rusting) Most methods of rust prevention involve coating the metal with some material to prevent oxygen and water coming in contact with the metal. The most important methods include: Painting-bridges, ships, bicycles etc. Greasing and oiling-car engines- bicycle chains, moving parts Galvanising- coating the iron with a layer of zinc that does not rust. Steel buckets, corrugated iron, wire for fencing. Chromium Plating- taps, car bumpers etc.

Painting-bridges, ships, bicycles etc.

Greasing and oiling-car engines- bicycle chains, moving parts

Galvanising- coating the iron with a layer of zinc that does not rust. Steel buckets, corrugated iron, wire for fencing. .

Chromium Plating- taps, car bumpers etc.

Metal Alloys An alloy is a mixture of metals. The alloys are usually much harder and more resistant to corrosion than either of the metals they contain. Examples of alloys include – Steel-Iron and Carbon – Bronze-Copper and Tin – Solder-Lead and Tin – Brass-Copper and Zinc

The Activity Series Most reactive Potassium(K) • The reactivity series is a list of metals in order of how reactive they are. Sodium (Na) Calcium (Ca) Magnesium (Mg) Aluminium (Al) • The most reactive metals are at the top. • The metals are arranged on the basis of how quickly they react in three tests (with Oxygen, Water and dilute HCl) Zinc (Zn) Iron (Fe) Lead (Pb) Hydrogen (H) Copper (Cu) Mercury (Hg) Silver (Ag) Gold (Au) Least reactive

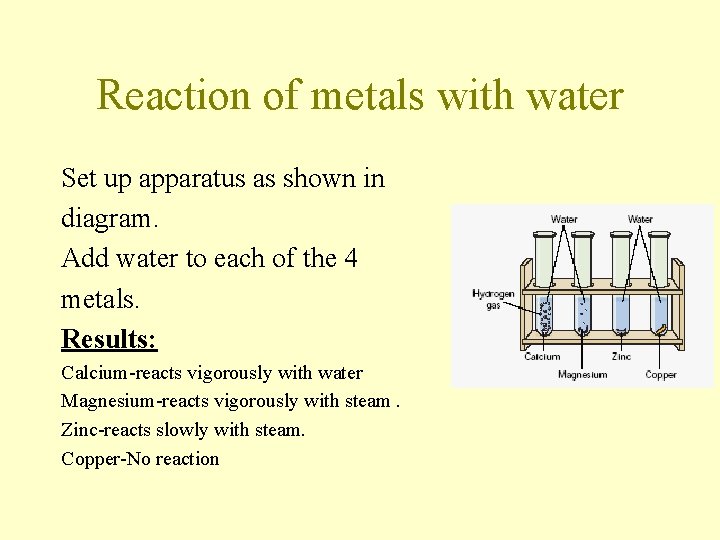
Reaction of metals with water Set up apparatus as shown in diagram. Add water to each of the 4 metals. Results: Calcium-reacts vigorously with water Magnesium-reacts vigorously with steam. Zinc-reacts slowly with steam. Copper-No reaction

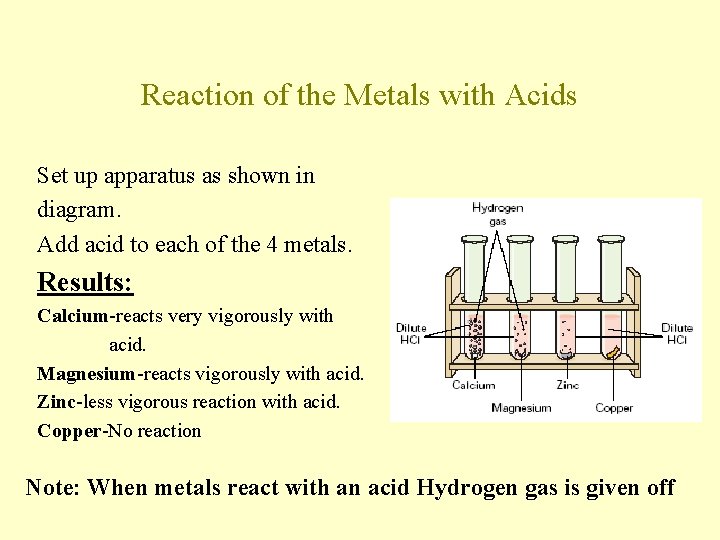
Reaction of the Metals with Acids Set up apparatus as shown in diagram. Add acid to each of the 4 metals. Results: Calcium-reacts very vigorously with acid. Magnesium-reacts vigorously with acid. Zinc-less vigorous reaction with acid. Copper-No reaction Note: When metals react with an acid Hydrogen gas is given off

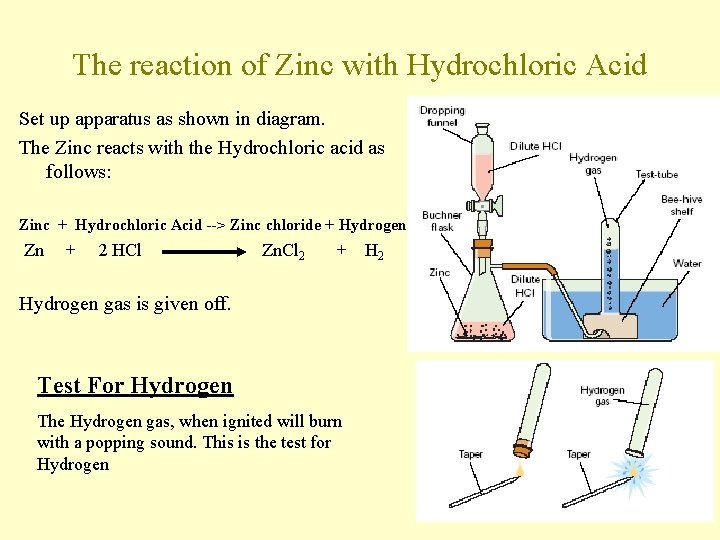
The reaction of Zinc with Hydrochloric Acid Set up apparatus as shown in diagram. The Zinc reacts with the Hydrochloric acid as follows: Zinc + Hydrochloric Acid --> Zinc chloride + Hydrogen Zn + 2 HCl Zn. Cl 2 + Hydrogen gas is given off. Test For Hydrogen The Hydrogen gas, when ignited will burn with a popping sound. This is the test for Hydrogen H 2