Ionization Energy Ionization energy is the amount of







- Slides: 15

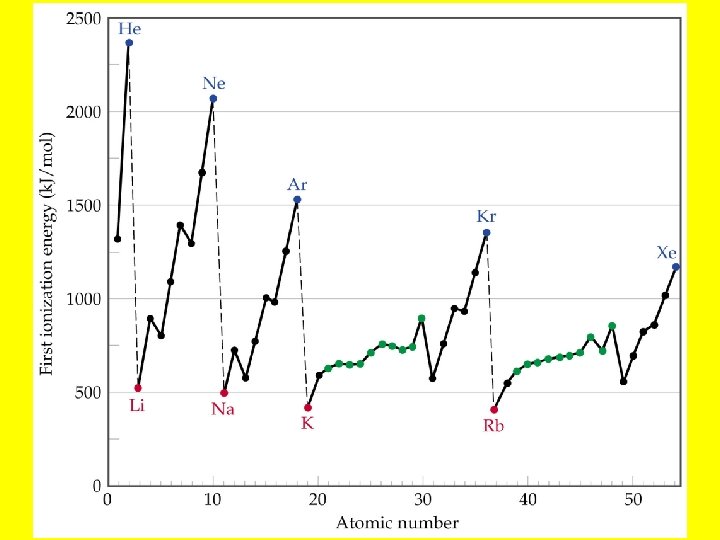
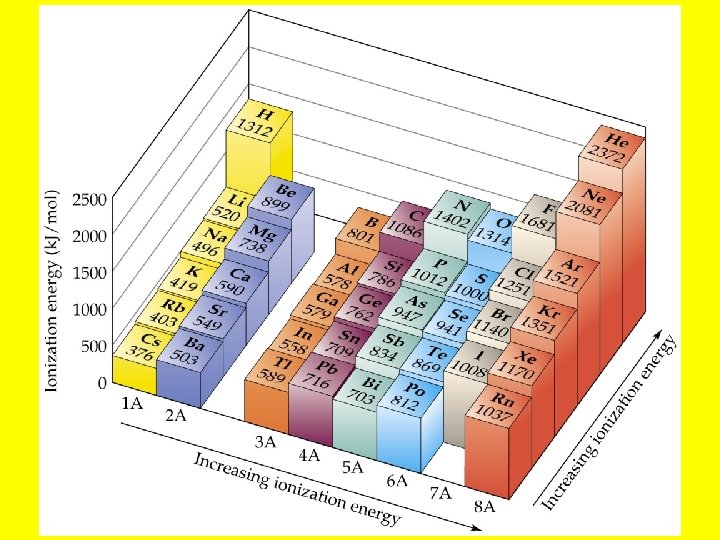
Ionization Energy • Ionization energy, is the amount of energy required to remove an electron from a gaseous atom: Na(g) Na+(g) + e-. • The larger ionization energy, the more difficult it is to remove the electron.

Periodic Trends in Ionization Energies • Ionization energy decreases down a group. • This means that the outermost electron is more readily removed as we go down a group. • As the atom gets bigger, it becomes easier to remove an electron from the most spatially extended orbital. • Ionization energy generally increases across a period. • As we move across a period, the number of protons increases. Therefore, it becomes more difficult to remove an electron.



Examples – Put each set in order of increasing first ionization energy: 1. P, Cl, Al, Na, S, Mg 2. Ca, Be, Ba, Mg, Sr 3. Ca, F, As, Rb, O, K, S, Ga

Examples – Put each set in order of increasing first ionization energy: 1. P, Cl, Al, Na, S, Mg 2. Ca, Be, Ba, Mg, Sr 3. Ca, F, As, Rb, O, K, S, Ga 4. 1. Na < Mg < Al < P < S < Cl 5. 2. Ba < Sr < Ca < Mg < Be 6. 3. Rb < K < Ca < Ga < As < S < O < F

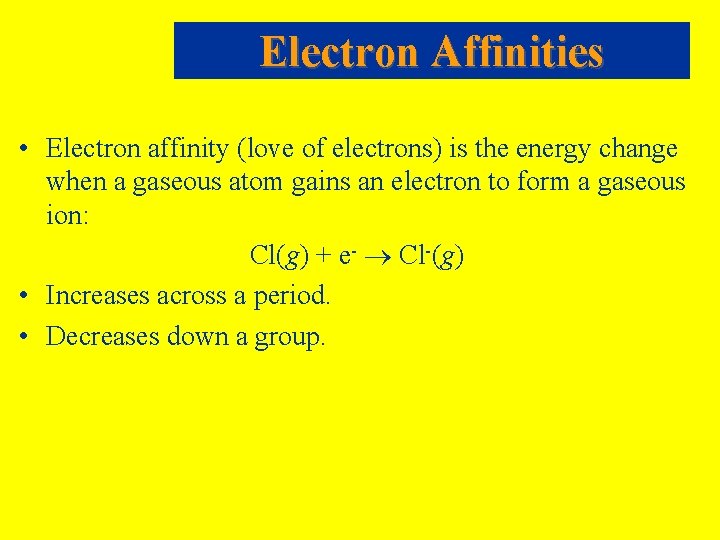
Electron Affinities • Electron affinity (love of electrons) is the energy change when a gaseous atom gains an electron to form a gaseous ion: Cl(g) + e- Cl-(g) • Increases across a period. • Decreases down a group.

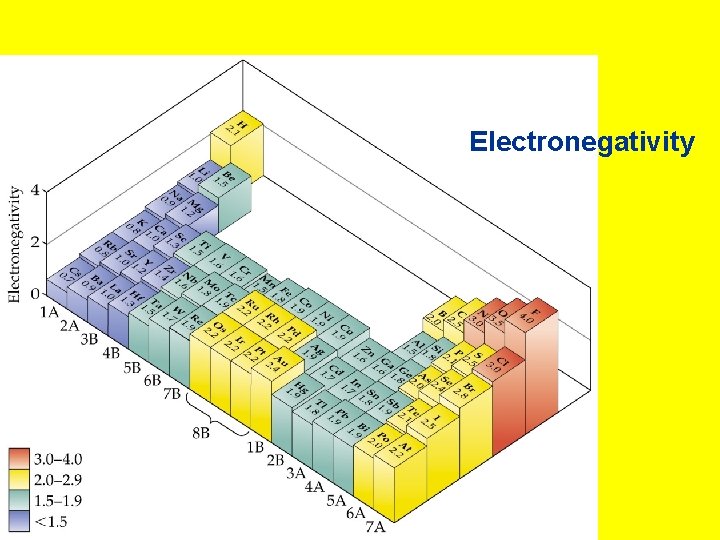
• Electronegativity: The ability of an atom in a molecule to attract electrons to itself. • Pauling set electronegativities on a scale from 0. 7 (Cs) to 4. 0 (F). Values are calculated from ionization energies and electron affinities. • Electronegativity increases • across a period and • up a group.

Electronegativity

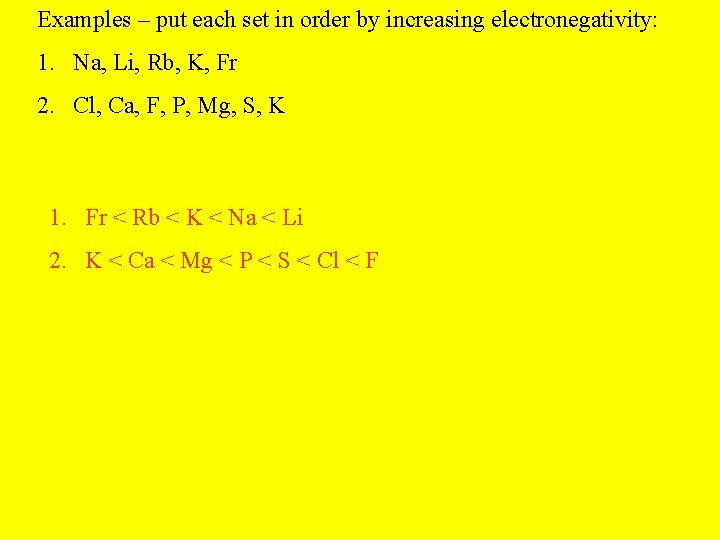
Examples – put each set in order by increasing electronegativity: 1. Na, Li, Rb, K, Fr 2. Cl, Ca, F, P, Mg, S, K

Examples – put each set in order by increasing electronegativity: 1. Na, Li, Rb, K, Fr 2. Cl, Ca, F, P, Mg, S, K 1. Fr < Rb < K < Na < Li 2. K < Ca < Mg < P < S < Cl < F

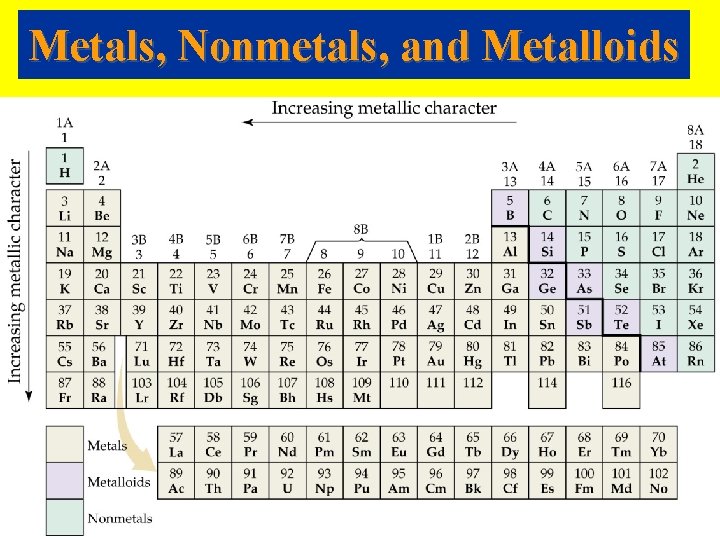
Metals, Nonmetals, and Metalloids

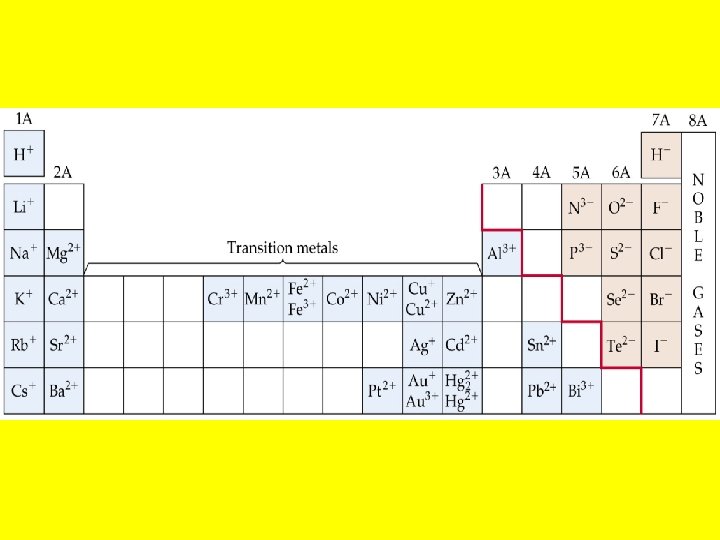
• • • Metals Metallic character refers to the properties of metals (shiny or lustrous, malleable and ductile, oxides form basic ionic solids, and tend to form cations in aqueous solution). Metallic character increases down a group. Metallic character decreases across a period. Metals have low ionization energies. Metals form positive ions.


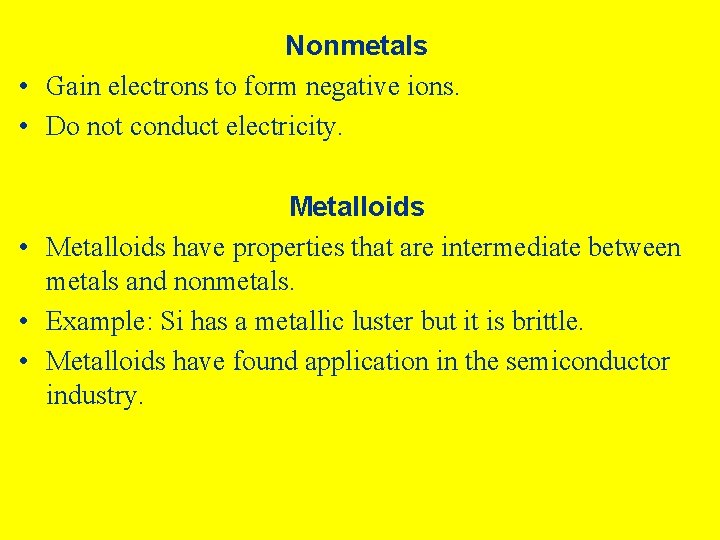
Nonmetals • Gain electrons to form negative ions. • Do not conduct electricity. Metalloids • Metalloids have properties that are intermediate between metals and nonmetals. • Example: Si has a metallic luster but it is brittle. • Metalloids have found application in the semiconductor industry.