Fblock elements Lanthanides and Actinides Dr U B

F-block elements Lanthanides and Actinides Dr U B Kosurkar Assistant Professor Email id: - ukosurkar@gmail. com
Introduction The elements constituting the f block are those in which the 4 f and 5 f orbitally progressively filled. These elements are the member of group 3. The f block elements are also termed as inner transition elements. It is because literally speaking they constitute transition series within transition series (d-block elements). In addition to incomplete d-subshell, their f-subshell is also incomplete. There are seven f-orbitals in a given shell. As much as fourteen electrons can be occupied in a given f block series. The general electronic configuration of the f block elements is (n– 2)f 1 -14 (n– 1)d 0 -1 ns 2
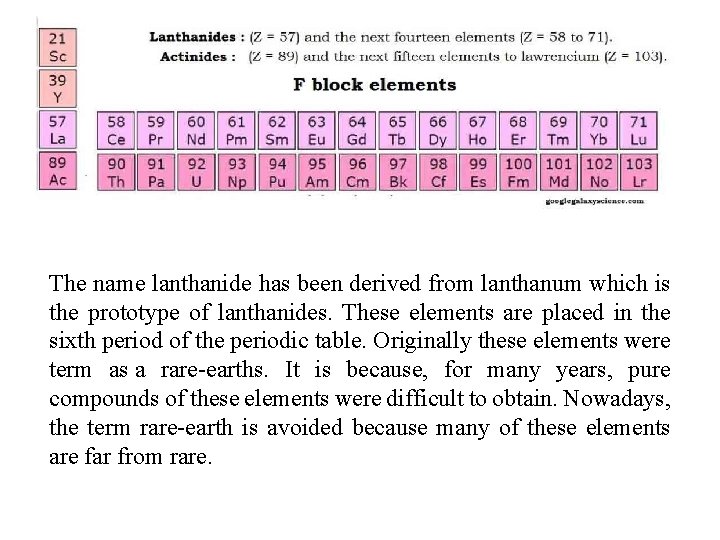
The name lanthanide has been derived from lanthanum which is the prototype of lanthanides. These elements are placed in the sixth period of the periodic table. Originally these elements were term as a rare-earths. It is because, for many years, pure compounds of these elements were difficult to obtain. Nowadays, the term rare-earth is avoided because many of these elements are far from rare.
5 f Block elements, Second inner transition series, Actinides or Actinones In these elements differentiating electron goes to 5 f orbitals. This series includes fifteen elements from actinium (Z = 89) to lawrencium (Z = 103). The name actinide is derived from actinium, the very first member of the series. Why f-block elements placed outside separately: - It is interesting to note that the f block elements (lanthanides and actinides) are placed outside the body of the periodic table. The reason for this is the remarkable similarities among the chemical properties of the lanthanides and also among the various members of actinides. The similarities in properties, in turn, is due to the similar electronic configuration of the outermost shell. These elements differ only in the number of f-electrons which do not take part in chemical bonding (difference from d-block elements in which differentiating d-electrons are involved in chemical interaction).
Characteristics of Lanthanides Although La 57 and the following elements up to Lu 71 resemble each other in their properties, lanthanum itself is studied in d-block elements and not f block. It is because here f-orbital has no electron. The f-orbital actually starts filling at cerium Ce (Z = 58) and is completely filled at lutetium, Lu (Z = 71) and hence elements from cerium (Z = 58) to Lu (Z = 71) are actually grouped as 4 f block elements. Further, since these fourteen elements follow lanthanum, these are also termed as lanthanides or lanthanones. Electronic configuration: The electronic configuration represented as: [Xe] 4 f n+1 5 d° 6 s 2 or [Xe] 4 fn 5 d 1 6 s 2 The valence shell electronic configuration is 4 f 1− 14 6 s 2 Oxidation states: The lanthanides too display variable oxidation states but much less than those displayed by the transition elements. The characteristic and the most stable oxidation state of the lanthanides is +3 (Ln 3+).
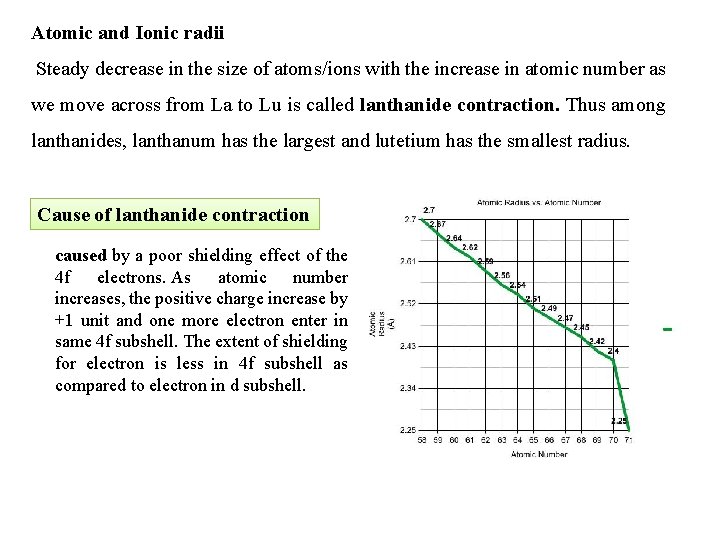
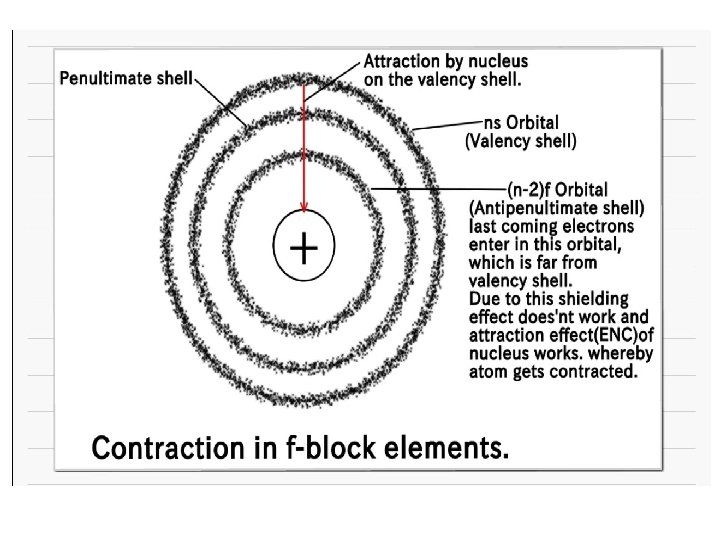
Atomic and Ionic radii Steady decrease in the size of atoms/ions with the increase in atomic number as we move across from La to Lu is called lanthanide contraction. Thus among lanthanides, lanthanum has the largest and lutetium has the smallest radius. Cause of lanthanide contraction caused by a poor shielding effect of the 4 f electrons. As atomic number increases, the positive charge increase by +1 unit and one more electron enter in same 4 f subshell. The extent of shielding for electron is less in 4 f subshell as compared to electron in d subshell.

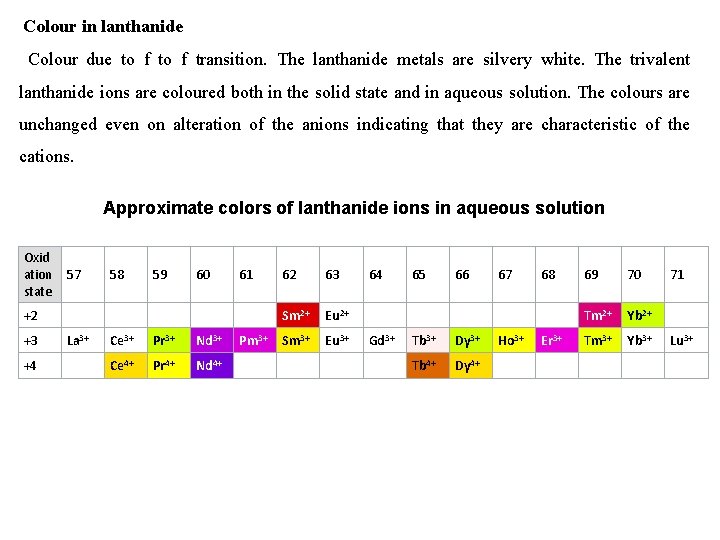
Colour in lanthanide Colour due to f transition. The lanthanide metals are silvery white. The trivalent lanthanide ions are coloured both in the solid state and in aqueous solution. The colours are unchanged even on alteration of the anions indicating that they are characteristic of the cations. Approximate colors of lanthanide ions in aqueous solution Oxid ation 57 state 58 59 60 61 +2 +3 +4 La 3+ Ce 3+ Pr 3+ Nd 3+ Ce 4+ Pr 4+ Nd 4+ Pm 3+ 62 63 Sm 2+ Eu 2+ Sm 3+ Eu 3+ 64 Gd 3+ 65 66 Tb 3+ Dy 3+ Tb 4+ Dy 4+ 67 Ho 3+ 68 Er 3+ 69 70 Tm 2+ Yb 2+ Tm 3+ Yb 3+ 71 Lu 3+

Magnetic Properties La 3+ (4 f 0) and Lu 3+ (4 f 14), having no unpaired electron; these do not show paramagnetism while all other tri positive ions of lanthanides are paramagnetic. Complexes Although the lanthanide ions have a high charge (+3), their large size (0. 85 – 1: 03 Angstrom) imparts them low charges density (charge to size ratio) with the result they cannot cause much polarisation and hence do not have much tendency to form complexes. Their complexes with unidentate ligands like β-diketones, oximes and ethylene diamine tetra-acetate (EDTA) are fairly common.
Occurrence and separation of Lanthanides Occurrence Cerium is the 26 th most abundant element in the Earth's crust, neodymium is more abundant than gold and even thulium (the least common naturally occurring lanthanide) is more abundant than iodine. Cerium earth/light earth - At. No. 57 to 63 Ytterium earth/heavy earth - At No. 64 to 71 Lanthanide minerals - Monozite, Bastaesite, Euxenite, Gadolinite, Xenotime These minerals found in Sweden, Norway, Brazil, Astralia, Texas, florida, canada 1) Ion-Exchange Ln 3+(aq) are strongly adsorbed by a cation-exchange resin Chelating ligand- EDTA

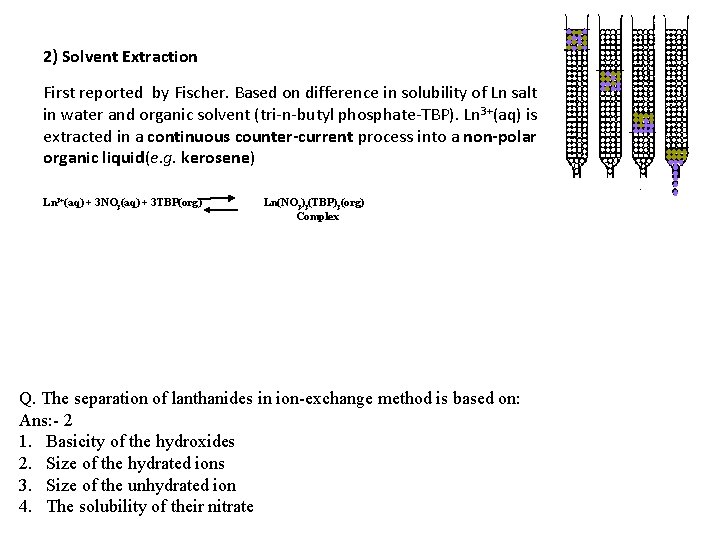
2) Solvent Extraction First reported by Fischer. Based on difference in solubility of Ln salt in water and organic solvent (tri-n-butyl phosphate-TBP). Ln 3+(aq) is extracted in a continuous counter-current process into a non-polar organic liquid(e. g. kerosene) Ln 3+(aq) + 3 NO 3(aq) + 3 TBP(org) Ln(NO 3)3(TBP)3(org) Complex Q. The separation of lanthanides in ion-exchange method is based on: Ans: - 2 1. Basicity of the hydroxides 2. Size of the hydrated ions 3. Size of the unhydrated ion 4. The solubility of their nitrate

Uses of Lanthanoids 1. Used for the production of alloy steels for plates and pipes. e. g. mischmetal which consists of lanthanoid metal (~95%) and iron (-5%) and traces of S, C, Ca and AI. Misch metal is used in Mg based alloy to produce bullets, shell and lighter int. 2. Mixed oxides of lanthanoids are employed as a catalyst in petroleum cracking 3. Some individual Ln oxides are used as phosphors in television screens and similar uorescing surfaces. 4. Because of their paramagnetic and ferromagnetic character, their compounds are used in making magnetic & electronic devices. 5. Ceric sulphate is an oxidising agent in volumetric analysis.
Characteristics of Actinoids (5 f-series): The actinoids include the fourteen elements from Th to Lr. The actinoids are radioactive elements and the earlier members have relatively long half lives, the latter ones have half life values ranging from a day to 3 minutes for lawrencium (Z=103). Electronic Configuration All the actinoids are believed to have the electronic configuration of 7 s 2 and variable occupancy of the 5 f and 6 d subshell. The fourteen electrons are formally added to 5 f, though not in thorium (Z= 90) from Pa onwards, the 5 f orbitals are complete at element 103. The irregularities in the electronic con guration of the actinoid, like those in the lanthanoids, are related to the stabilities of the f 0 , f 7 and f 14 occupancies of the 5 f orbitals. Thus the confi gurations of Am and Cm are (Rn) 5 f 7 s 2 and [Rn] 5 f 7 6 d 1 7 s 2 .
Atomic and Ionic radii The general trend in lanthanoids is observable in the actinoids as well. There is a gradual decrease 3+ in the size of atoms or M ions across the series. This may be referred to as the actinoids contraction (like lanthanoids contraction). The contraction is, however, greater from elements to an element in this series resulting from poor shielding by 5 f electrons. Oxidation states There is a greater range of oxidation states, which is in part attributed to the fact that the 5 f, 6 d and 7 s levels are of comparable energies. The actinoids show in general +3 oxidation state. The elements, in the rest half to the series frequently exhibit higher oxidation state. e. g. The maximum oxidation state increases from +4 in Th to +5, +6 and +7 respectively in Pa, U and Np but decreases in succeeding elements. The actinoids resemble the lanthanoids in having more compounds in +3 state than in the +4 state. However, +3 and +4 ions tend to hydrolyze.

Uses of Actinoids 1. Thorium is used in atomic reactors and in the treatment of cancer. Its salts are used in making incandescent gas mantles. 2. Uranium is used as a nuclear fuel. Its salts are used in glass industry (for imparting green colour), textile industry, ceramic industry and in medicines 3. Plutonium it is used as a fuel for atomic reactors as well as for making atomic bombs.
- Slides: 17