MS Interpretation II Fragmentation Ionization OE Electron Ionization


- Slides: 32

MS Interpretation II Fragmentation

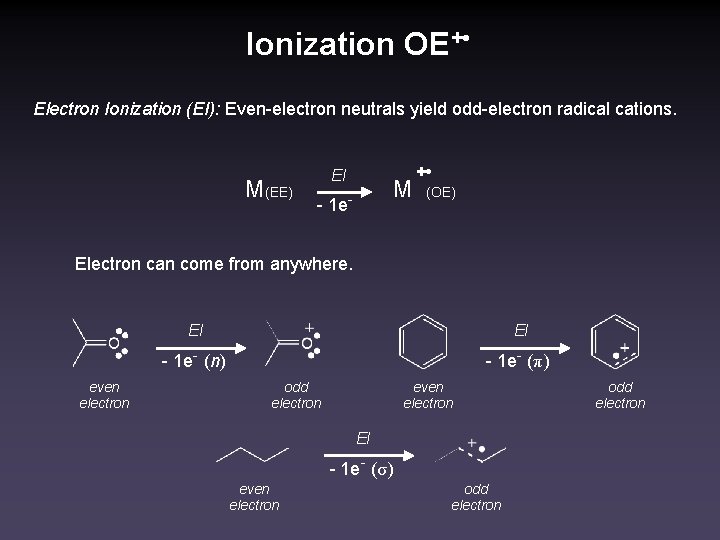
Ionization OE+ • Electron Ionization (EI): Even-electron neutrals yield odd-electron radical cations. M(EE) EI - 1 e- M + • (OE) Electron can come from anywhere. EI EI - 1 e- (n) even electron - 1 e- (π) odd electron even electron EI - 1 e- (σ) even electron odd electron

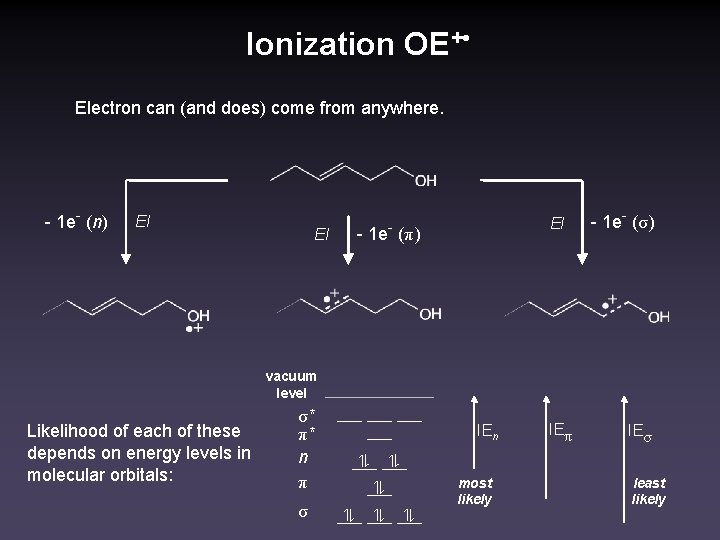
Ionization OE+ • Electron can (and does) come from anywhere. - 1 e- (n) EI EI EI - 1 e- (π) - 1 e- (σ) vacuum level Likelihood of each of these depends on energy levels in molecular orbitals: σ* π* n π σ IEn most likely IEπ IEσ least likely

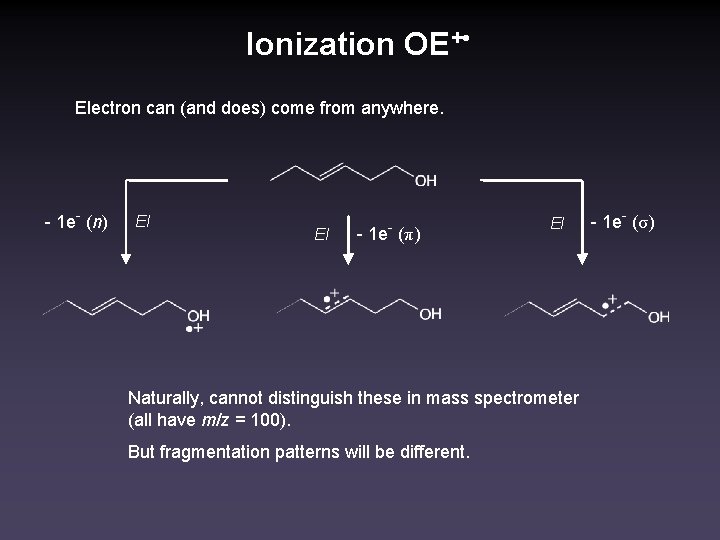
Ionization OE+ • Electron can (and does) come from anywhere. - 1 e- (n) EI EI - 1 e- (π) EI Naturally, cannot distinguish these in mass spectrometer (all have m/z = 100). But fragmentation patterns will be different. - 1 e- (σ)

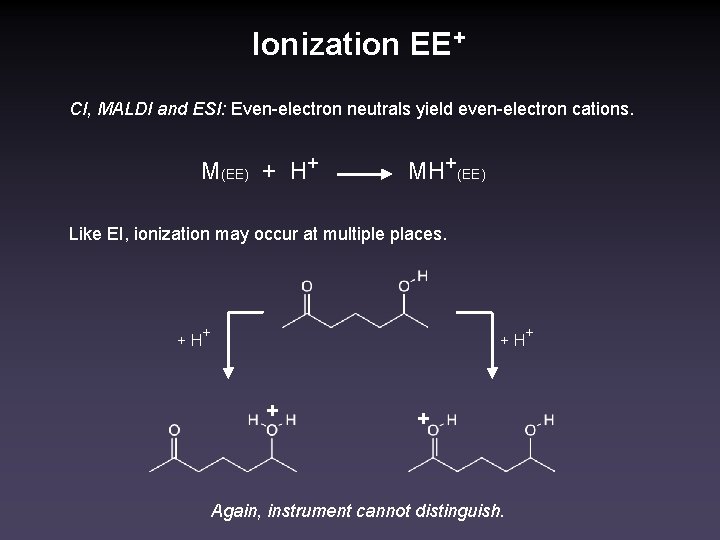
Ionization EE+ CI, MALDI and ESI: Even-electron neutrals yield even-electron cations. M(EE) + H+ MH+(EE) Like EI, ionization may occur at multiple places. + H+ + + Again, instrument cannot distinguish.

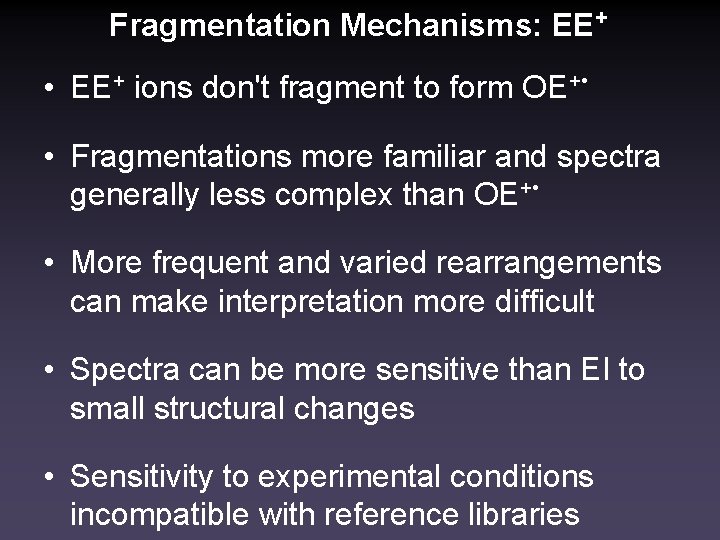
Fragmentation Mechanisms: EE+ • EE+ ions don't fragment to form OE+ • • Fragmentations more familiar and spectra generally less complex than OE+ • • More frequent and varied rearrangements can make interpretation more difficult • Spectra can be more sensitive than EI to small structural changes • Sensitivity to experimental conditions incompatible with reference libraries

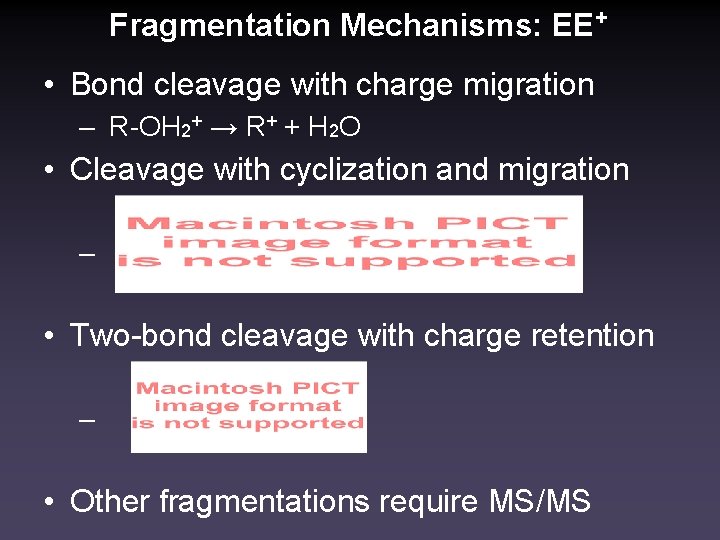
Fragmentation Mechanisms: EE+ • Bond cleavage with charge migration – R-OH 2+ → R+ + H 2 O • Cleavage with cyclization and migration – • Two-bond cleavage with charge retention – • Other fragmentations require MS/MS

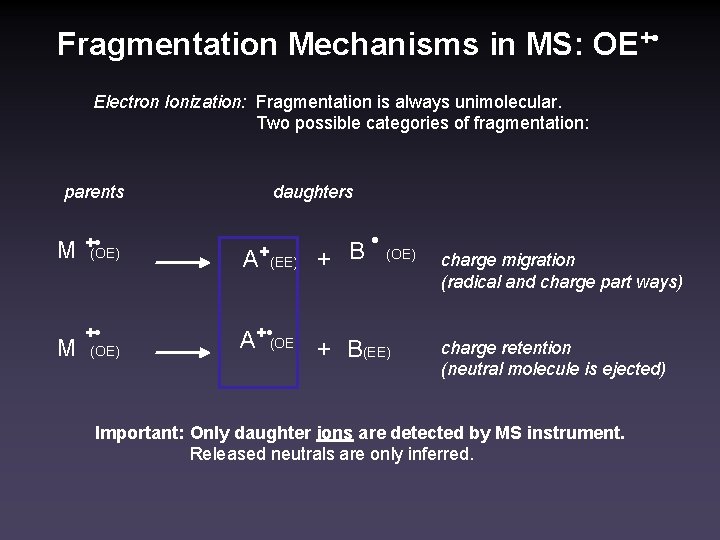
Fragmentation Mechanisms in MS: OE+ • Electron Ionization: Fragmentation is always unimolecular. Two possible categories of fragmentation: parents M M daughters • (OE) B + + • A+(EE) + • A+ • (OE) + B(EE) (OE) charge migration (radical and charge part ways) charge retention (neutral molecule is ejected) Important: Only daughter ions are detected by MS instrument. Released neutrals are only inferred.

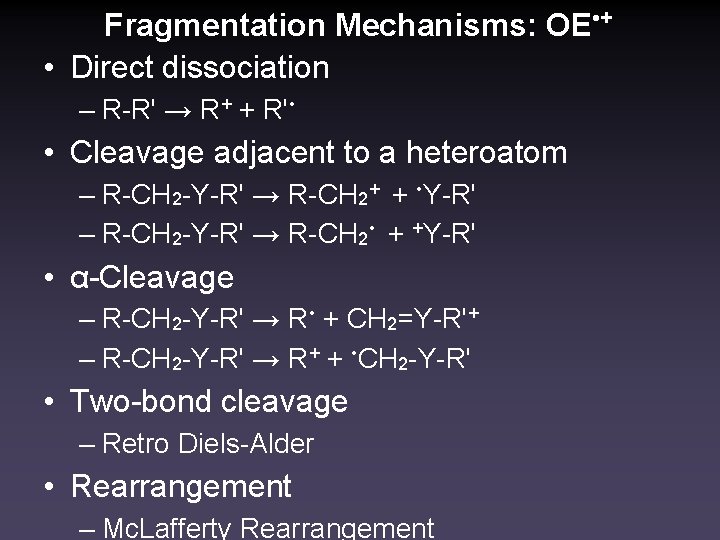
Fragmentation Mechanisms: OE • + • Direct dissociation – R-R' → R+ + R' • • Cleavage adjacent to a heteroatom – R-CH 2 -Y-R' → R-CH 2+ + • Y-R' – R-CH 2 -Y-R' → R-CH 2 • + +Y-R' • α-Cleavage – R-CH 2 -Y-R' → R • + CH 2=Y-R'+ – R-CH 2 -Y-R' → R+ + • CH 2 -Y-R' • Two-bond cleavage – Retro Diels-Alder • Rearrangement – Mc. Lafferty Rearrangement

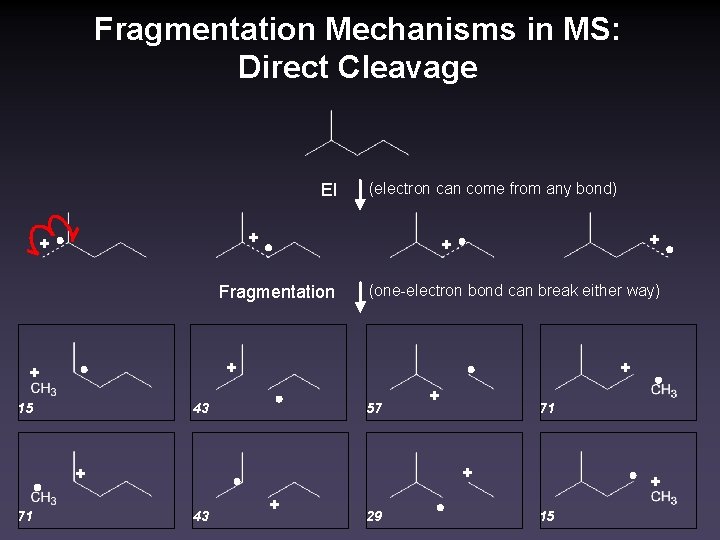
Fragmentation Mechanisms in MS: Direct Cleavage EI (electron can come from any bond) + + Fragmentation (one-electron bond can break either way) + + + 15 43 57 + 71 + + 43 + 29 + 15

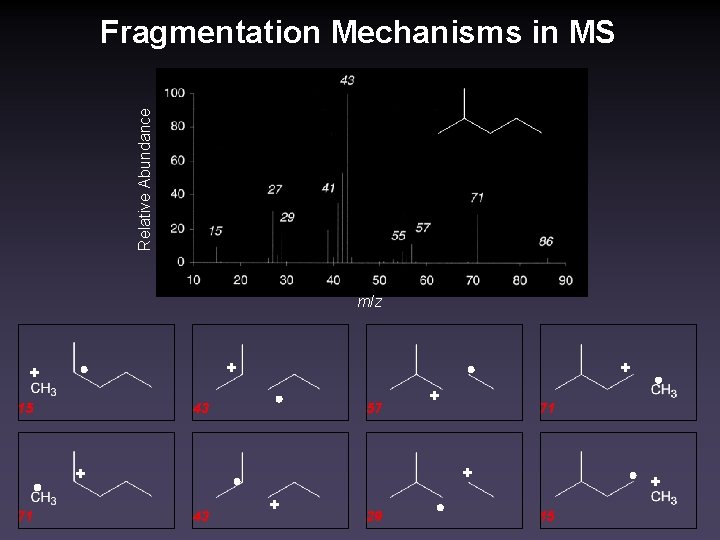
Relative Abundance Fragmentation Mechanisms in MS m/z + + + 15 43 57 71 + + 71 + 43 + 29 + 15

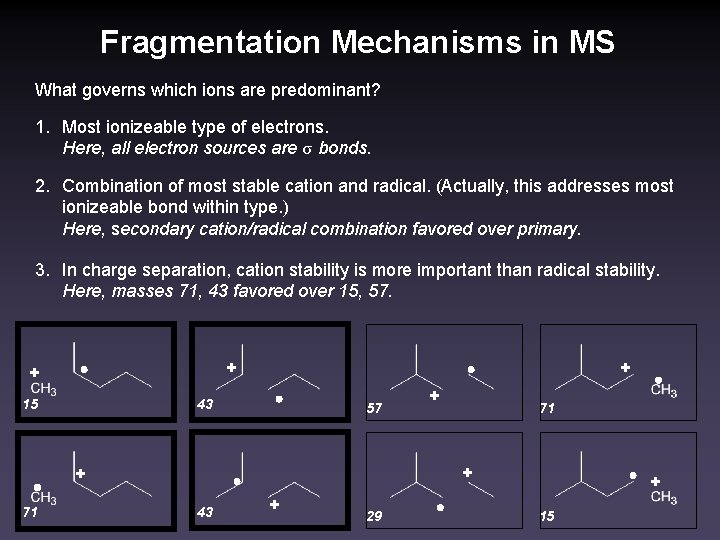
Fragmentation Mechanisms in MS What governs which ions are predominant? 1. Most ionizeable type of electrons. Here, all electron sources are σ bonds. 2. Combination of most stable cation and radical. (Actually, this addresses most ionizeable bond within type. ) Here, secondary cation/radical combination favored over primary. 3. In charge separation, cation stability is more important than radical stability. Here, masses 71, 43 favored over 15, 57. + + + 15 43 57 71 + + 71 + 43 + 29 + 15

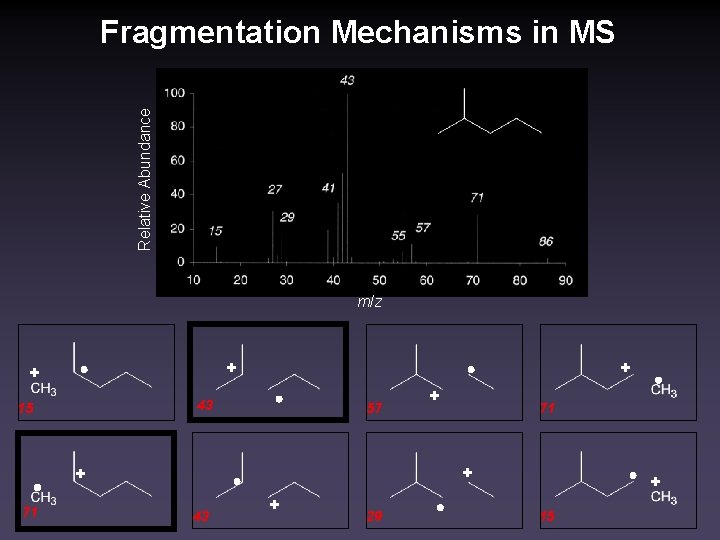
Relative Abundance Fragmentation Mechanisms in MS m/z + + + 43 15 57 71 + + 71 + 43 + 29 + 15

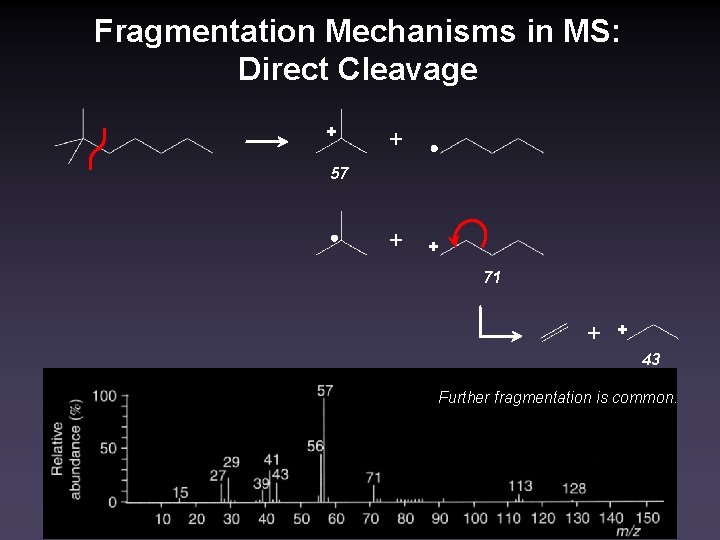
Fragmentation Mechanisms in MS: Direct Cleavage + + 57 + + 71 + + 43 Further fragmentation is common.

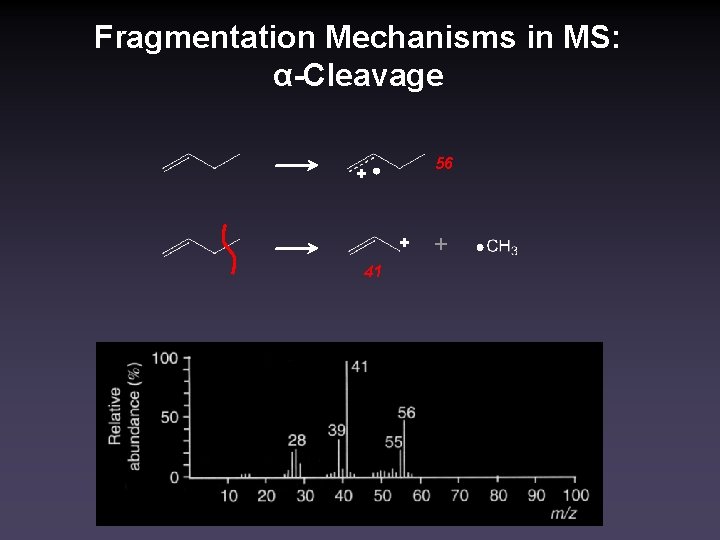
Fragmentation Mechanisms in MS: α-Cleavage 56 + + 41 +

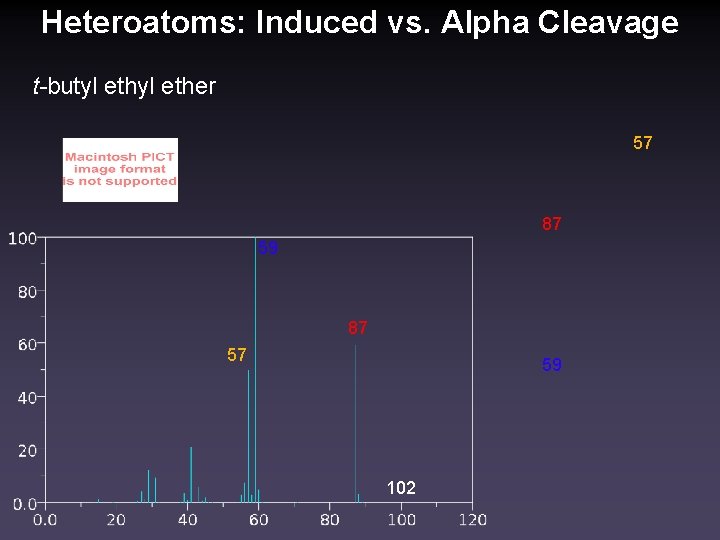
Heteroatoms: Induced vs. Alpha Cleavage t-butyl ether 57 87 59 87 57 59 102

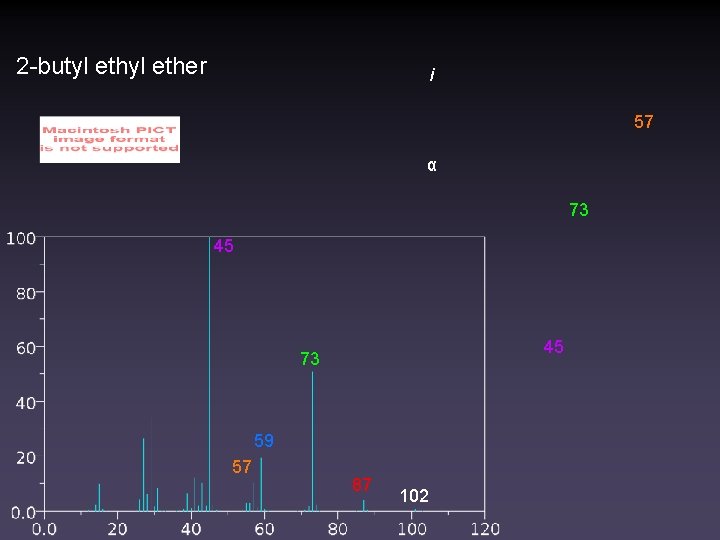
2 -butyl ether i 57 α 73 45 45 73 59 57 87 102

Competition: i vs α Cleavage • Induced cleavage is favored for larger heteroatoms • α-cleavage is favored for electron donating substituents • Br, Cl < R • , π bond, S, O < N

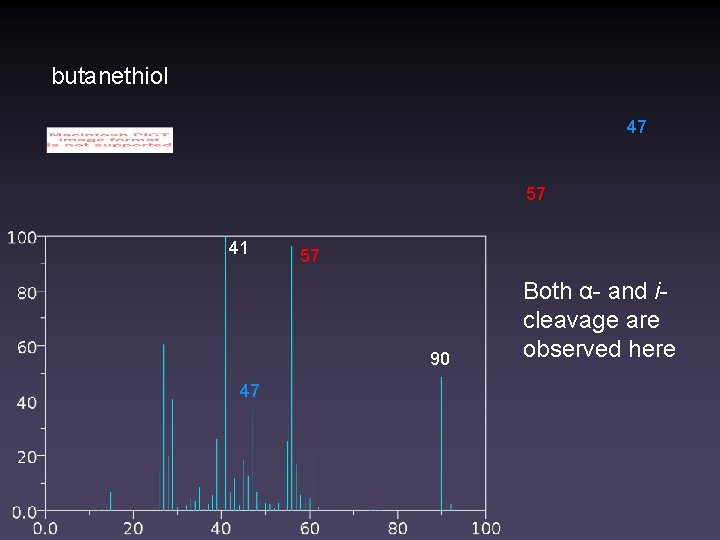
butanethiol 47 57 41 57 90 47 Both α- and icleavage are observed here

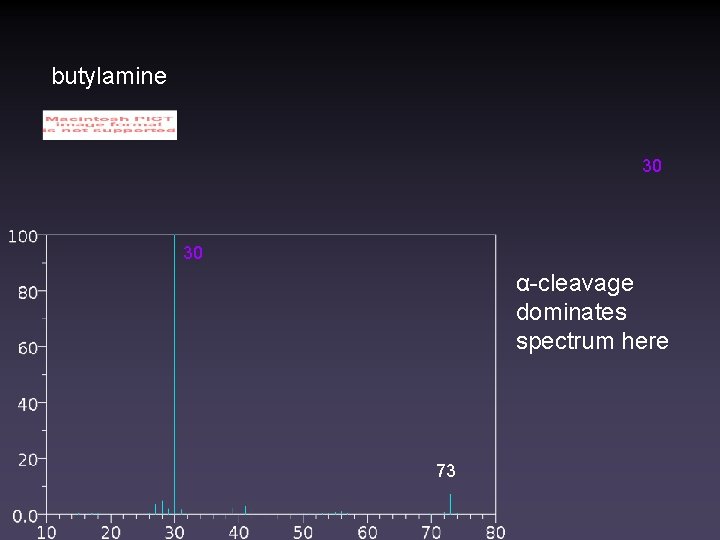
butylamine 30 30 α-cleavage dominates spectrum here 73

OE+ • Fragments • Odd electron fragments are less common, especially at low mass – Even mass ions at low mass likely contain N • These fragments arise from two-bond cleavages, or rearrangements • Even mass OE+ • fragments are important ions, as they can be highly sensitive to structural changes

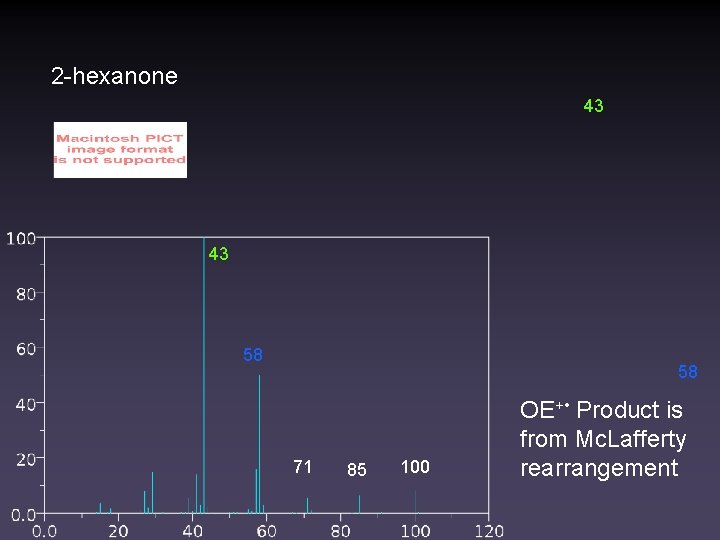
2 -hexanone 43 43 58 58 71 85 100 OE+ • Product is from Mc. Lafferty rearrangement

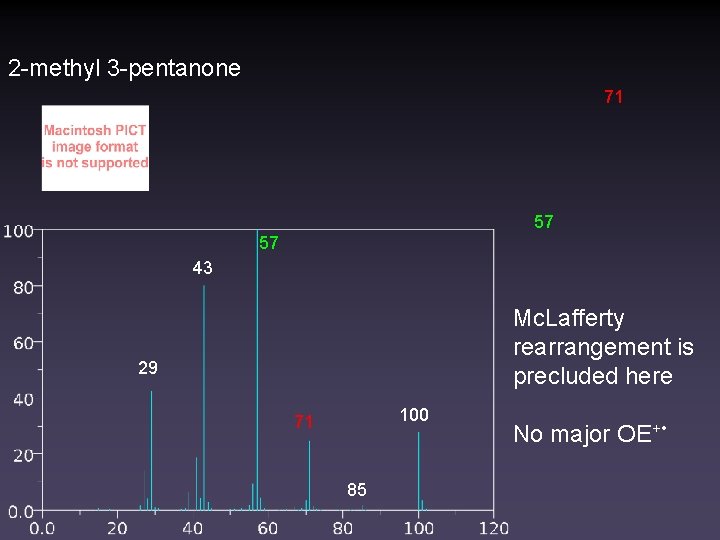
2 -methyl 3 -pentanone 71 57 57 43 Mc. Lafferty rearrangement is precluded here 29 100 71 85 No major OE+ •

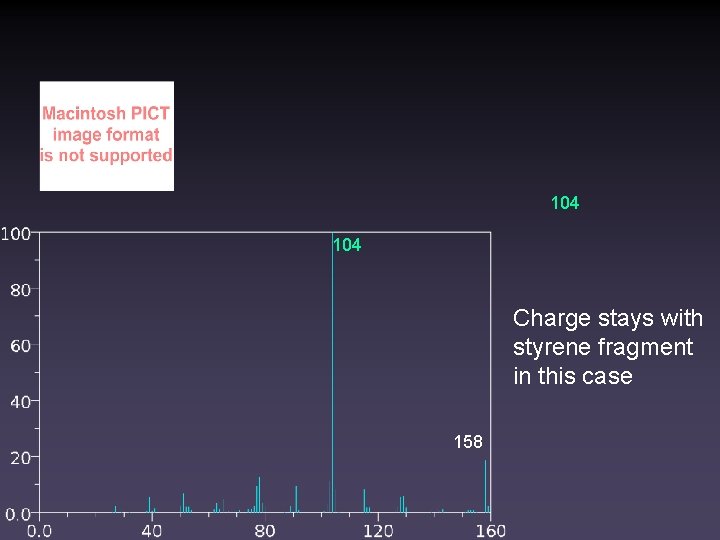
104 Charge stays with styrene fragment in this case 158

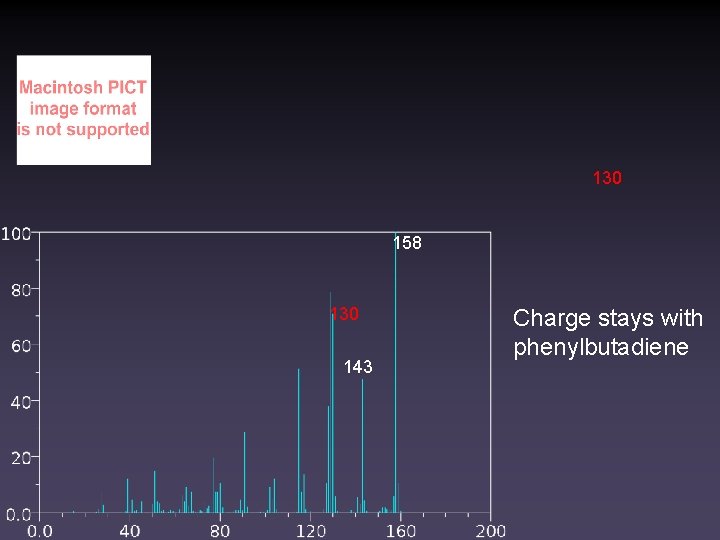
130 158 130 143 Charge stays with phenylbutadiene

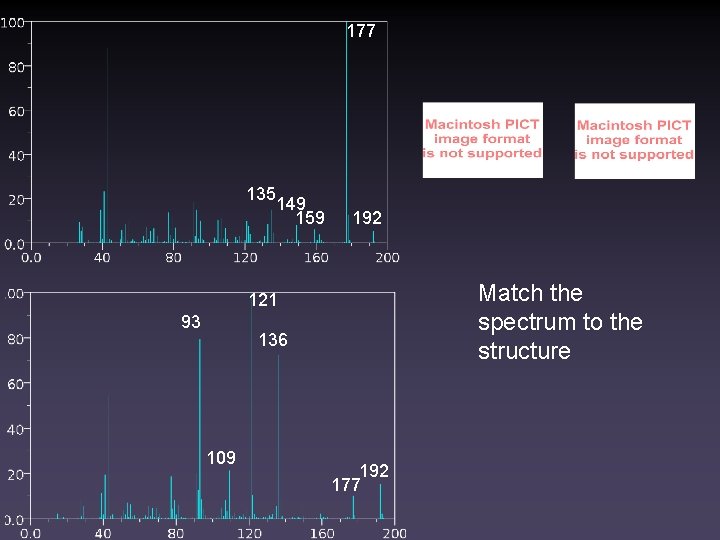
177 135 149 159 192 Match the spectrum to the structure 121 93 136 109 192 177

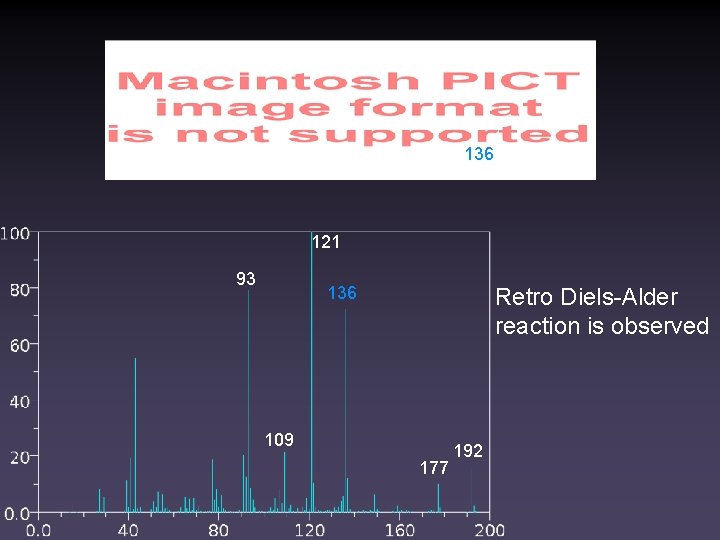
136 121 93 136 Retro Diels-Alder reaction is observed 109 177 192

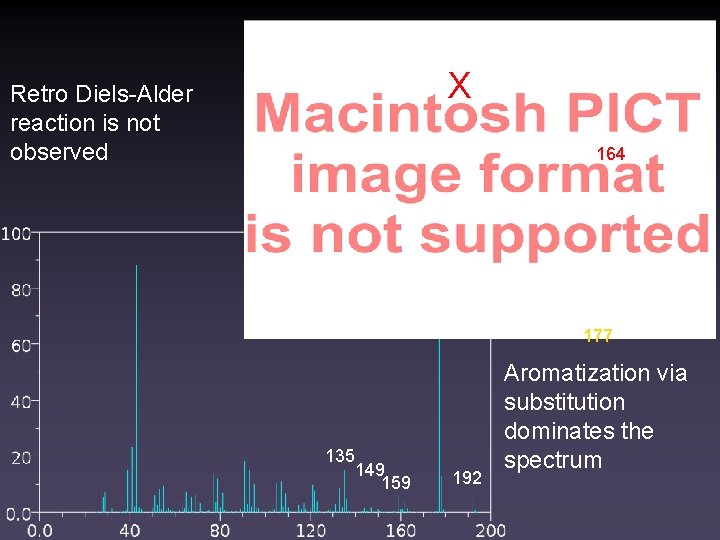
X Retro Diels-Alder reaction is not observed 164 177 135 149 159 192 Aromatization via substitution dominates the spectrum

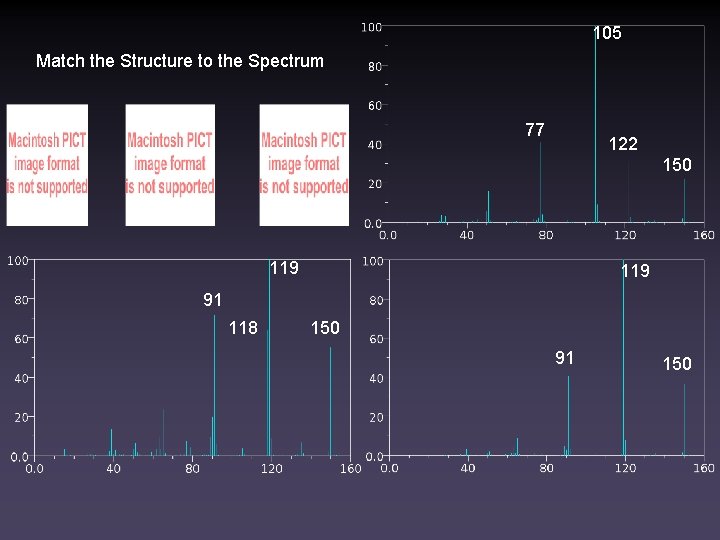
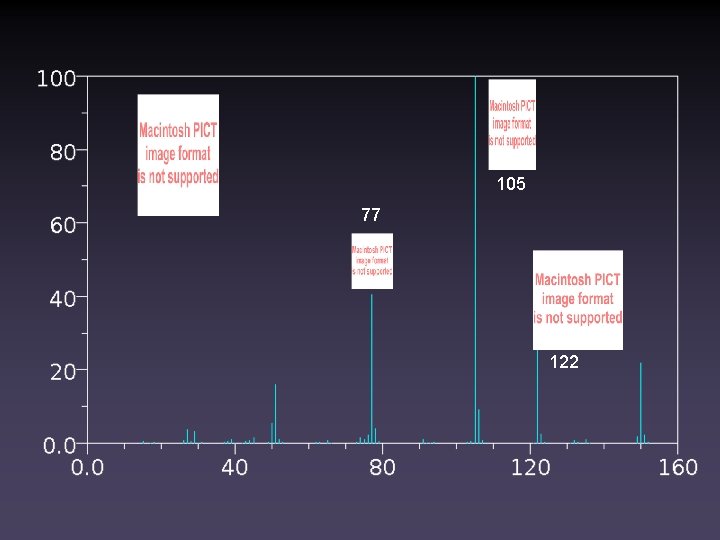
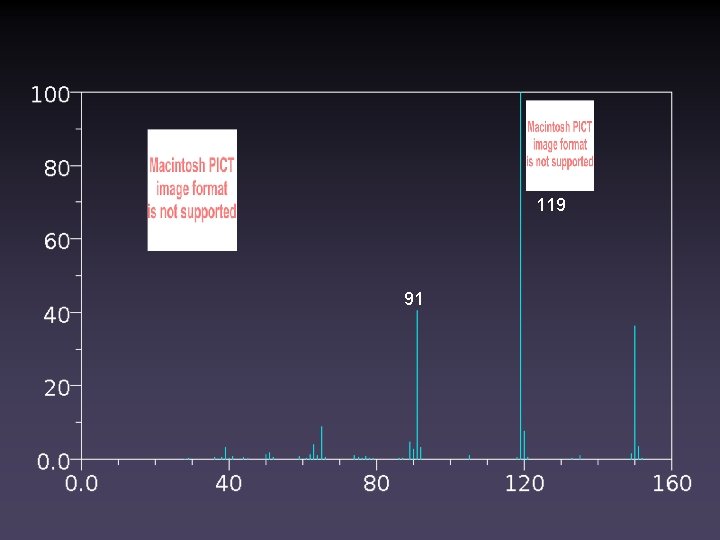
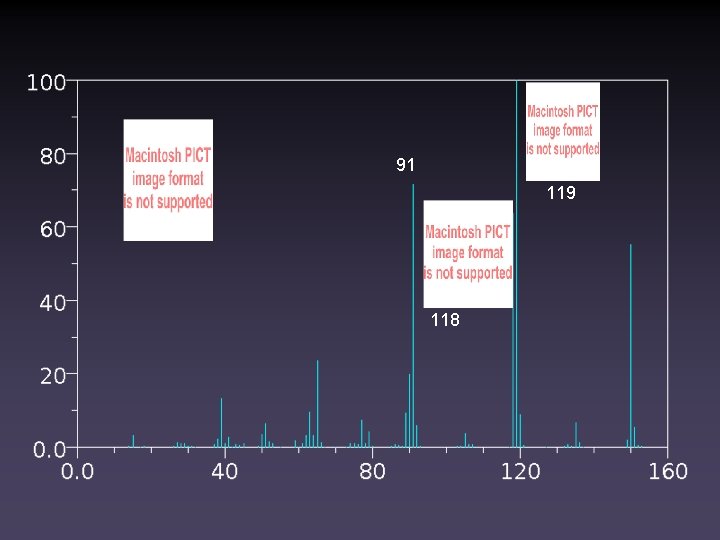
105 Match the Structure to the Spectrum 77 122 150 119 91 118 150 91 150

105 77 122

119 91

91 119 118