Group 1 Alkali Metals Contains Metals l Valence









- Slides: 9

Group 1: Alkali Metals Contains: Metals l Valence Electrons: 1 l Reactivity: Very Reactive l Properties: l l solids l soft l react violently with water l shiny l low density

Group 2: Alkaline-Earth Metals Contains: Metals l Valence Electrons: 2 l Reactivity: very reactive but less reactive than alkali metals (Group 1) l Properties: l l Solids l Silver colored l More dense than alkali metals

Group 13: Boron Group l Contains: 1 metalloid and 4 metals l Valence Electrons: 3 l Reactivity: Reactive l Other shared properties: l l Solid at room temperature

Group 14: Carbon Group Contains: 1 non-metal, 2 metalloids, and 3 metals l Valence Electrons: 4 l Reactivity: Varies l Other shared properties: l l Solid at room temperature

Group 15: Nitrogen Group Contains: 2 non-metals, 2 metalloids, and 1 metal l Valence electrons: 5 l Reactivity: Varies l Other shared properties: l l All but N are solid at room temperature

Group 16: Oxygen Group Contains: 3 non-metals, 1 metalloid, and 2 metals l Valence Electrons: 6 l Reactivity: Reactive l Other shared properties: l l All but O are solid at room temperature.

Groups 17 : Halogens Contain: Nonmetals l Valence Electrons: 7 l Reactivity: Very reactive l Other shared properties l ● ● ● Poor conductors of electric current React violently with alkali metals to form salts Never found uncombined in nature

Group 18 Noble Gases Contains: Nonmetals l Valence Electrons: 8 (2 for He) l Reactivity: Unreactive (least reactive group) l Other shared properties: l l Colorless, odorless gases at room temperature l Outermost energy level full l All found in atmosphere

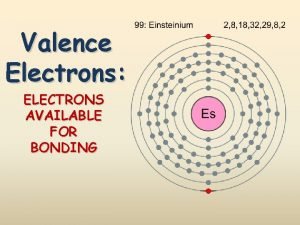
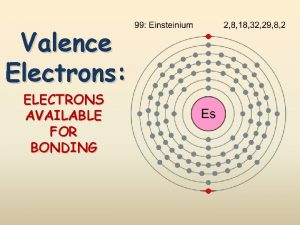
Hydrogen Stands Apart H set apart because its properties don’t match any single group. l Valence electrons: 1 l Reactivity: very but loses the 1 electron easily l Properties: l l Similar metals to those of non-metals rather than
 Alkali earth metals properties
Alkali earth metals properties Which region contains the halogen family of elements?
Which region contains the halogen family of elements? Which substance contains free-floating valence electrons?
Which substance contains free-floating valence electrons? Valence electron
Valence electron Group with 6 valence electrons
Group with 6 valence electrons Whats an alkali metal
Whats an alkali metal Group 16 period 6
Group 16 period 6 Reactivity alkali metals
Reactivity alkali metals Periodic table element families
Periodic table element families Are halogens representative elements
Are halogens representative elements