Metals Alkali Alkaline Transition Metals in Mixed Group













- Slides: 17

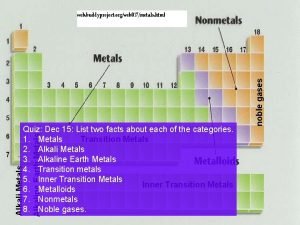
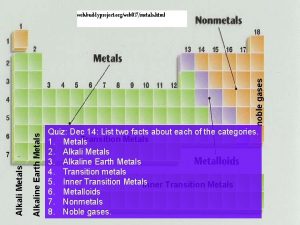
Metals!! Alkali, Alkaline, Transition, Metals in Mixed Group, Lanthanides, and Actinides


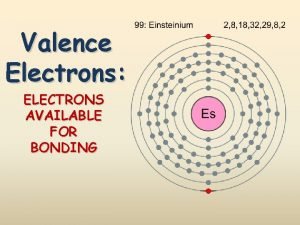
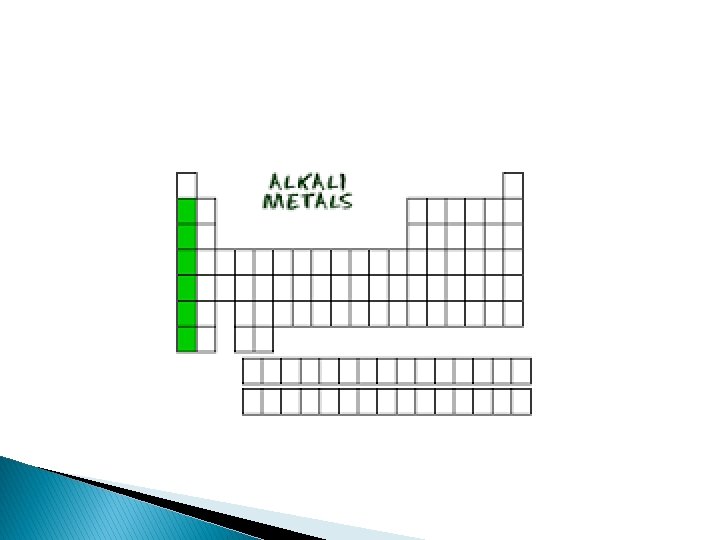
Alkali Metals � Group � Alkali 1 on the periodic table metals react with other elements by losing one electron � They are soooo REACTIVE that they are never found alone in nature. � Soft and shiny, you could cut it with a plastic knife.

� Examples: � **Lithium – found in batteries and medicines. � **Sodium and potassium are good for our health. � -Sodium is found in seawater and salt beds. � -Potassium is in spinach, baked potato with skin, plain yogurt and fish


� Group 2/starts with Berylium) � Reacts by losing 2 electrons � Also found combined in nature. � Gray and white � Fairly hard � Good conductors of electricity

� ** Calcium – is found in yogurt, milk, all dairy products, green leafy vegetables. It gives us strong bones. � ** Magnesium – When we mix Mg with aluminum it creates a strong but lightweight material that’s used to make ladders, airplane parts, and car parts.


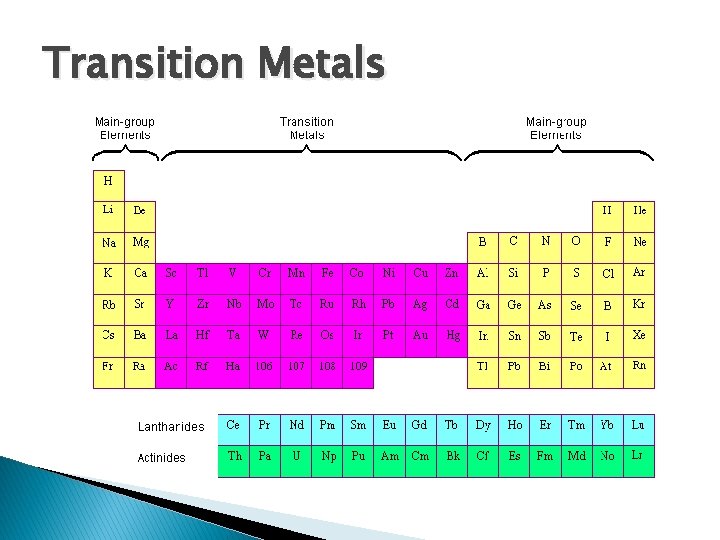
Transition Metals

� � Groups 3 -12. Some famous transition metals are: gold, platinum, silver, iron, mercury, � Hard and shiny � Good conductors of electricity � Form colorful compounds � Not so reactive

� Gold: very precious metal because it’s not reactive � **Iron: iron produces hemoglobin which carries oxygen into our bloodstream.

Metals in Mixed Group � Groups 13 -15 � Some are metals- not all! ( MIXED) � Less reactive � Aluminum, tin, lead

� Aluminum – lightweight metal used in beverage cans and airplane parts � Tin - a thin coating of tin protects steel from corrosion in cans of food. � Lead – it was once used in paints and water pipes. Since it is poisonous, it is no longer anymore. Now, it is used in car batteries and tires.


Lanthanides Actinides

Lanthanides � Soft � Malleable � Shiny � High � They conductivity are mixed with more common metals to make alloys. � Alloys- 2 or more metals mixed together � They are difficult to separate because they all have very similar properties.

Actinides � Only 4 actinides are found in nature. Uranium 238 is used in a power plant to create nuclear energy. The rest of the actinides are not found in nature. They are created in a lab, they produce a great amount of energy, and then they fizzle out. The energy is captured by scientists.
 Common properties of alkali metals
Common properties of alkali metals Group 1
Group 1 Group 2 reactions
Group 2 reactions Halogens valence electrons
Halogens valence electrons Periodic table of elements families
Periodic table of elements families Halides
Halides Order of mobility of alkali metal ions
Order of mobility of alkali metal ions General characteristics of alkali metals
General characteristics of alkali metals Periodic table color coded by families
Periodic table color coded by families Alkali metals colour
Alkali metals colour Fun facts about alkali metals
Fun facts about alkali metals Reaction of alkali metals with water
Reaction of alkali metals with water Youtube alkali metals
Youtube alkali metals Alkali metals bohr diagrams
Alkali metals bohr diagrams Chlorine bohr rutherford
Chlorine bohr rutherford Alkali metals reacting with water
Alkali metals reacting with water Alkali metals reacting with water
Alkali metals reacting with water Li reaction with oxygen
Li reaction with oxygen