Alkali earth metals Alkali metals Anions Atomic Atomic



- Slides: 37


Alkali earth metals Alkali metals Anions Atomic # Atomic number Atomic Theory Atoms Bohr diagram Cations Chemical Change Chemical reaction Compound Covalent bonding Covalent Compound Electrons Element Family/Group Halogens Ionic bonding Ionic compounds Ions Lewis Diagram Matter Metalloids Mixture Molecule Neutron Noble gases Non-Metal Nucleus Period Proton Pure Substance Stable outer shell Subatomic particle Transition metals Valence electrons

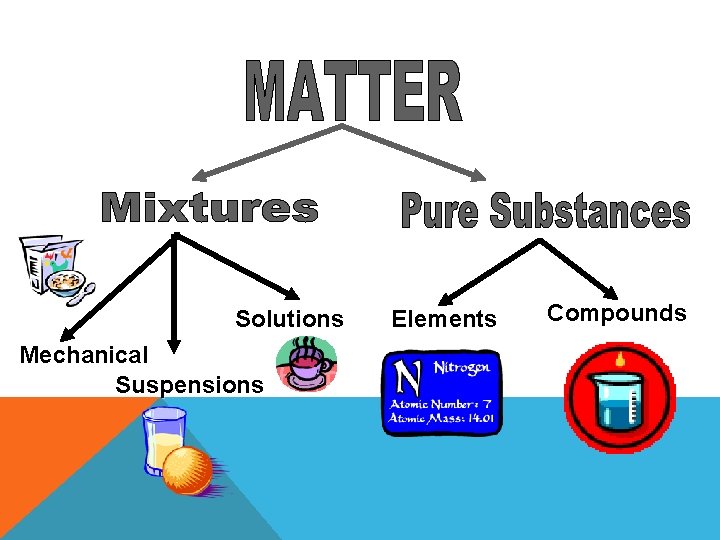
Solutions Mechanical Suspensions Elements Compounds

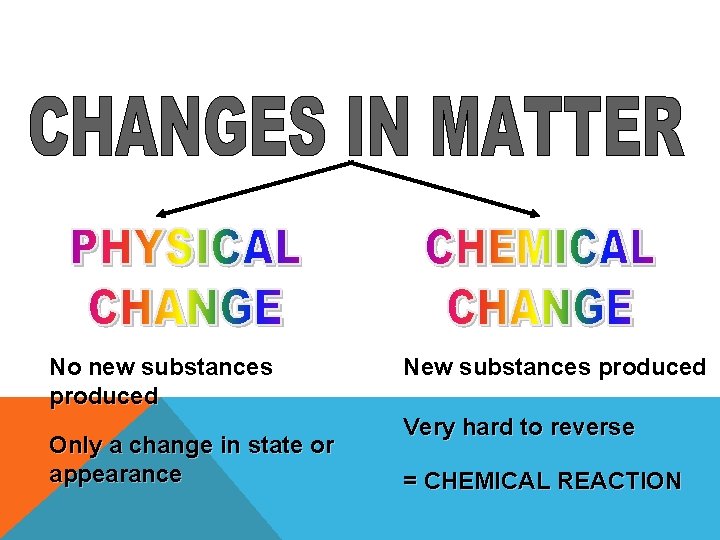
No new substances produced Only a change in state or appearance New substances produced Very hard to reverse = CHEMICAL REACTION

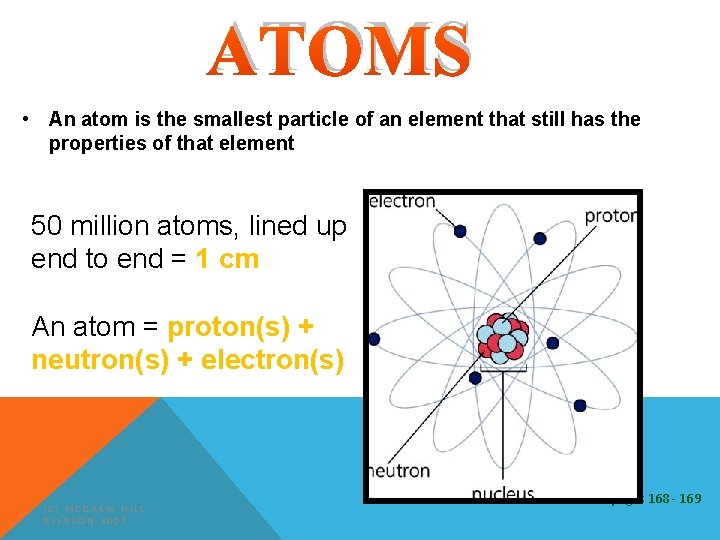
ATOMS • An atom is the smallest particle of an element that still has the properties of that element 50 million atoms, lined up end to end = 1 cm An atom = proton(s) + neutron(s) + electron(s) (C) MCGRAW HILL RYERSON 2007 See pages 168 - 169

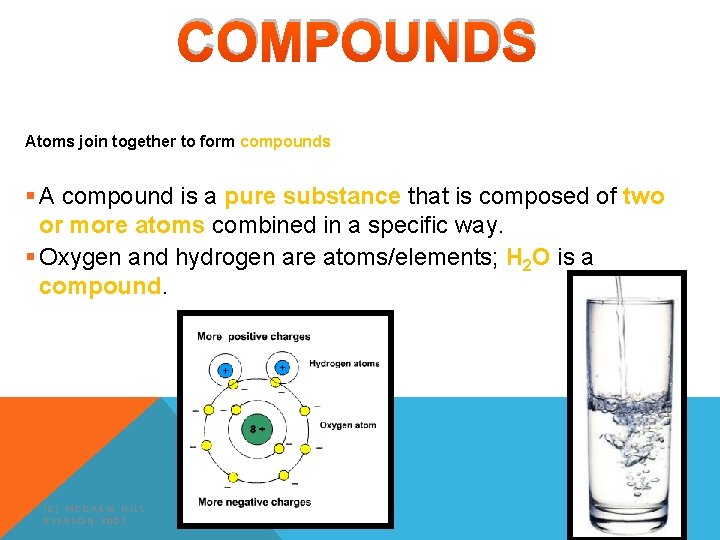
COMPOUNDS Atoms join together to form compounds § A compound is a pure substance that is composed of two or more atoms combined in a specific way. § Oxygen and hydrogen are atoms/elements; H 2 O is a compound. (C) MCGRAW HILL RYERSON 2007

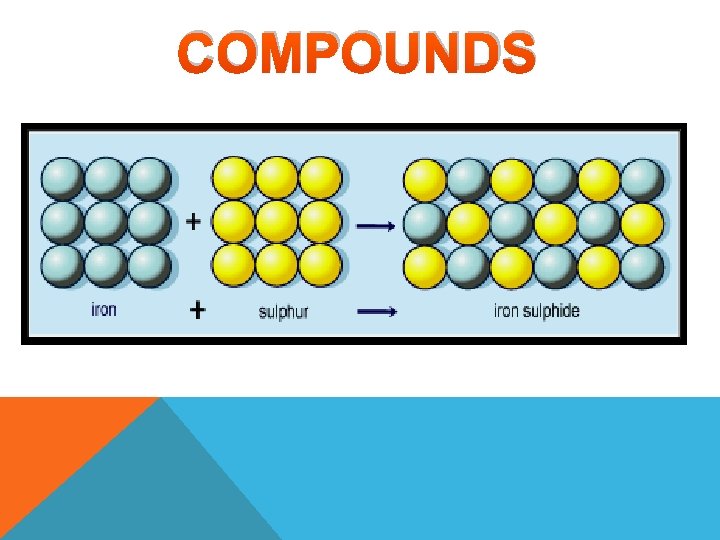
COMPOUNDS

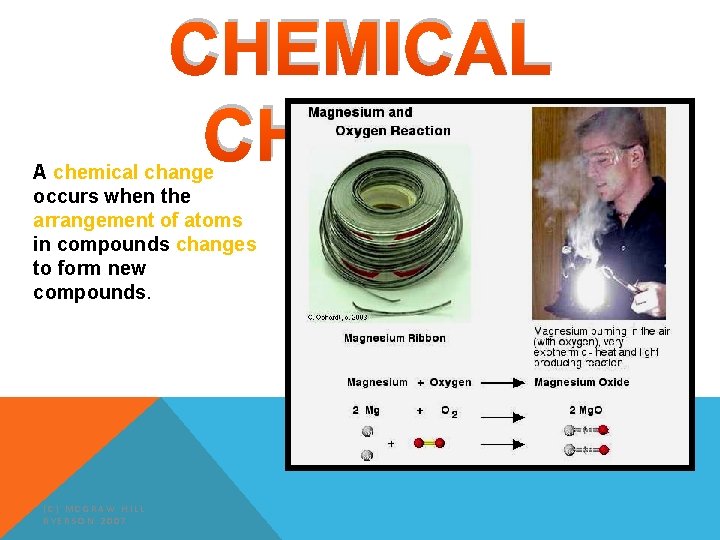
CHEMICAL CHANGE A chemical change occurs when the arrangement of atoms in compounds changes to form new compounds. (C) MCGRAW HILL RYERSON 2007

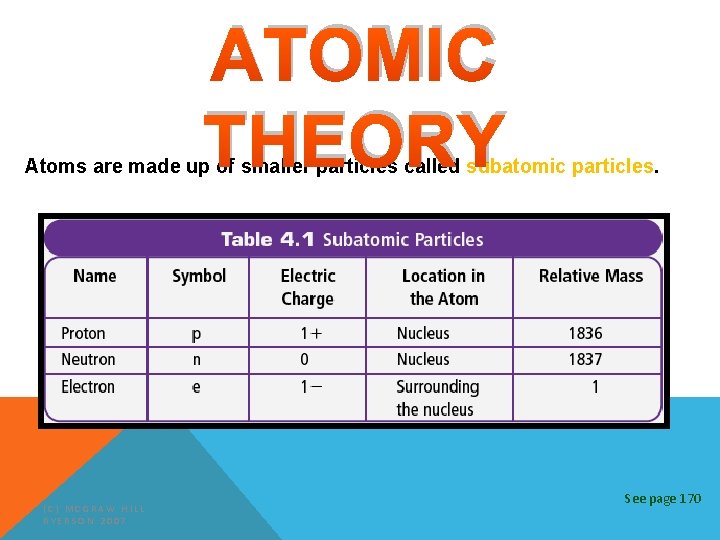
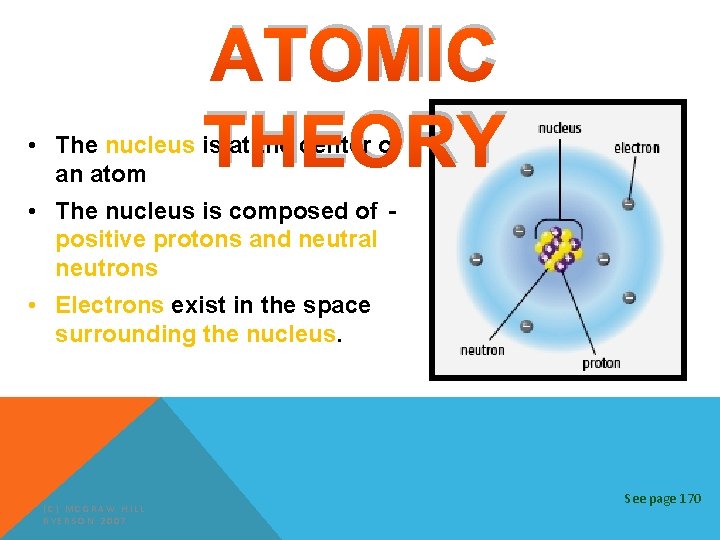
ATOMIC THEORY Atoms are made up of smaller particles called subatomic particles. (C) MCGRAW HILL RYERSON 2007 See page 170

ATOMIC THEORY • The nucleus is at the center of an atom • The nucleus is composed of positive protons and neutral neutrons • Electrons exist in the space surrounding the nucleus. (C) MCGRAW HILL RYERSON 2007 See page 170

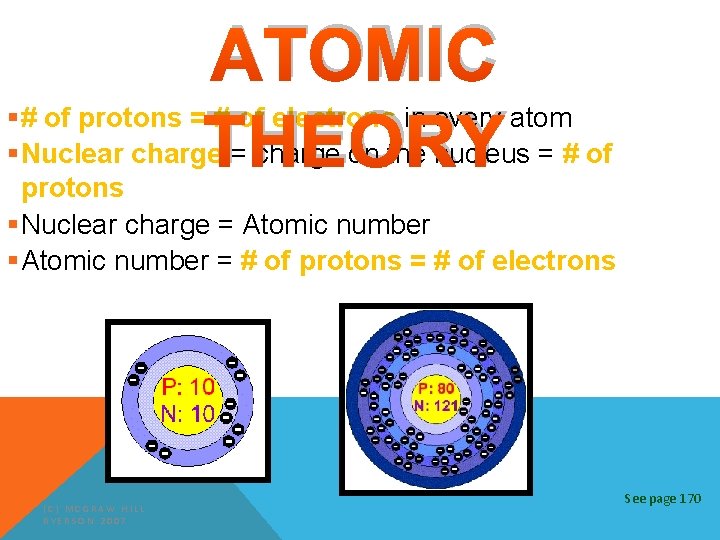
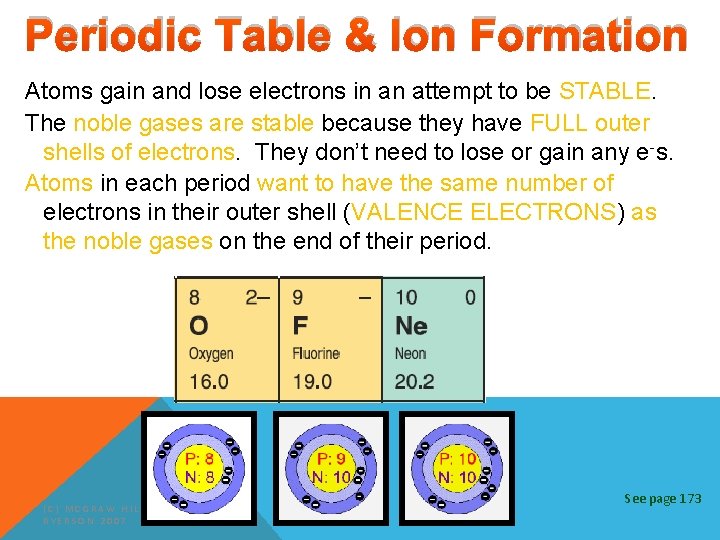
ATOMIC THEORY §# of protons = # of electrons in every atom §Nuclear charge = charge on the nucleus = # of protons §Nuclear charge = Atomic number §Atomic number = # of protons = # of electrons (C) MCGRAW HILL RYERSON 2007 See page 170

INCREASING REACTIVITY

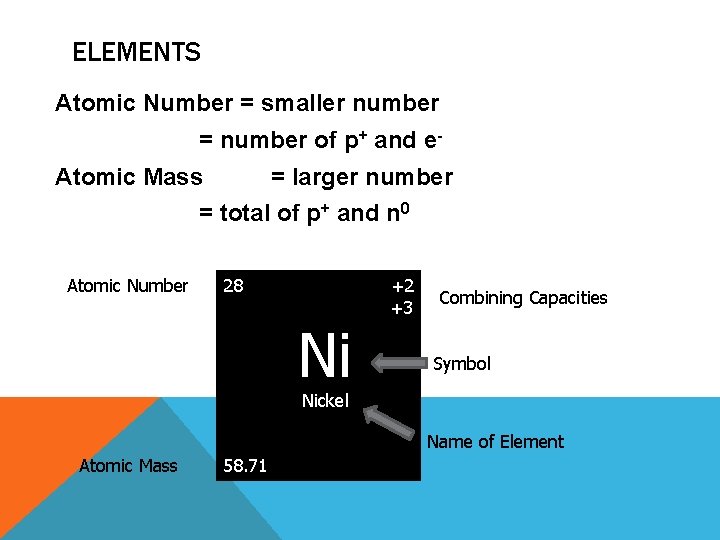
ELEMENTS Atomic Number = smaller number = number of p+ and e. Atomic Mass = larger number = total of p+ and n 0 Atomic Number 28 Ni +2 +3 Combining Capacities Symbol Nickel Name of Element Atomic Mass 58. 71

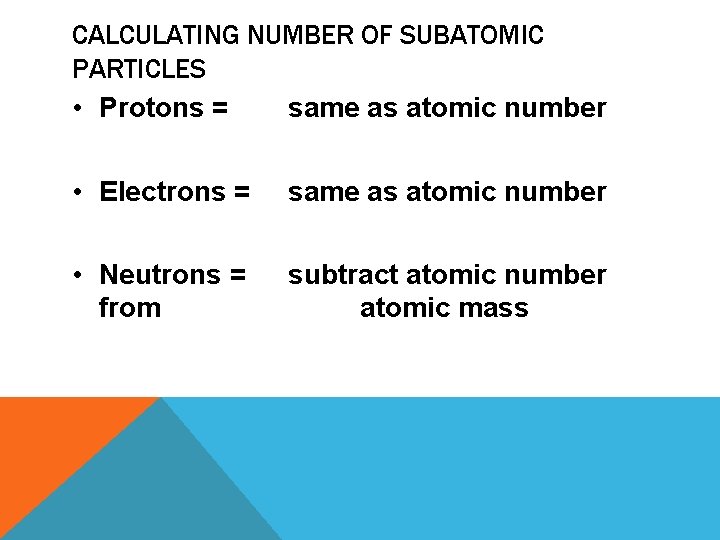
CALCULATING NUMBER OF SUBATOMIC PARTICLES • Protons = same as atomic number • Electrons = same as atomic number • Neutrons = from subtract atomic number atomic mass

PERIODIC TABLE PRACTICE Element Hydrogen Beryllium Carbon Cobalt Nickel Krypton Symbol Atomic Number Atomic Mass Number of Protons Number of Neutrons Number of Electrons

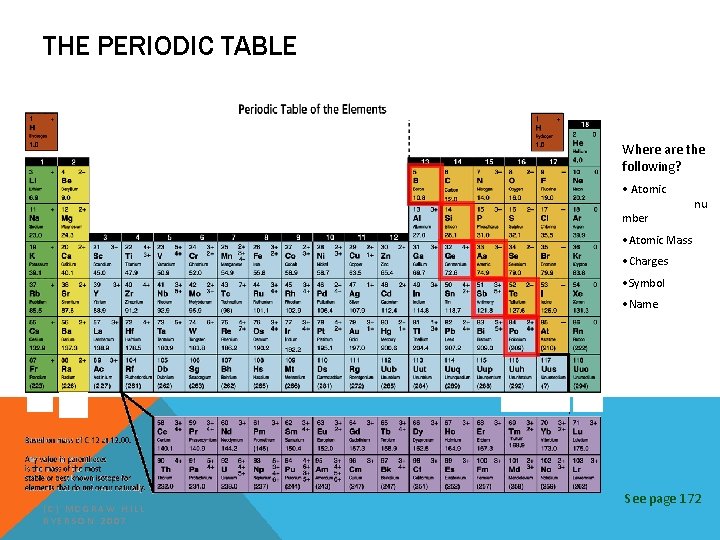
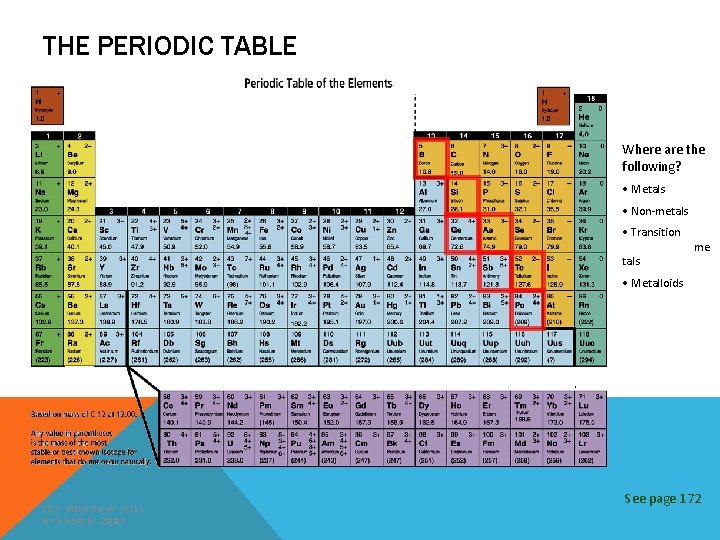
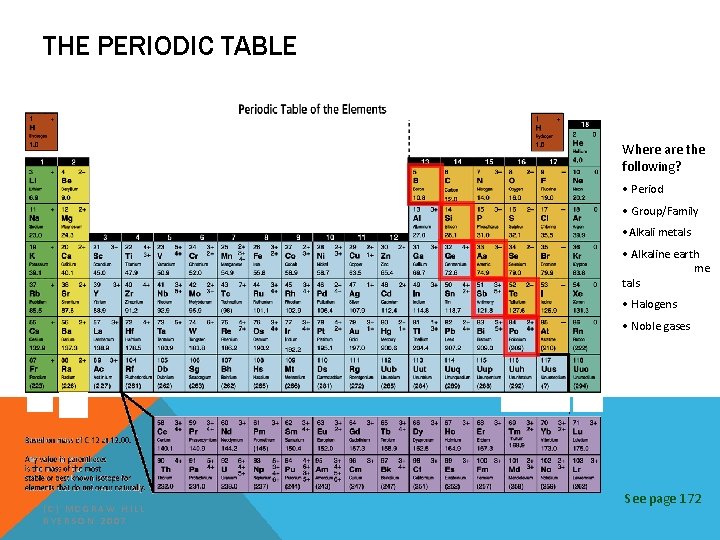
THE PERIODIC TABLE Where are the following? • Atomic INCREASING REACTIVITY mber nu • Atomic Mass • Charges • Symbol • Name (C) MCGRAW HILL RYERSON 2007 See page 172

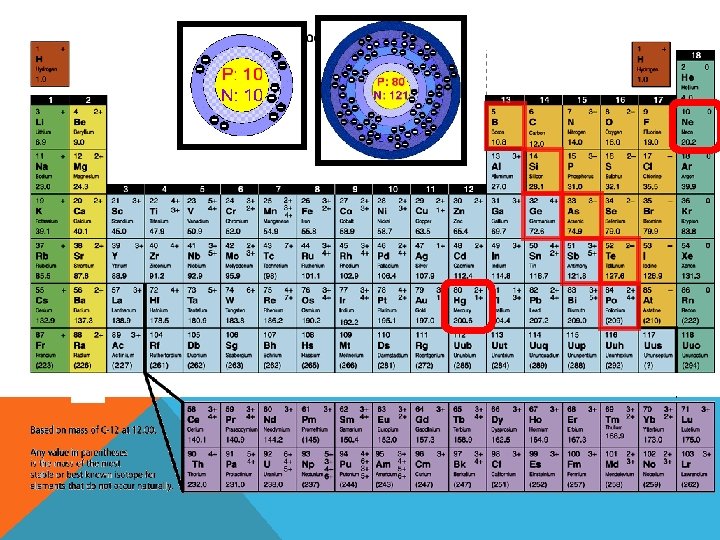
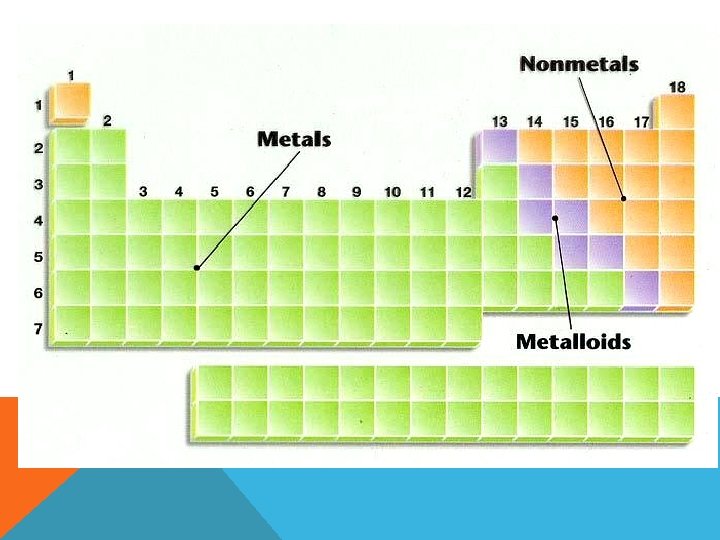
Organization of the Periodic Table In the periodic table elements are listed in order by their atomic number. §Metals are on the left §The transition metals range from group 3 -12 §Non-metals are on the right §Metalloids form a “staircase” toward the right side. (C) MCGRAW HILL RYERSON 2007 See page 171

Metals (left of zig zag line) Physical Properties of Metals: Shiny, good conductors of heat and electricity, ductile (make wires) and malleable (thin sheets). Easily lose electrons. Like to join with non-metals. Corrode (tarnish/rust). Nonmetals (right of zig zag line) Physical Properties of Nonmetals: dull appearance, poor conductor, brittle (breaks easily), not ductile or malleable. Easily gain electrons. Like to join with metals, but will bond to other non-metals. Metalloids (on both sides of zigzag line) Physical Properties of Metalloids: have properties of both metals and nonmetals. Solid, shiny or dull, ductile and malleable, conduct heat and electricity, but not very well.


THE PERIODIC TABLE Where are the following? INCREASING REACTIVITY • Metals • Non-metals • Transition tals me • Metalloids (C) MCGRAW HILL RYERSON 2007 See page 172

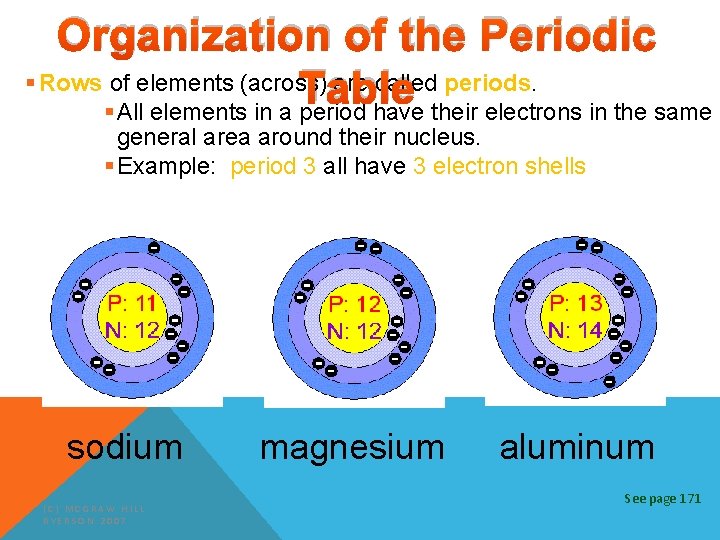
Organization of the Periodic § Rows of elements (across) are called periods. Table § All elements in a period have their electrons in the same general area around their nucleus. § Example: period 3 all have 3 electron shells sodium (C) MCGRAW HILL RYERSON 2007 magnesium aluminum See page 171

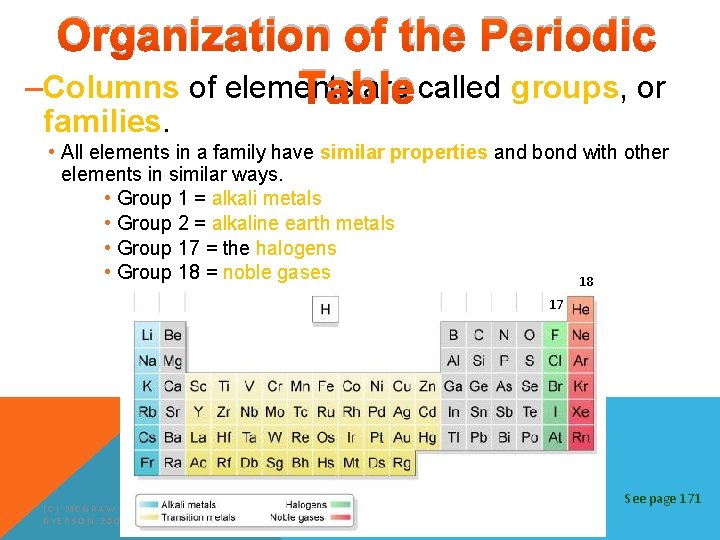
Organization of the Periodic –Columns of elements are called groups, or Table families. • All elements in a family have similar properties and bond with other elements in similar ways. • Group 1 = alkali metals • Group 2 = alkaline earth metals • Group 17 = the halogens • Group 18 = noble gases 18 17 (C) MCGRAW HILL RYERSON 2007 See page 171

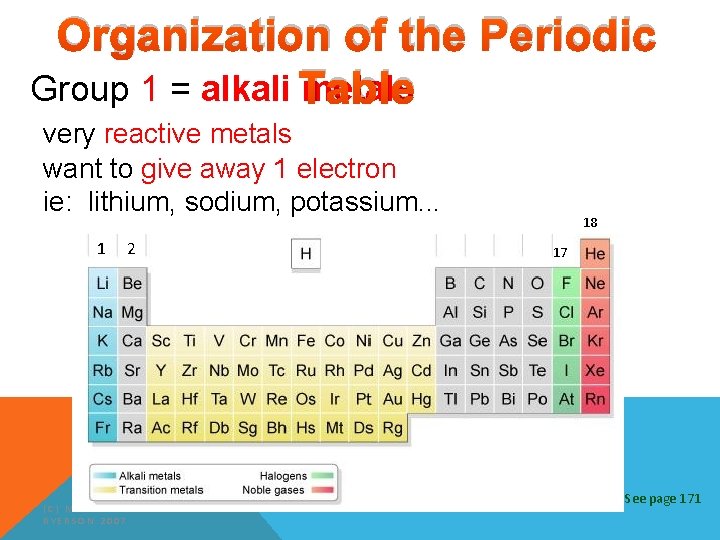
Organization of the Periodic Group 1 = alkali Table metals very reactive metals want to give away 1 electron ie: lithium, sodium, potassium. . . 1 2 (C) MCGRAW HILL RYERSON 2007 18 17 See page 171

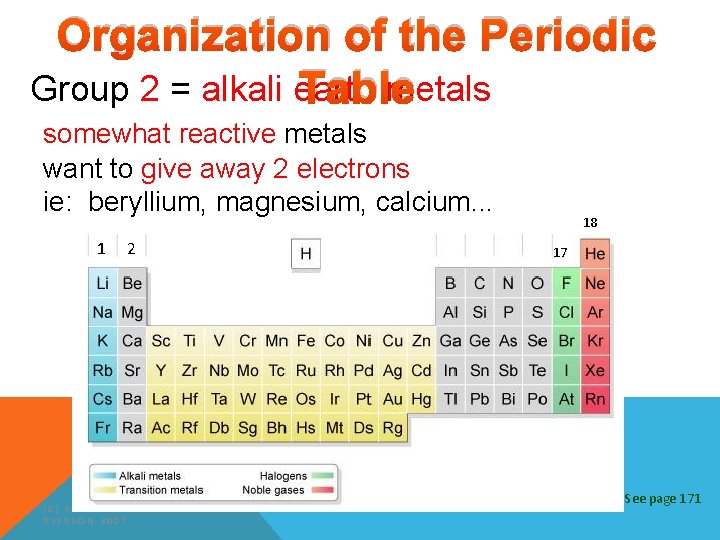
Organization of the Periodic Table Group 2 = alkali earth metals somewhat reactive metals want to give away 2 electrons ie: beryllium, magnesium, calcium. . . 1 2 (C) MCGRAW HILL RYERSON 2007 18 17 See page 171

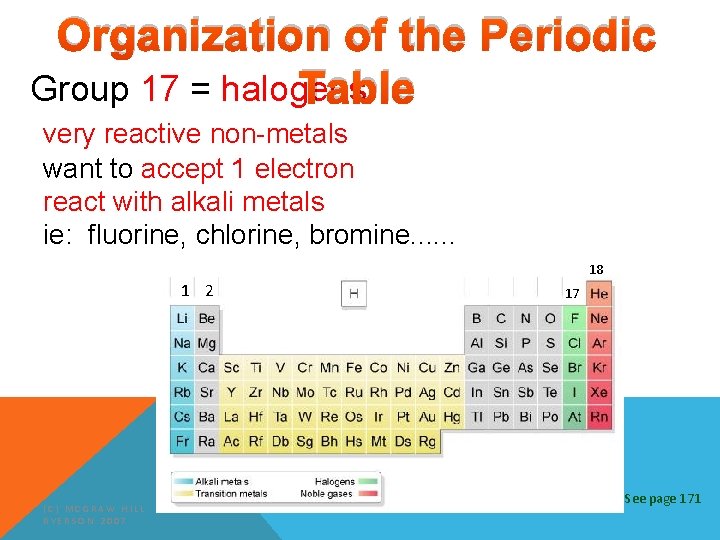
Organization of the Periodic Table Group 17 = halogens very reactive non-metals want to accept 1 electron react with alkali metals ie: fluorine, chlorine, bromine. . . 18 1 2 (C) MCGRAW HILL RYERSON 2007 17 See page 171

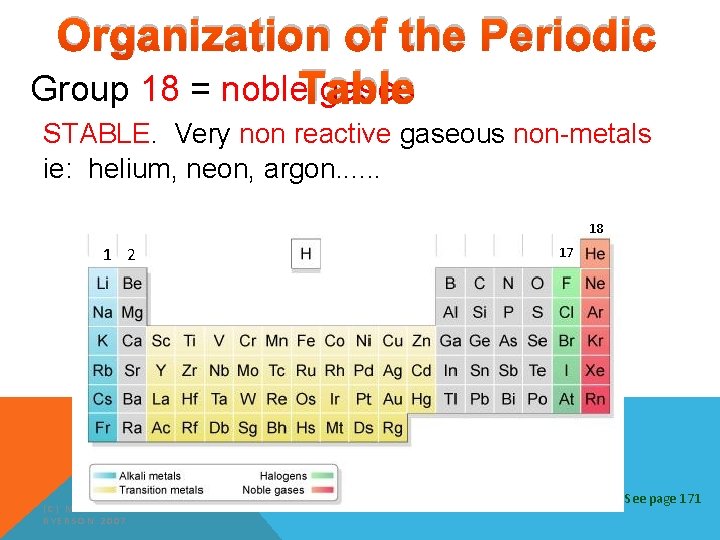
Organization of the Periodic Group 18 = noble. Table gases STABLE. Very non reactive gaseous non-metals ie: helium, neon, argon. . . 18 1 2 (C) MCGRAW HILL RYERSON 2007 17 See page 171

THE PERIODIC TABLE Where are the following? • Period INCREASING REACTIVITY • Group/Family • Alkali metals • Alkaline earth me tals • Halogens • Noble gases (C) MCGRAW HILL RYERSON 2007 See page 172

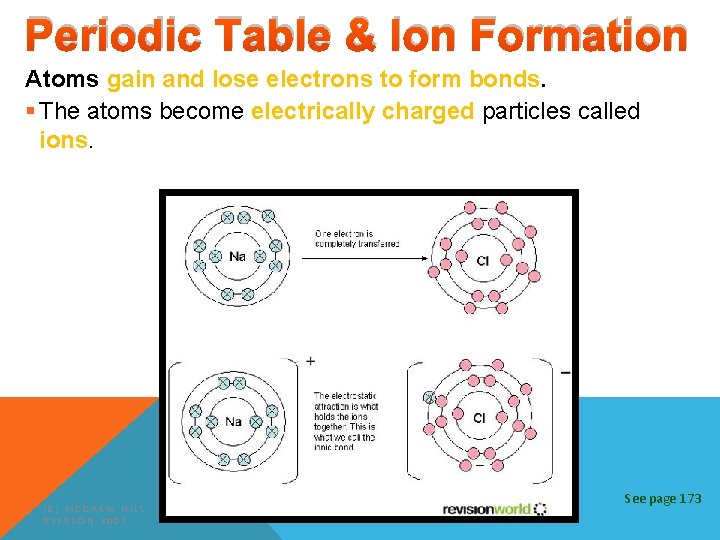
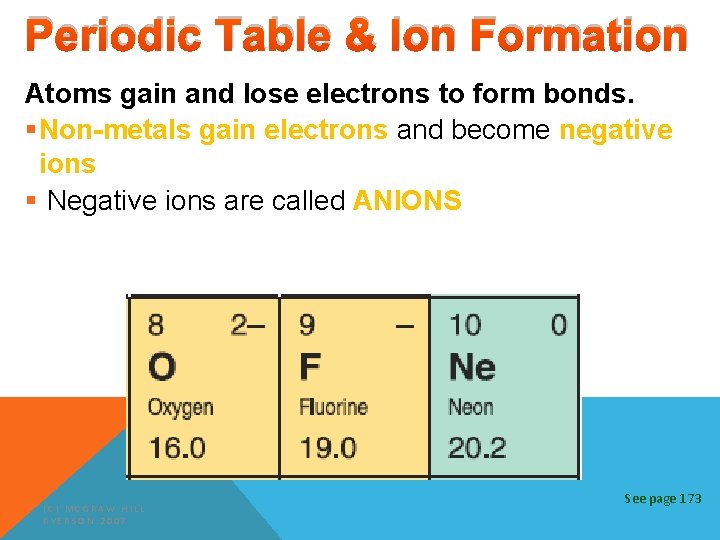
Periodic Table & Ion Formation Atoms gain and lose electrons to form bonds. § The atoms become electrically charged particles called ions. (C) MCGRAW HILL RYERSON 2007 See page 173

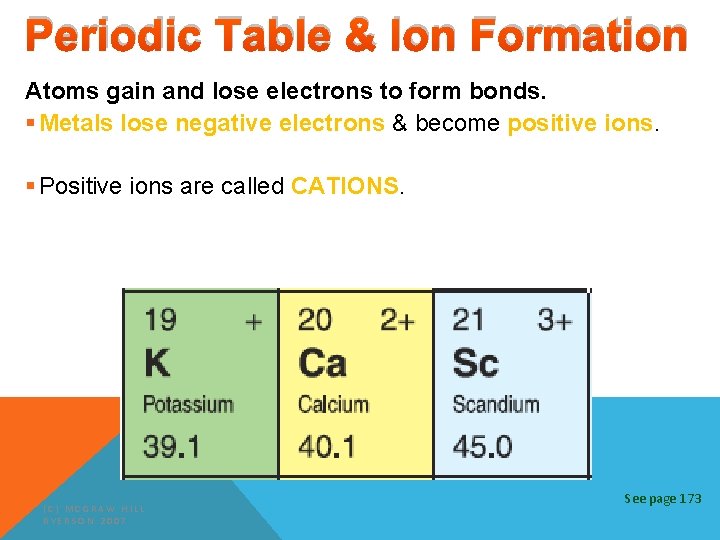
Periodic Table & Ion Formation Atoms gain and lose electrons to form bonds. § Metals lose negative electrons & become positive ions. § Positive ions are called CATIONS. (C) MCGRAW HILL RYERSON 2007 See page 173


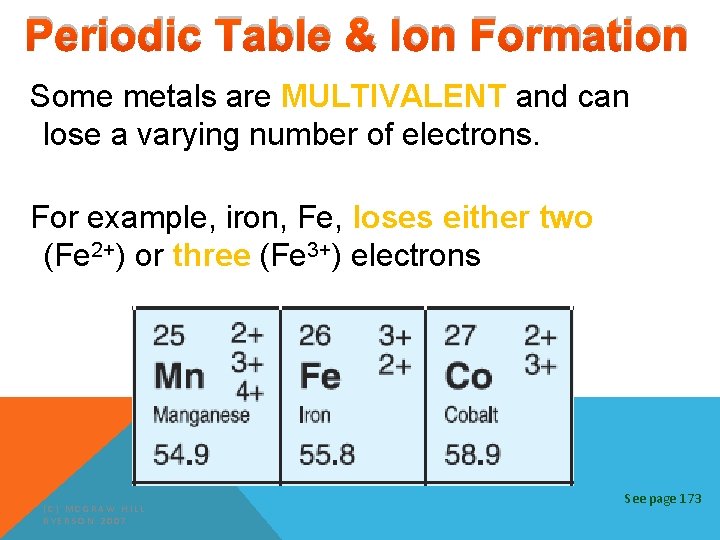
Periodic Table & Ion Formation Some metals are MULTIVALENT and can lose a varying number of electrons. For example, iron, Fe, loses either two (Fe 2+) or three (Fe 3+) electrons (C) MCGRAW HILL RYERSON 2007 See page 173

Periodic Table & Ion Formation Atoms gain and lose electrons to form bonds. §Non-metals gain electrons and become negative ions § Negative ions are called ANIONS (C) MCGRAW HILL RYERSON 2007 See page 173

Periodic Table & Ion Formation Atoms gain and lose electrons in an attempt to be STABLE. The noble gases are stable because they have FULL outer shells of electrons. They don’t need to lose or gain any e-s. Atoms in each period want to have the same number of electrons in their outer shell (VALENCE ELECTRONS) as the noble gases on the end of their period. (C) MCGRAW HILL RYERSON 2007 See page 173

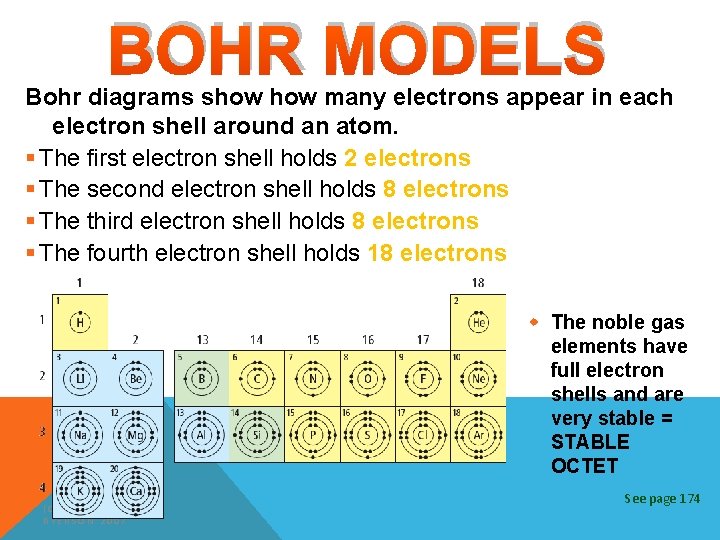
BOHR MODELS Bohr diagrams show many electrons appear in each electron shell around an atom. § The first electron shell holds 2 electrons § The second electron shell holds 8 electrons § The third electron shell holds 8 electrons § The fourth electron shell holds 18 electrons w The noble gas elements have full electron shells and are very stable = STABLE OCTET (C) MCGRAW HILL RYERSON 2007 See page 174

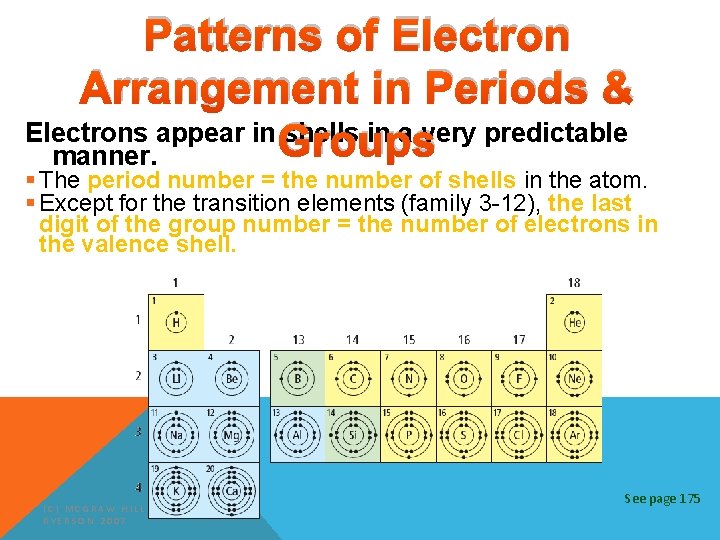
Patterns of Electron Arrangement in Periods & Electrons appear in Groups shells in a very predictable manner. § The period number = the number of shells in the atom. § Except for the transition elements (family 3 -12), the last digit of the group number = the number of electrons in the valence shell. (C) MCGRAW HILL RYERSON 2007 See page 175

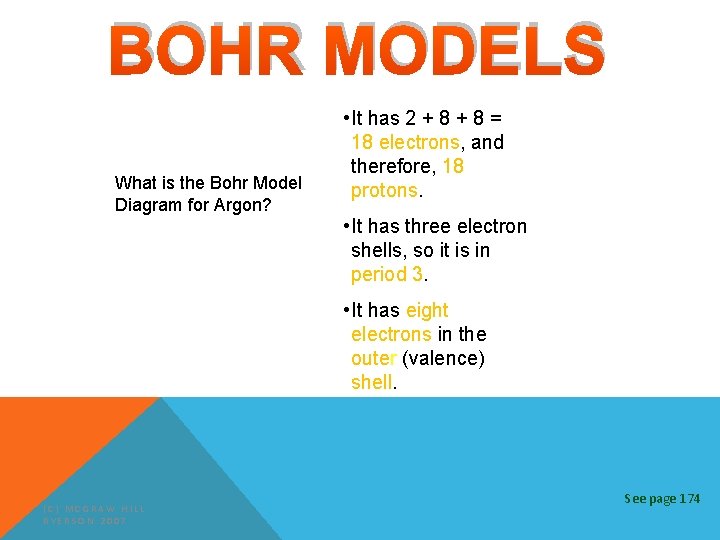
BOHR MODELS What is the Bohr Model Diagram for Argon? • It has 2 + 8 = 18 electrons, and therefore, 18 protons. • It has three electron shells, so it is in period 3. • It has eight electrons in the outer (valence) shell. (C) MCGRAW HILL RYERSON 2007 See page 174
