Energy Pooling and Ionization in dense Alkali Metal


- Slides: 20

Energy Pooling and Ionization in dense Alkali Metal Vapor Sean Bresler, Joonbum Park, Michael Heaven ISMS 2017

Motivation • 1: Knize, R. J. , Zhdanov, B. V. , & Shaffer, M. K. (2011). Photoionization in alkali lasers. Optics Express, 19(8), 7894. https: //doi. org/10. 1364/OE. 19. 007894

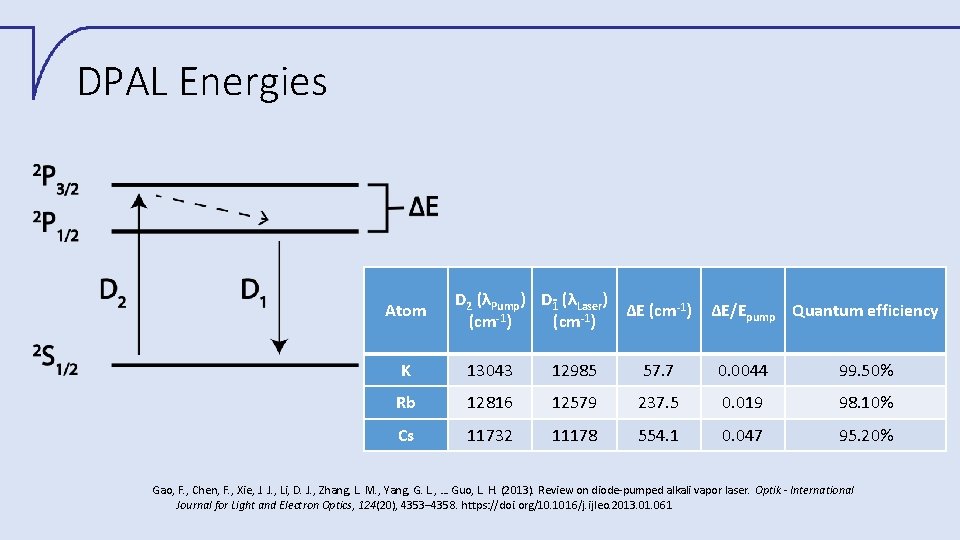
DPAL Energies Atom D 2 (λPump) D 1 (λLaser) ΔE (cm 1) 1 1 (cm ) ΔE/Epump Quantum efficiency K 13043 12985 57. 7 0. 0044 99. 50% Rb 12816 12579 237. 5 0. 019 98. 10% Cs 11732 11178 554. 1 0. 047 95. 20% Gao, F. , Chen, F. , Xie, J. J. , Li, D. J. , Zhang, L. M. , Yang, G. L. , … Guo, L. H. (2013). Review on diode-pumped alkali vapor laser. Optik - International Journal for Light and Electron Optics, 124(20), 4353– 4358. https: //doi. org/10. 1016/j. ijleo. 2013. 01. 061

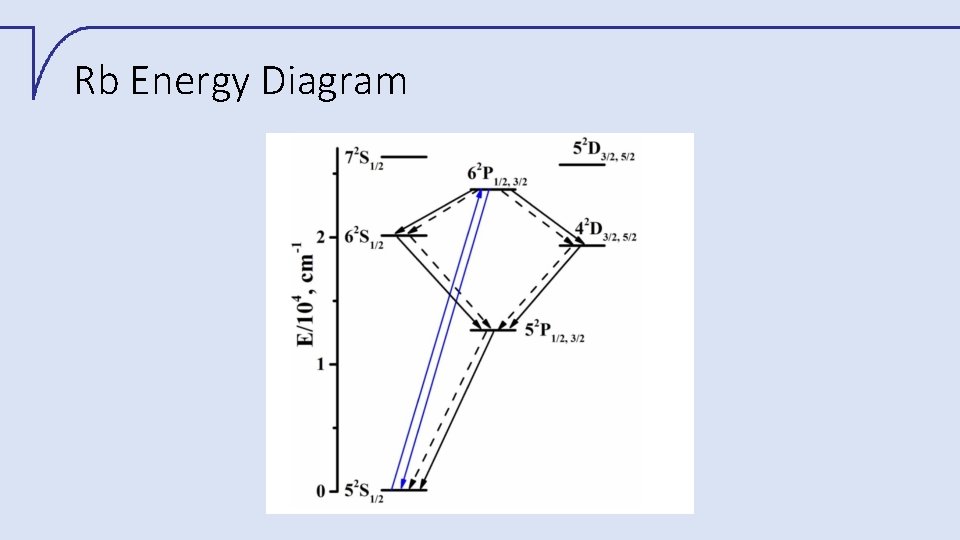
Rb Energy Diagram

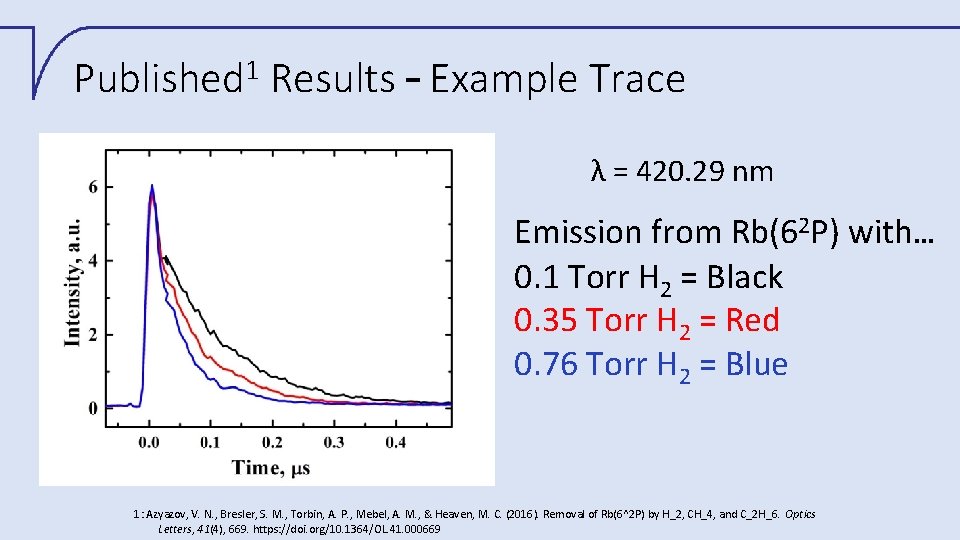
1 Published Results – Example Trace λ = 420. 29 nm Emission from Rb(62 P) with… 0. 1 Torr H 2 = Black 0. 35 Torr H 2 = Red 0. 76 Torr H 2 = Blue 1: Azyazov, V. N. , Bresler, S. M. , Torbin, A. P. , Mebel, A. M. , & Heaven, M. C. (2016). Removal of Rb(6^2 P) by H_2, CH_4, and C_2 H_6. Optics Letters, 41(4), 669. https: //doi. org/10. 1364/OL. 41. 000669

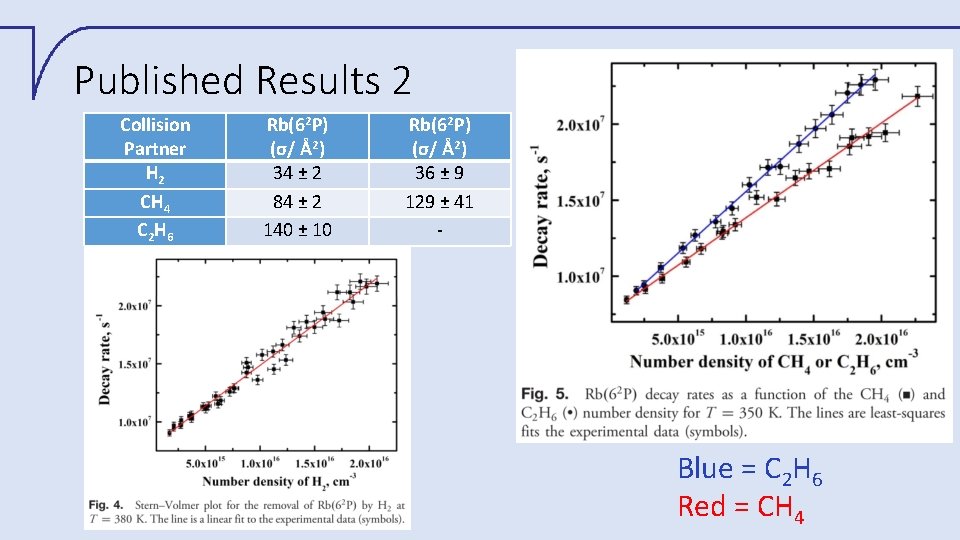
Published Results 2 Collision Partner H 2 CH 4 C 2 H 6 Rb(62 P) (σ/ Å2) 34 ± 2 84 ± 2 140 ± 10 Rb(62 P) (σ/ Å2) 36 ± 9 129 ± 41 - Blue = C 2 H 6 Red = CH 4

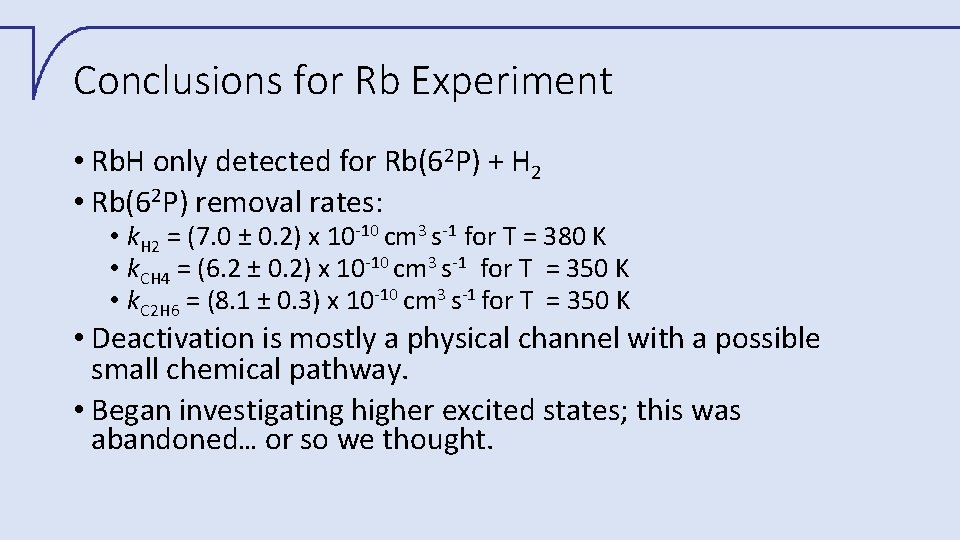
Conclusions for Rb Experiment • Rb. H only detected for Rb(62 P) + H 2 • Rb(62 P) removal rates: • k. H 2 = (7. 0 ± 0. 2) x 10 -10 cm 3 s-1 for T = 380 K • k. CH 4 = (6. 2 ± 0. 2) x 10 -10 cm 3 s-1 for T = 350 K • k. C 2 H 6 = (8. 1 ± 0. 3) x 10 -10 cm 3 s-1 for T = 350 K • Deactivation is mostly a physical channel with a possible small chemical pathway. • Began investigating higher excited states; this was abandoned… or so we thought.

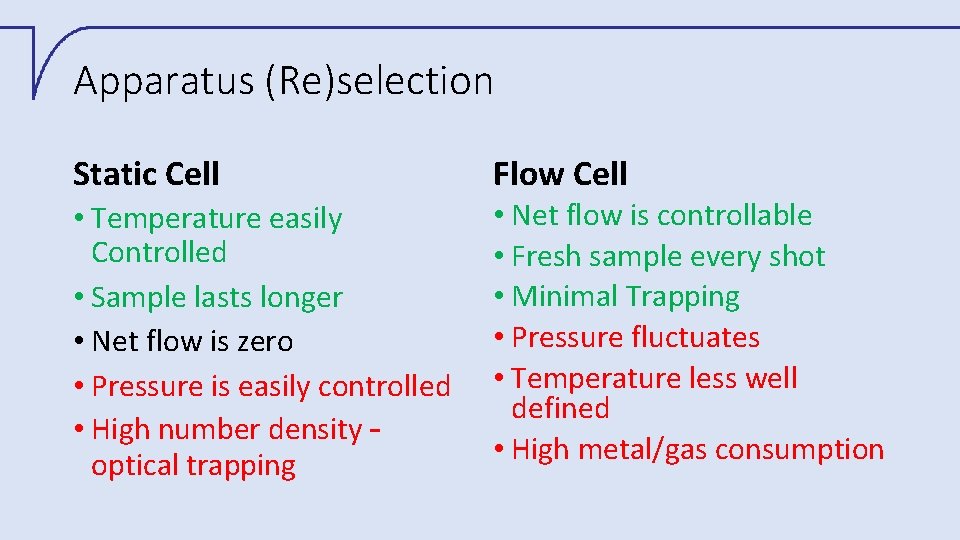
Apparatus (Re)selection Static Cell • Temperature easily Controlled • Sample lasts longer • Net flow is zero • Pressure is easily controlled • High number density – optical trapping Flow Cell • Net flow is controllable • Fresh sample every shot • Minimal Trapping • Pressure fluctuates • Temperature less well defined • High metal/gas consumption

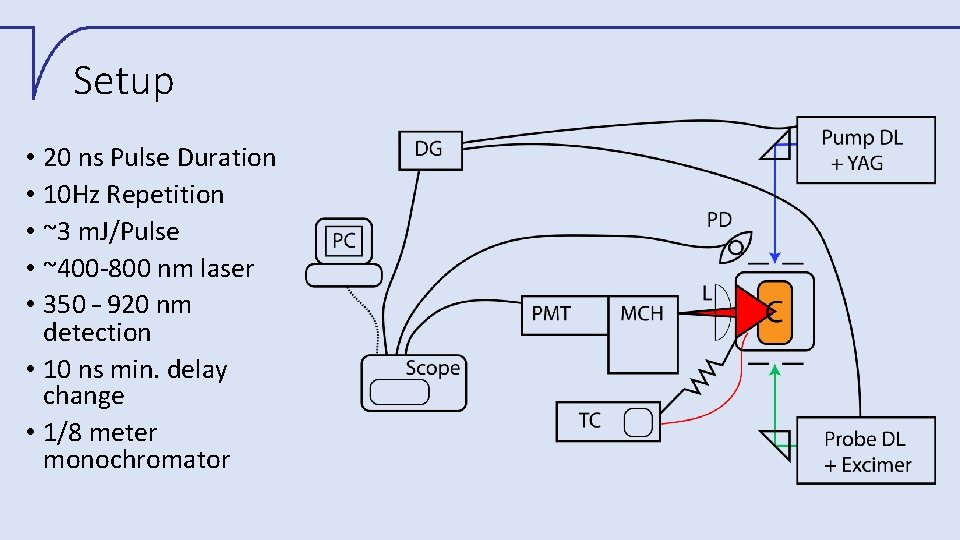
Setup • 20 ns Pulse Duration • 10 Hz Repetition • ~3 m. J/Pulse • ~400 -800 nm laser • 350 – 920 nm detection • 10 ns min. delay change • 1/8 meter monochromator

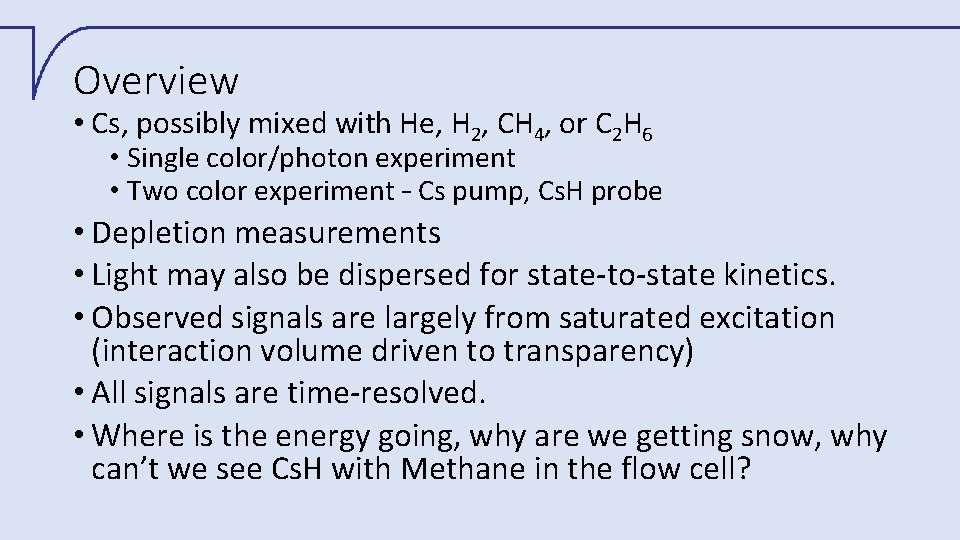
Overview • Cs, possibly mixed with He, H 2, CH 4, or C 2 H 6 • Single color/photon experiment • Two color experiment – Cs pump, Cs. H probe • Depletion measurements • Light may also be dispersed for state-to-state kinetics. • Observed signals are largely from saturated excitation (interaction volume driven to transparency) • All signals are time-resolved. • Where is the energy going, why are we getting snow, why can’t we see Cs. H with Methane in the flow cell?


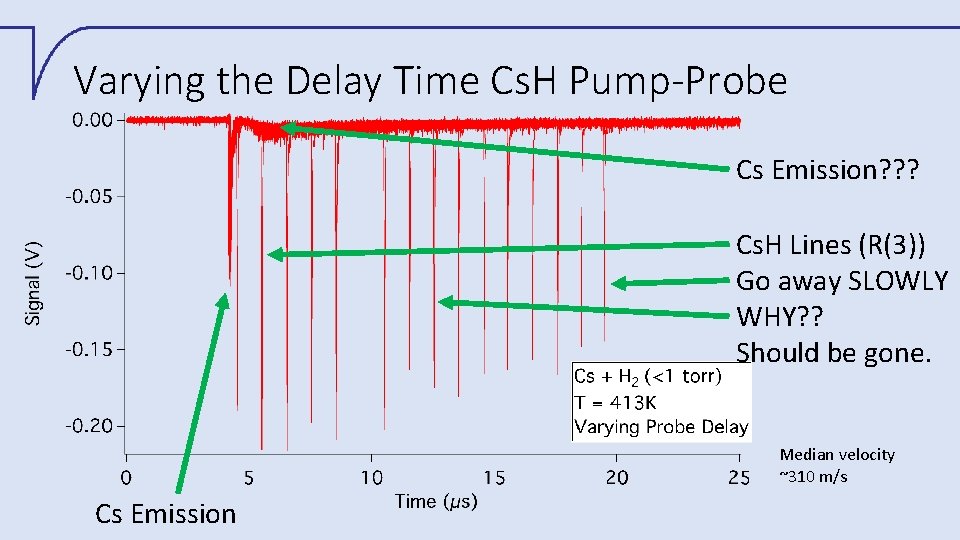
Varying the Delay Time Cs. H Pump-Probe Cs Emission? ? ? Cs. H Lines (R(3)) Go away SLOWLY WHY? ? Should be gone. Median velocity ~310 m/s Cs Emission

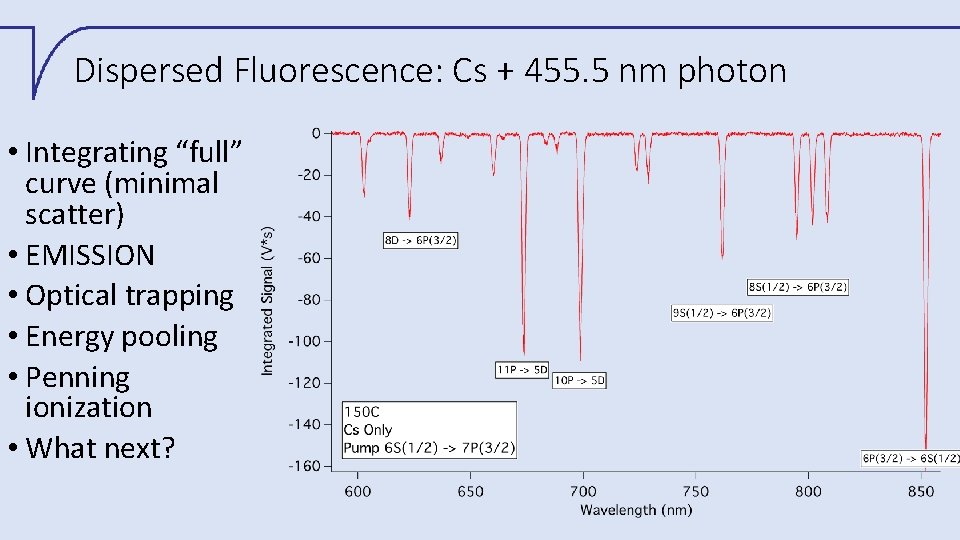
Dispersed Fluorescence: Cs + 455. 5 nm photon • Integrating “full” curve (minimal scatter) • EMISSION • Optical trapping • Energy pooling • Penning ionization • What next?

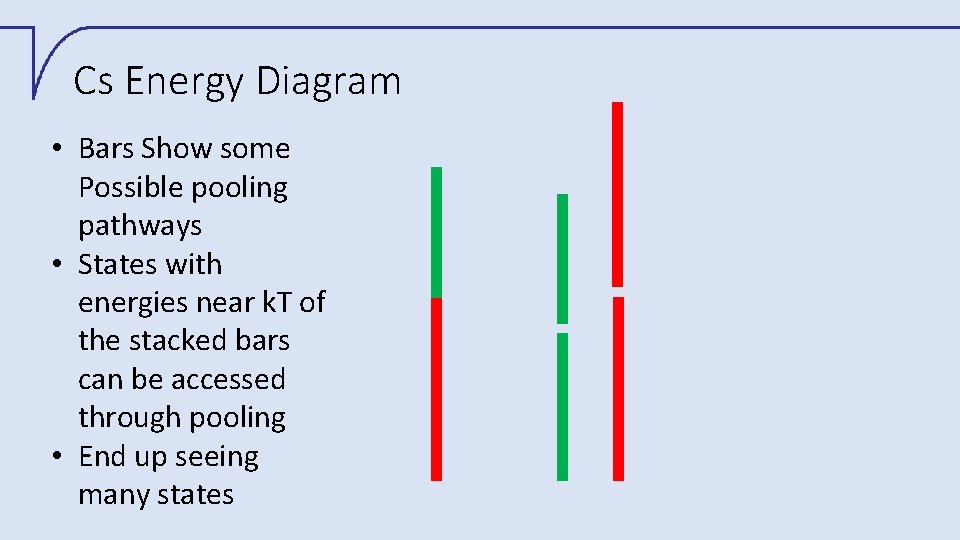
Cs Energy Diagram • Bars Show some Possible pooling pathways • States with energies near k. T of the stacked bars can be accessed through pooling • End up seeing many states

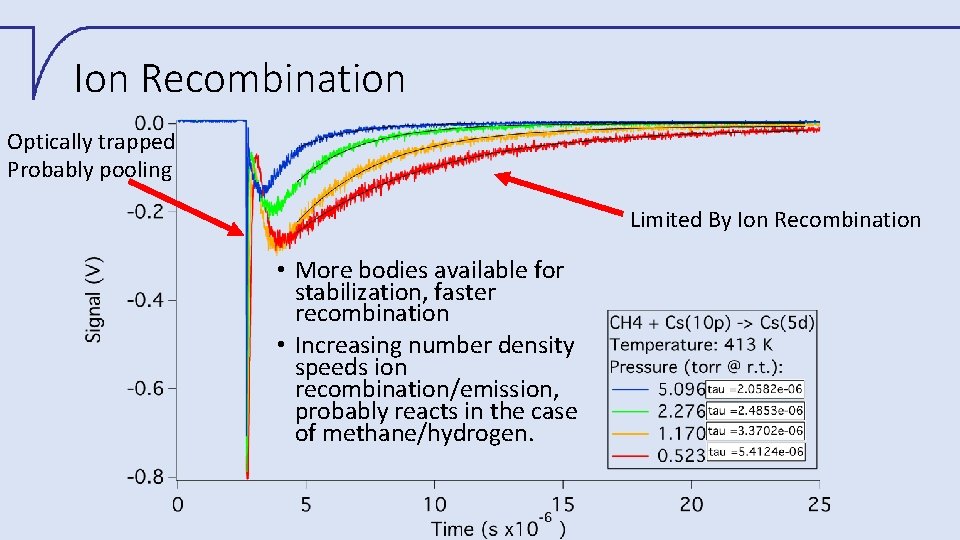
Ion Recombination Optically trapped Probably pooling Limited By Ion Recombination • More bodies available for stabilization, faster recombination • Increasing number density speeds ion recombination/emission, probably reacts in the case of methane/hydrogen.

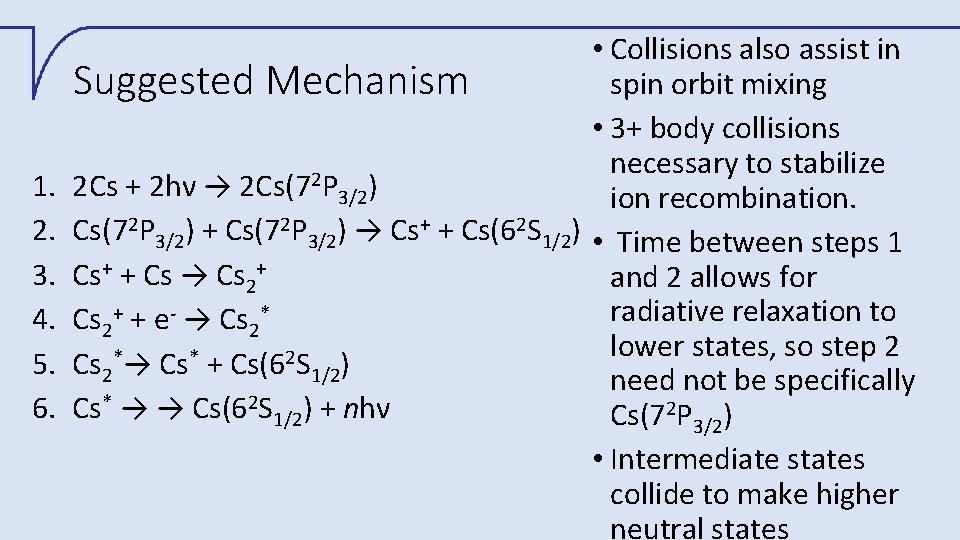
1. 2. 3. 4. 5. 6. • Collisions also assist in spin orbit mixing Suggested Mechanism • 3+ body collisions necessary to stabilize 2 2 Cs + 2 hν → 2 Cs(7 P 3/2) ion recombination. Cs(72 P 3/2) + Cs(72 P 3/2) → Cs+ + Cs(62 S 1/2) • Time between steps 1 Cs+ + Cs → Cs 2+ and 2 allows for radiative relaxation to Cs 2+ + e- → Cs 2* lower states, so step 2 * * 2 Cs 2 → Cs + Cs(6 S 1/2) need not be specifically Cs* → → Cs(62 S 1/2) + nhν Cs(72 P 3/2) • Intermediate states collide to make higher neutral states

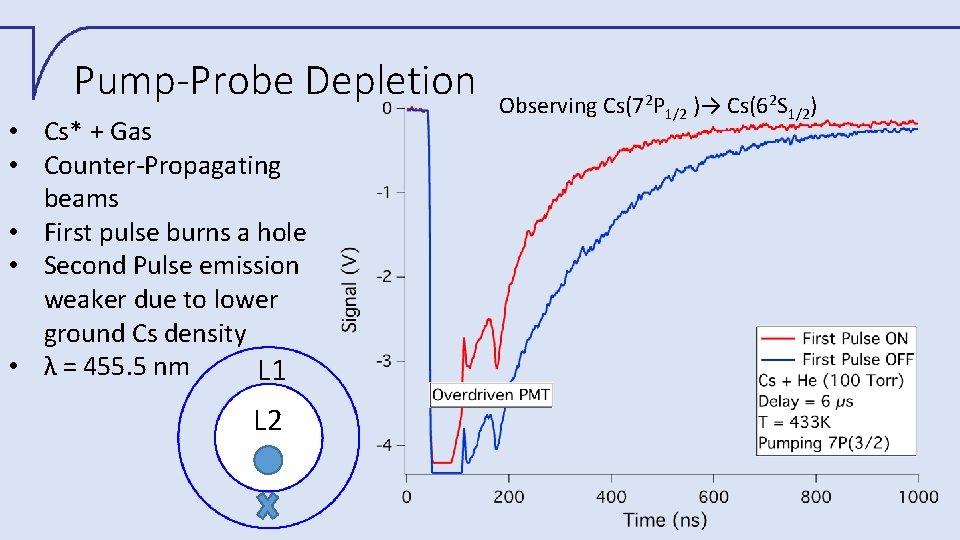
Pump-Probe Depletion • Cs* + Gas • Counter-Propagating beams • First pulse burns a hole • Second Pulse emission weaker due to lower ground Cs density • λ = 455. 5 nm L 1 L 2 Observing Cs(72 P 1/2 )→ Cs(62 S 1/2)

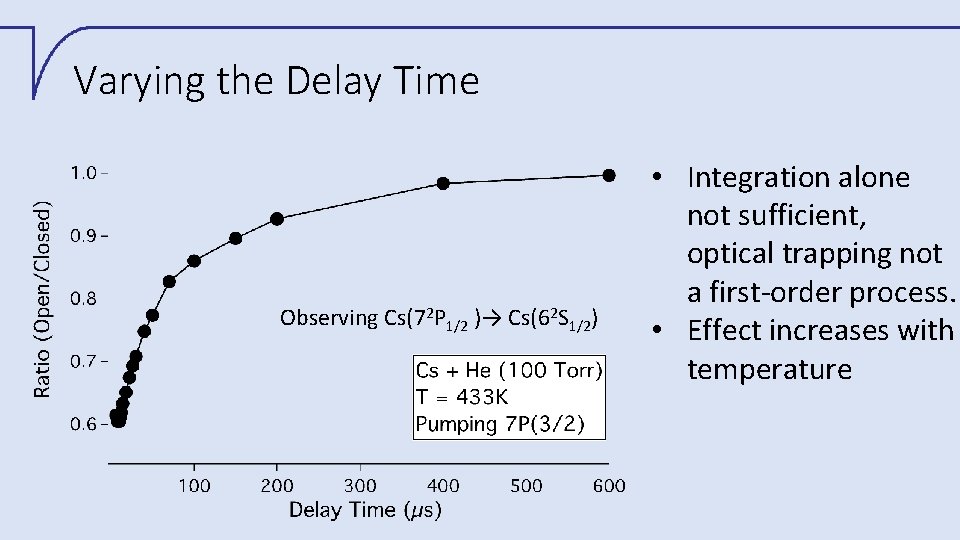
Varying the Delay Time Observing Cs(72 P 1/2 )→ Cs(62 S 1/2) • Integration alone not sufficient, optical trapping not a first-order process. • Effect increases with temperature

Cs Conclusions and Future work • Rb(62 P) removal in the presence of H 2, CH 4, C 2 H 6 has been quantified • Energy Pooling is extremely efficient in the Cs Case, and is an easy way to make Cs+ Ions. REMPI type schemes or UV photodeatchment has been attempted, doesn’t work. • Cs* DOES react with H 2, CH 4 to form Cs. H. Cannot Rule out the possibility that Cs(72 P 3/2) is NOT the primary reactive partner with CH 4 • Goal is to get branching ratios. Complicated, need model. working on differential equations. Want repeatability. • Cs* + C 2 H 6 will be determined soon (ethane shortage)

Thanks • • • Michael C. Heaven, Ph. D (PI) Joonbum Park, Ph. D (partner) Jiande Han, Ph. D Daniel Frohman, Ph. D Kyle Mascaritolo, Ph. D (gone) Joshua Bartlett, Ph. D (gone) Michael Sullivan, Ph. D (going) Robert Van. Gundy Amanda Dermer Mallory Theis Jessica Cifuentes (undergrad) • Wafaa Fawzy, Ph. D (Visiting Prof. Murray State) • Jacob Stewart, Ph. D (taught me how to use an oscilloscope) • Ian Finneran, Ph. D (Inspired me on the bus back last year, Cal. Tech) • Joint Technology Office; Air Force Office of Scientific Research (AFOSR) (FA 9550 -13 -1 -0002)
 Crust outer core inner core mantle
Crust outer core inner core mantle Earth's layers most dense to least dense
Earth's layers most dense to least dense The earth's layers foldable
The earth's layers foldable Dense ball of solid metal
Dense ball of solid metal Which atoms has the largest atomic radius
Which atoms has the largest atomic radius Atomic radius in periodic table
Atomic radius in periodic table Ionization energy atomic radius
Ionization energy atomic radius Trends for atomic radius
Trends for atomic radius Atomic size vs atomic radius
Atomic size vs atomic radius Reactivity group 1
Reactivity group 1 Alkali metals colour
Alkali metals colour Whats an ionic bond
Whats an ionic bond Order of mobility of alkali metal ions
Order of mobility of alkali metal ions Group 7 elements properties
Group 7 elements properties Whats atomic radius
Whats atomic radius What is successive ionisation energy
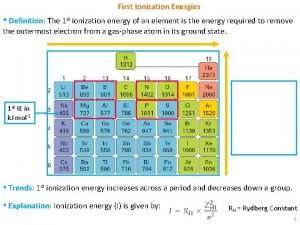
What is successive ionisation energy First ionization energy of calcium
First ionization energy of calcium Ionization energy
Ionization energy Decreasing metallic character
Decreasing metallic character Chapter 6 the periodic table and periodic law
Chapter 6 the periodic table and periodic law Largest atomic radius
Largest atomic radius