The Management of Alzheimers Disease and Related Dementias





![Correlation between CSF [Phospho-tau] and MMSE Decrease in MCI Annual MMSE Decrease 10 0 Correlation between CSF [Phospho-tau] and MMSE Decrease in MCI Annual MMSE Decrease 10 0](https://slidetodoc.com/presentation_image_h/bcfa456fd29dff1e3eddc2f55ff24c75/image-42.jpg)

- Slides: 46

The Management of Alzheimer’s Disease and Related Dementias The Treatment of Alzheimer’s Disease and Disease Progression Modification

Alzheimer’s Disease (AD): Overview n Progressive, degenerative CNS disorder n Characterized by memory impairment plus one or more additional cognitive disturbances n Gradual decline in three key symptom domains – Activities of daily living (ADL) – Behavior and personality – Cognition n Most common cause of dementia in people aged 65 and over

Alzheimer’s Disease: Economic Consequences n Third most expensive disease in the US n Costs over $100 billion/year n Further $33 billion in lost productivity and other employer costs n 3/4 of patients admitted to residential care within 5 years of diagnosis Evans DA, Scherr PA, Smith LA, et al. Aging (Milano). 1990(Sept); 2(3): 298 -302; Ernst RL, Hay JW. Am J Public Health. 1994(Aug); 84(8): 1261 -1264; Alzheimer’s Association, 2002

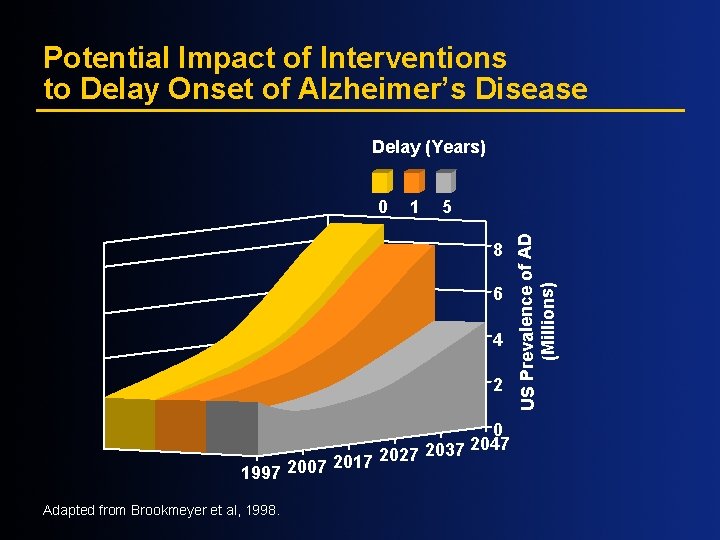
Potential Impact of Interventions to Delay Onset of Alzheimer’s Disease Delay (Years) 1 5 8 6 4 2 2017 2027 2007 1997 Adapted from Brookmeyer et al, 1998. 0 2047 2037 US Prevalence of AD (Millions) 0

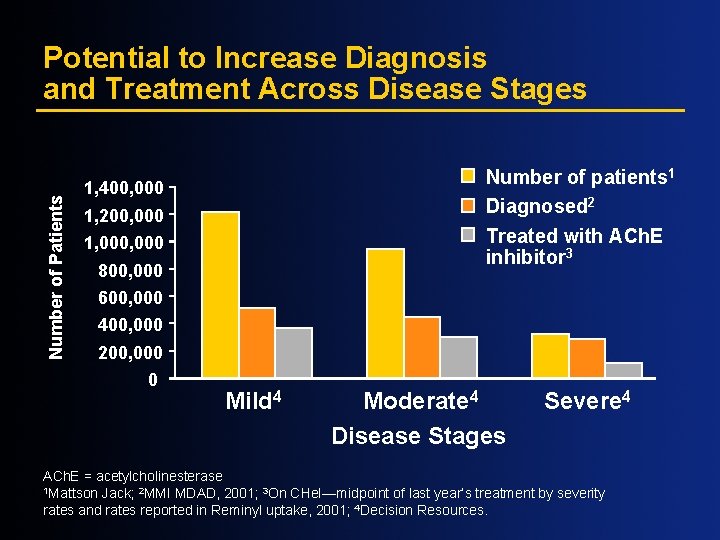
Number of Patients Potential to Increase Diagnosis and Treatment Across Disease Stages Number of patients 1 Diagnosed 2 Treated with ACh. E inhibitor 3 1, 400, 000 1, 200, 000 1, 000 800, 000 600, 000 400, 000 200, 000 0 Mild 4 Moderate 4 Disease Stages Severe 4 ACh. E = acetylcholinesterase 1 Mattson Jack; 2 MMI MDAD, 2001; 3 On CHe. I—midpoint of last year’s treatment by severity rates and rates reported in Reminyl uptake, 2001; 4 Decision Resources.

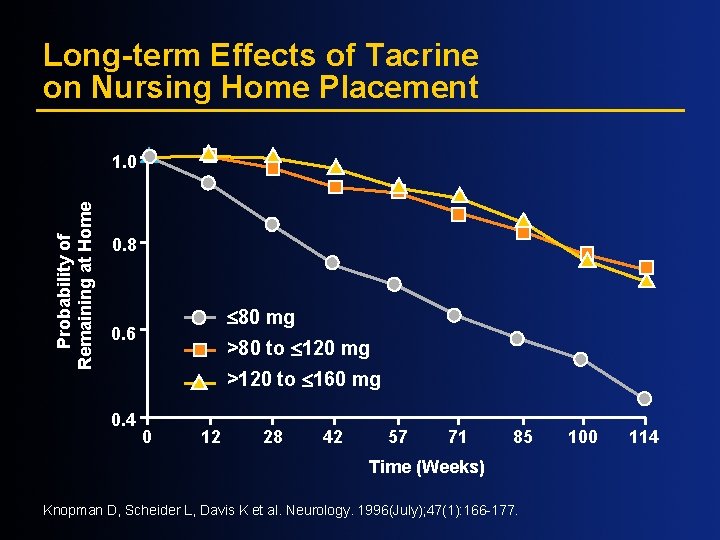
Long-term Effects of Tacrine on Nursing Home Placement Probability of Remaining at Home 1. 0 0. 8 £ 80 mg 0. 6 >80 to £ 120 mg >120 to £ 160 mg 0. 4 0 12 28 42 57 71 85 Time (Weeks) Knopman D, Scheider L, Davis K et al. Neurology. 1996(July); 47(1): 166 -177. 100 114

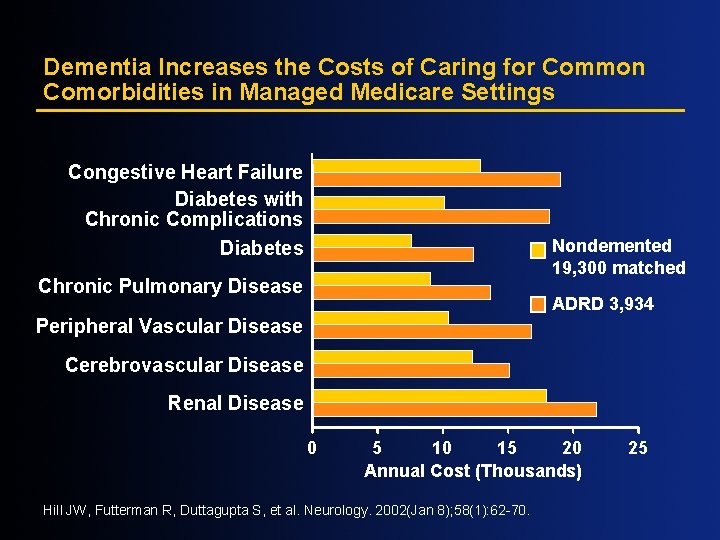
Dementia Increases the Costs of Caring for Common Comorbidities in Managed Medicare Settings Congestive Heart Failure Diabetes with Chronic Complications Diabetes Nondemented 19, 300 matched Chronic Pulmonary Disease ADRD 3, 934 Peripheral Vascular Disease Cerebrovascular Disease Renal Disease 0 5 10 15 20 Annual Cost (Thousands) Hill JW, Futterman R, Duttagupta S, et al. Neurology. 2002(Jan 8); 58(1): 62 -70. 25

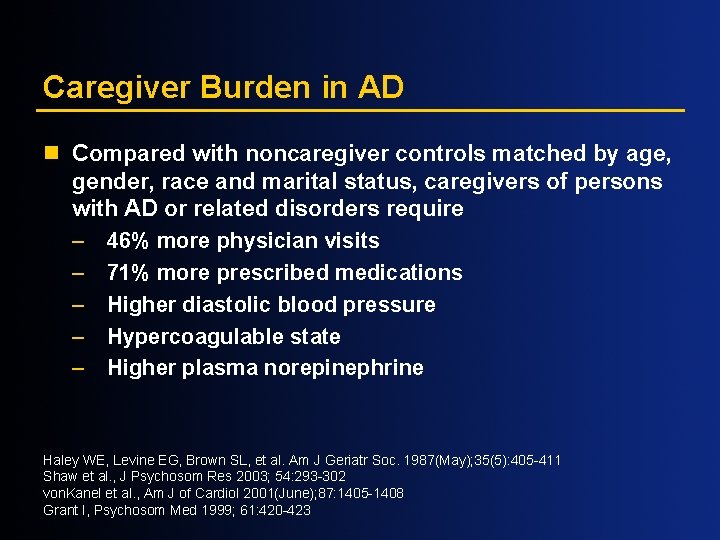
Caregiver Burden in AD n Compared with noncaregiver controls matched by age, gender, race and marital status, caregivers of persons with AD or related disorders require – 46% more physician visits – 71% more prescribed medications – Higher diastolic blood pressure – Hypercoagulable state – Higher plasma norepinephrine Haley WE, Levine EG, Brown SL, et al. Am J Geriatr Soc. 1987(May); 35(5): 405 -411 Shaw et al. , J Psychosom Res 2003; 54: 293 -302 von. Kanel et al. , Am J of Cardiol 2001(June); 87: 1405 -1408 Grant I, Psychosom Med 1999; 61: 420 -423

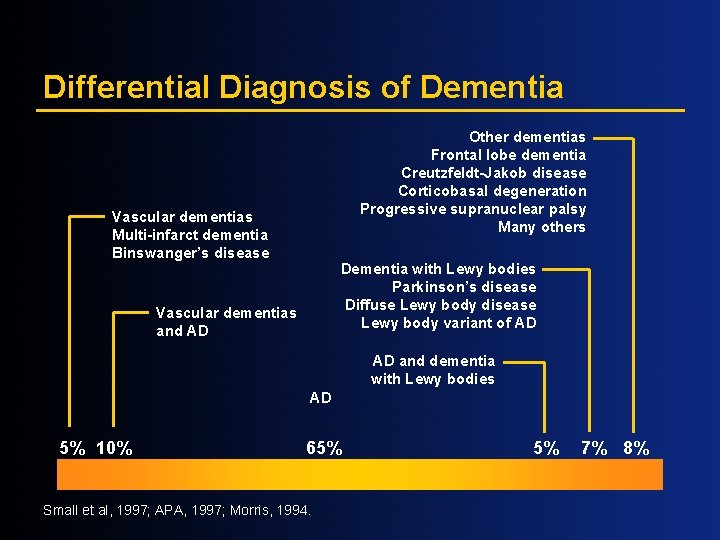
Differential Diagnosis of Dementia Other dementias Frontal lobe dementia Creutzfeldt-Jakob disease Corticobasal degeneration Progressive supranuclear palsy Many others Vascular dementias Multi-infarct dementia Binswanger’s disease Dementia with Lewy bodies Parkinson’s disease Diffuse Lewy body disease Lewy body variant of AD Vascular dementias and AD AD and dementia with Lewy bodies AD 5% 10% 65% Small et al, 1997; APA, 1997; Morris, 1994. 5% 7% 8%

Neuropathologic Changes Characteristic of AD Normal AD AP AP=amyloid plaques; NFT=neurofibrillary tangles Courtesy of George Grossberg M. D. ; St. Louis University. NFT

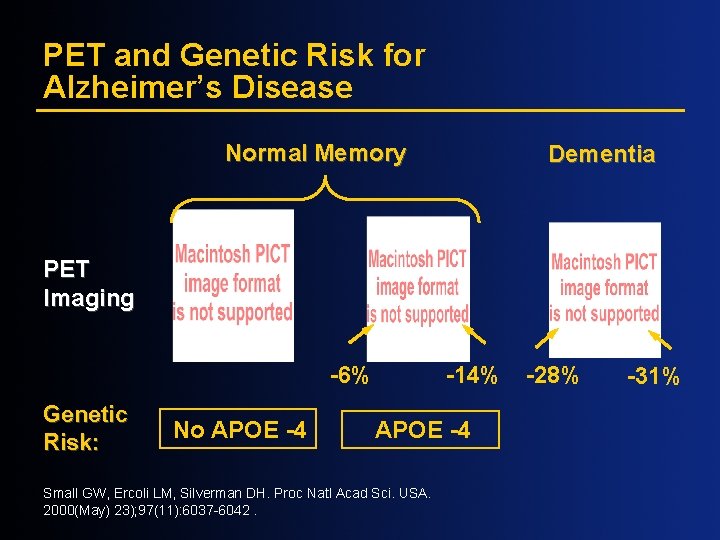
PET and Genetic Risk for Alzheimer’s Disease Normal Memory Dementia PET Imaging -6% Genetic Risk: No APOE -4 -14% APOE -4 Small GW, Ercoli LM, Silverman DH. Proc Natl Acad Sci. USA. 2000(May) 23); 97(11): 6037 -6042. -28% -31%

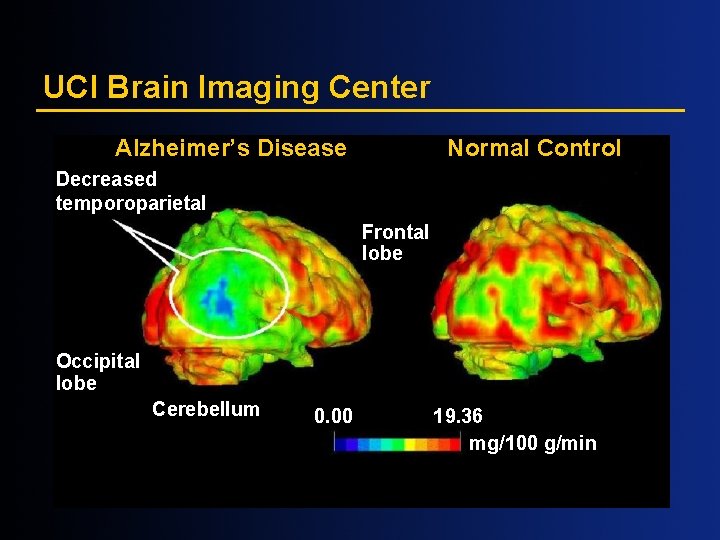
UCI Brain Imaging Center Alzheimer’s Disease Normal Control Decreased temporoparietal Frontal lobe Occipital lobe Cerebellum 0. 00 19. 36 mg/100 g/min

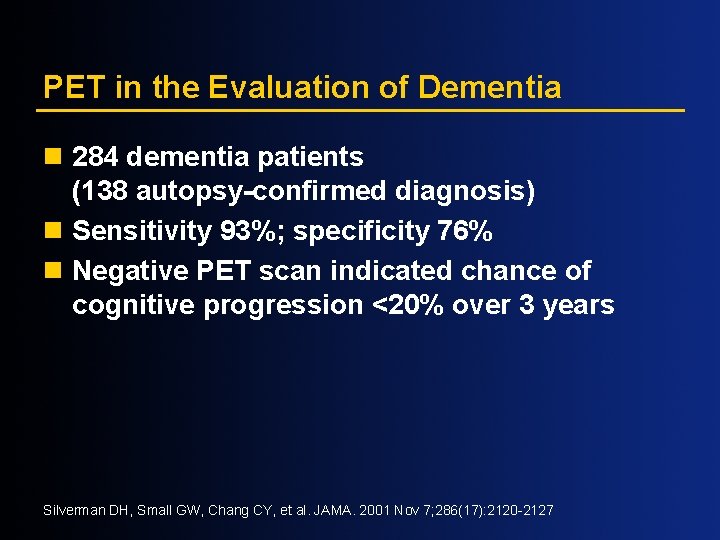
PET in the Evaluation of Dementia n 284 dementia patients (138 autopsy-confirmed diagnosis) n Sensitivity 93%; specificity 76% n Negative PET scan indicated chance of cognitive progression <20% over 3 years Silverman DH, Small GW, Chang CY, et al. JAMA. 2001 Nov 7; 286(17): 2120 -2127

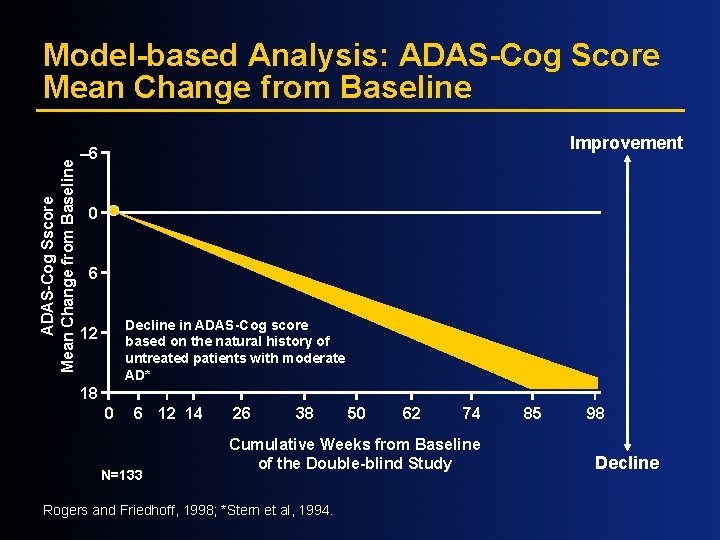
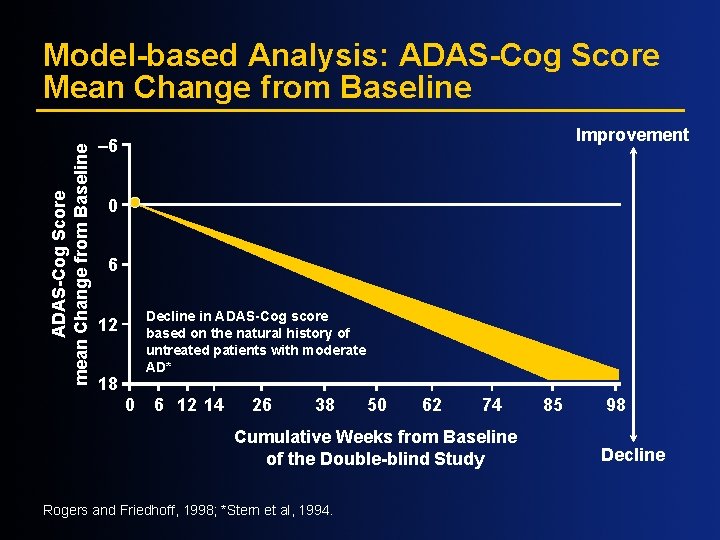
ADAS-Cog Sscore Mean Change from Baseline Model-based Analysis: ADAS-Cog Score Mean Change from Baseline Improvement – 6 0 6 Decline in ADAS-Cog score based on the natural history of untreated patients with moderate AD* 12 18 0 6 12 14 N=133 26 38 50 62 74 Cumulative Weeks from Baseline of the Double-blind Study Rogers and Friedhoff, 1998; *Stern et al, 1994. 85 98 Decline

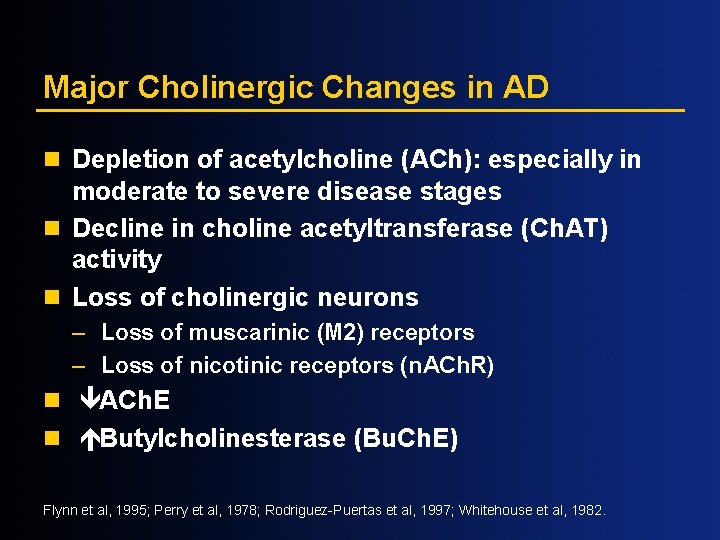
Major Cholinergic Changes in AD n Depletion of acetylcholine (ACh): especially in moderate to severe disease stages n Decline in choline acetyltransferase (Ch. AT) activity n Loss of cholinergic neurons – Loss of muscarinic (M 2) receptors – Loss of nicotinic receptors (n. ACh. R) n êACh. E n éButylcholinesterase (Bu. Ch. E) Flynn et al, 1995; Perry et al, 1978; Rodriguez-Puertas et al, 1997; Whitehouse et al, 1982.

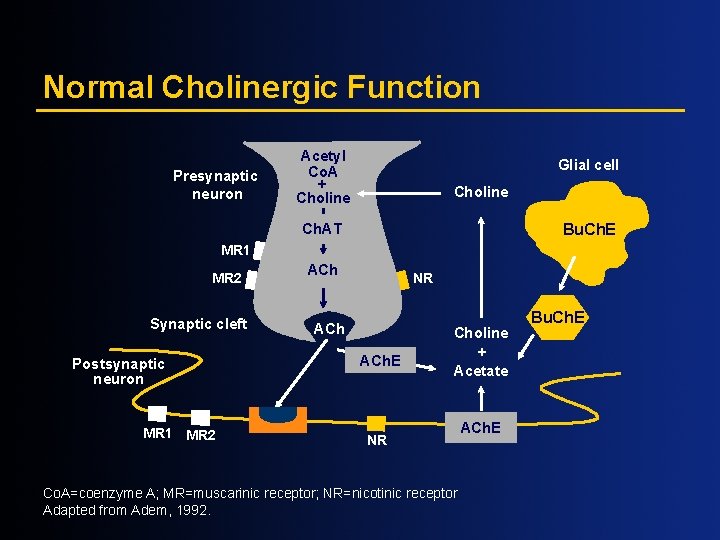
Normal Cholinergic Function Presynaptic neuron Acetyl Co. A + Choline Glial cell Choline Ch. AT Bu. Ch. E MR 1 MR 2 Synaptic cleft Postsynaptic neuron MR 1 MR 2 ACh NR ACh. E Choline + Acetate NR Co. A=coenzyme A; MR=muscarinic receptor; NR=nicotinic receptor Adapted from Adem, 1992. ACh. E Bu. Ch. E

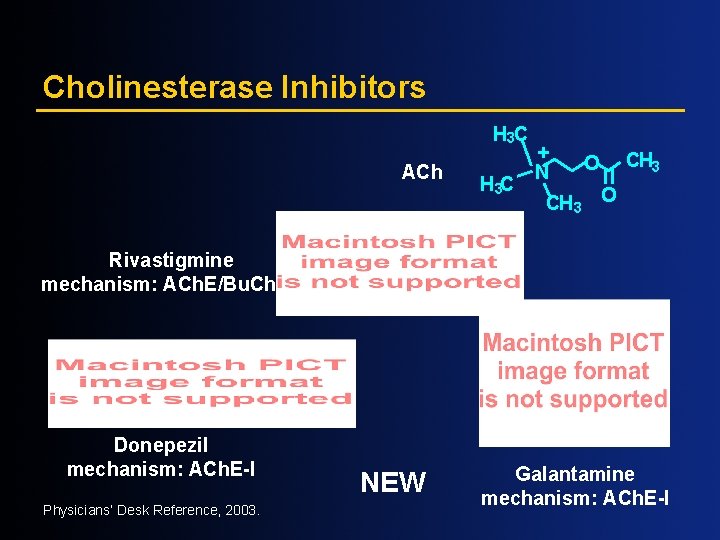
Cholinesterase Inhibitors H 3 C ACh H 3 C + N O CH 3 O Rivastigmine mechanism: ACh. E/Bu. Ch. E-I Donepezil mechanism: ACh. E-I Physicians’ Desk Reference, 2003. NEW Galantamine mechanism: ACh. E-I

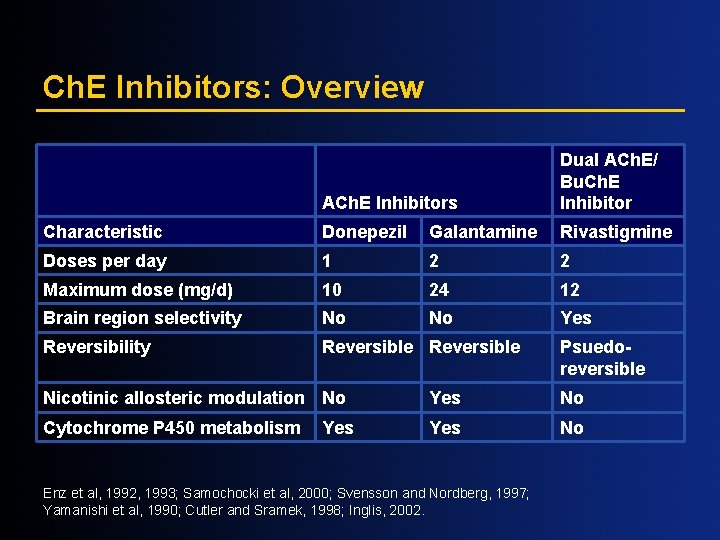
Ch. E Inhibitors: Overview ACh. E Inhibitors Dual ACh. E/ Bu. Ch. E Inhibitor Characteristic Donepezil Galantamine Rivastigmine Doses per day 1 2 2 Maximum dose (mg/d) 10 24 12 Brain region selectivity No No Yes Reversibility Reversible Psuedoreversible Nicotinic allosteric modulation No Yes No Cytochrome P 450 metabolism Yes No Yes Enz et al, 1992, 1993; Samochocki et al, 2000; Svensson and Nordberg, 1997; Yamanishi et al, 1990; Cutler and Sramek, 1998; Inglis, 2002.

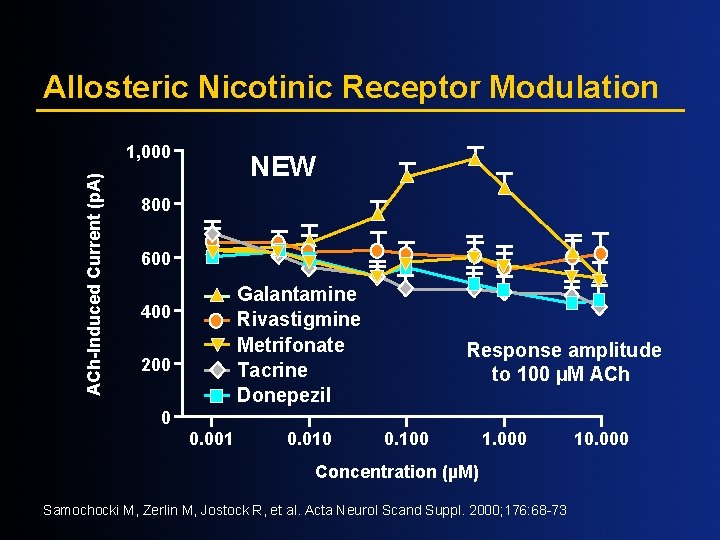
Allosteric Nicotinic Receptor Modulation ACh-Induced Current (p. A) 1, 000 NEW 800 600 Galantamine Rivastigmine Metrifonate Tacrine Donepezil 400 200 Response amplitude to 100 µM ACh 0 0. 001 0. 010 0. 100 1. 000 Concentration (µM) Samochocki M, Zerlin M, Jostock R, et al. Acta Neurol Scand Suppl. 2000; 176: 68 -73 10. 000

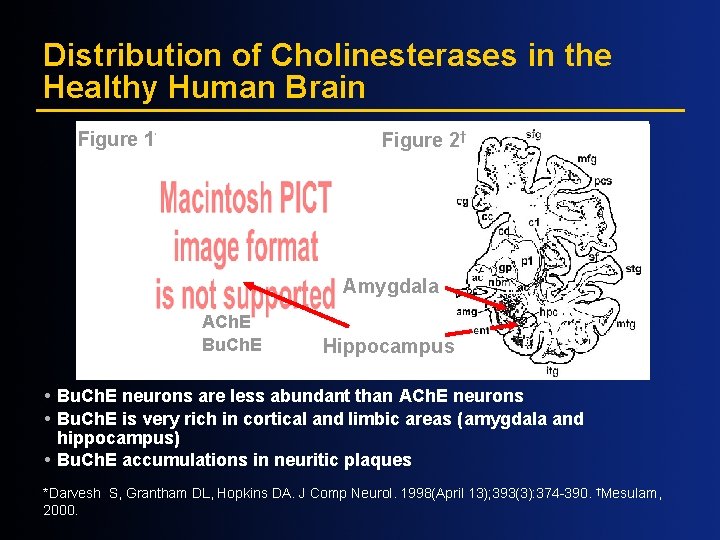
Distribution of Cholinesterases in the Healthy Human Brain Figure 1* Figure 2† Amygdala ACh. E Bu. Ch. E Hippocampus Bu. Ch. E neurons are less abundant than ACh. E neurons Bu. Ch. E is very rich in cortical and limbic areas (amygdala and hippocampus) Bu. Ch. E accumulations in neuritic plaques *Darvesh S, Grantham DL, Hopkins DA. J Comp Neurol. 1998(April 13); 393(3): 374 -390. †Mesulam, 2000.

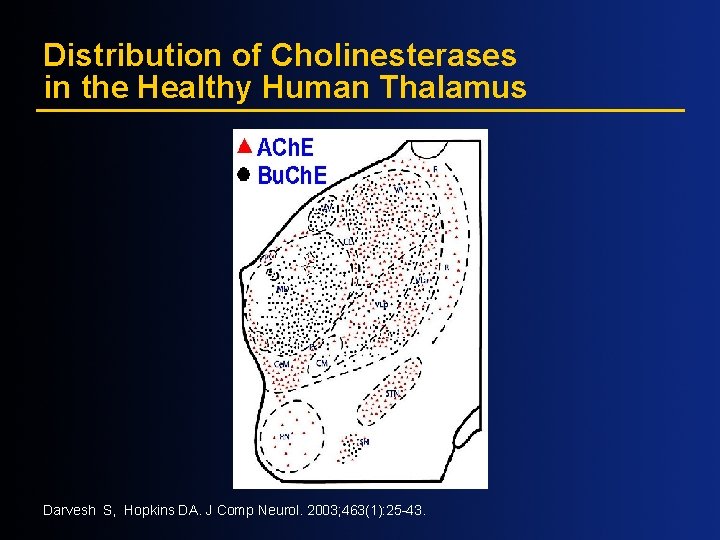
Distribution of Cholinesterases in the Healthy Human Thalamus Darvesh S, Hopkins DA. J Comp Neurol. 2003; 463(1): 25 -43.

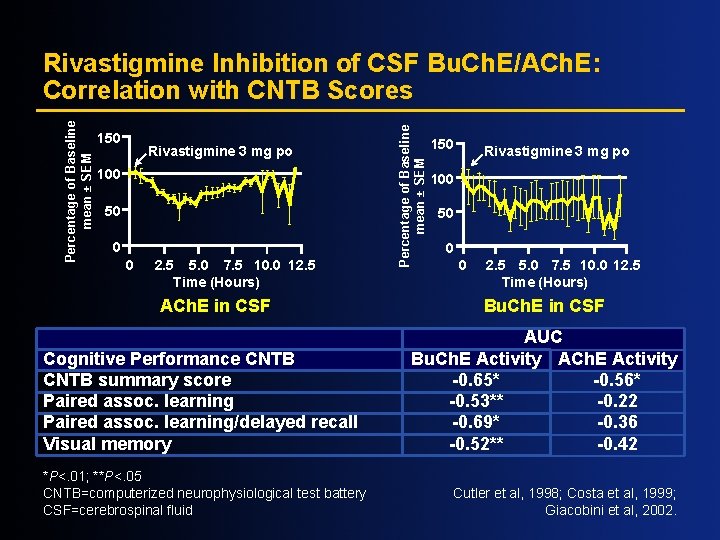
150 Rivastigmine 3 mg po 100 50 0 0 2. 5 5. 0 7. 5 10. 0 12. 5 Time (Hours) ACh. E in CSF Cognitive Performance CNTB summary score Paired assoc. learning/delayed recall Visual memory *P<. 01; **P<. 05 CNTB=computerized neurophysiological test battery CSF=cerebrospinal fluid Percentage of Baseline mean ± SEM Rivastigmine Inhibition of CSF Bu. Ch. E/ACh. E: Correlation with CNTB Scores 150 Rivastigmine 3 mg po 100 50 0 0 2. 5 5. 0 7. 5 10. 0 12. 5 Time (Hours) Bu. Ch. E in CSF AUC Bu. Ch. E Activity ACh. E Activity -0. 65* -0. 56* -0. 53** -0. 22 -0. 69* -0. 36 -0. 52** -0. 42 Cutler et al, 1998; Costa et al, 1999; Giacobini et al, 2002.

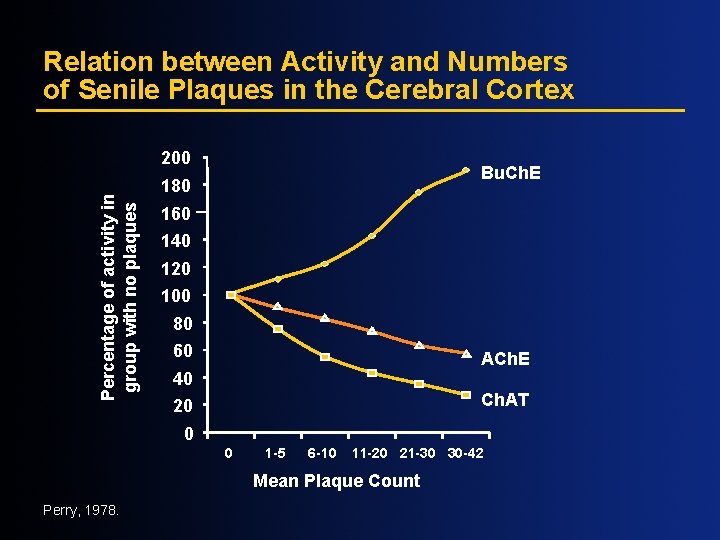
Relation between Activity and Numbers of Senile Plaques in the Cerebral Cortex Percentage of activity in group with no plaques 200 Bu. Ch. E 180 160 140 120 100 80 60 ACh. E 40 Ch. AT 20 0 0 1 -5 6 -10 11 -20 21 -30 30 -42 Mean Plaque Count Perry, 1978.

Compact Plaque Formation Guillozet et al, 1997.

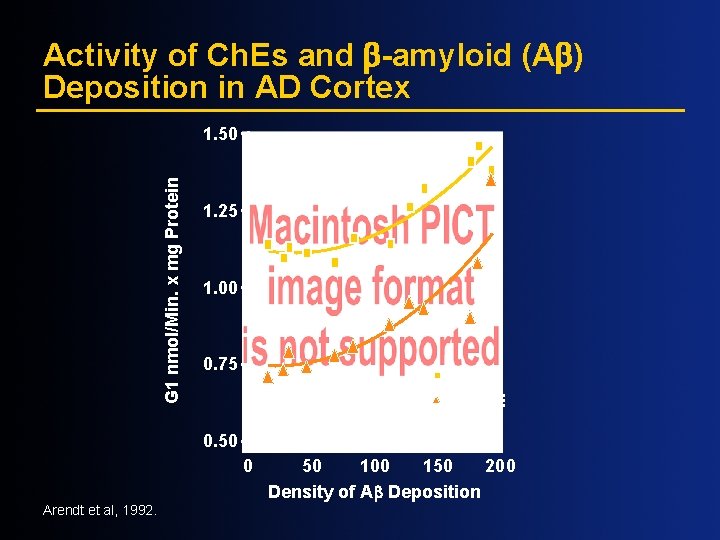
Activity of Ch. Es and -amyloid (A ) Deposition in AD Cortex G 1 nmol/Min. x mg Protein 1. 50 1. 25 1. 00 0. 75 ACh. E Bu. Ch. E 0. 50 0 Arendt et al, 1992. 50 100 150 200 Density of A Deposition

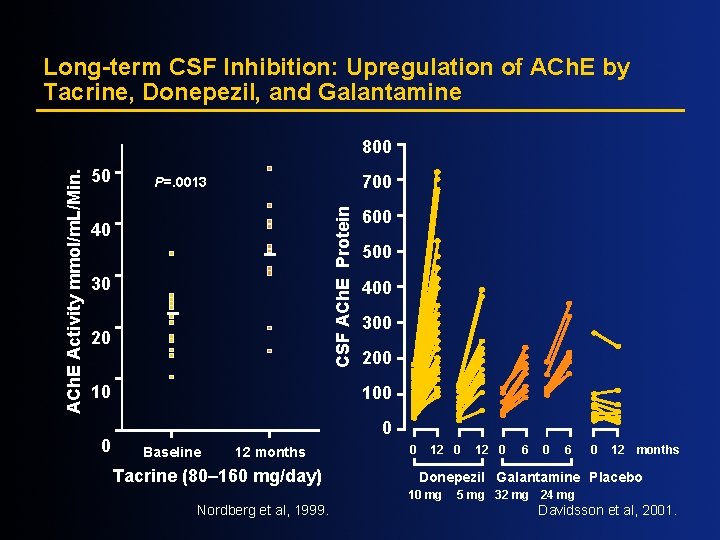
Long-term CSF Inhibition: Upregulation of ACh. E by Tacrine, Donepezil, and Galantamine 50 700 P=. 0013 CSF ACh. E Protein ACh. E Activity mmol/m. L/Min. 800 40 30 20 10 0 600 500 400 300 200 100 0 Baseline 12 months Tacrine (80– 160 mg/day) 0 12 0 6 0 12 months Donepezil Galantamine Placebo 10 mg Nordberg et al, 1999. 12 0 5 mg 32 mg 24 mg Davidsson et al, 2001.

Long-term CSF Inhibition: Sustained Inhibition of ACh. E and Bu. Ch. E by Rivastigmine 60 50 40 30 20 10 0 -10 0 3 6 9 12 Treatment Length (Months) Darreh-Shori et al, 2002. Inhibition of CSF Bu. Ch. E (%) Inhibition of CSF ACh. E (%) High-dose rivastigmine Low-dose rivastigmine 80 70 60 50 40 30 20 10 0 -10 0 3 6 9 12 Treatment Length (Months)

ADAS-Cog Score mean Change from Baseline Model-based Analysis: ADAS-Cog Score Mean Change from Baseline Improvement – 6 0 6 Decline in ADAS-Cog score based on the natural history of untreated patients with moderate AD* 12 18 0 6 12 14 26 38 50 62 74 Cumulative Weeks from Baseline of the Double-blind Study Rogers and Friedhoff, 1998; *Stern et al, 1994. 85 98 Decline

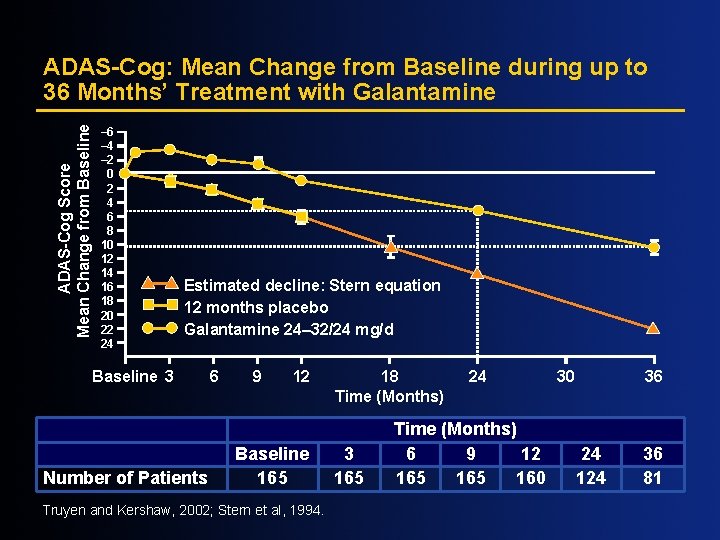
ADAS-Cog Score Mean Change from Baseline ADAS-Cog: Mean Change from Baseline during up to 36 Months’ Treatment with Galantamine – 6 – 4 – 2 0 2 4 6 8 10 12 14 16 18 20 22 24 Estimated decline: Stern equation 12 months placebo Galantamine 24– 32/24 mg/d Baseline 3 Number of Patients 6 9 12 Baseline 165 Truyen and Kershaw, 2002; Stern et al, 1994. 18 Time (Months) 3 165 24 Time (Months) 6 9 12 165 160 30 36 24 124 36 81

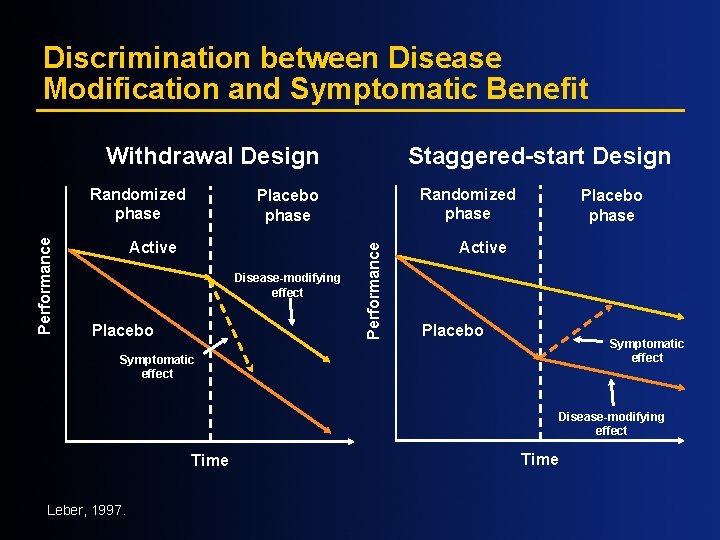
Discrimination between Disease Modification and Symptomatic Benefit Withdrawal Design Randomized phase Placebo phase Active Disease-modifying effect Placebo Performance Randomized phase Staggered-start Design Placebo phase Active Placebo Symptomatic effect Disease-modifying effect Time Leber, 1997. Time

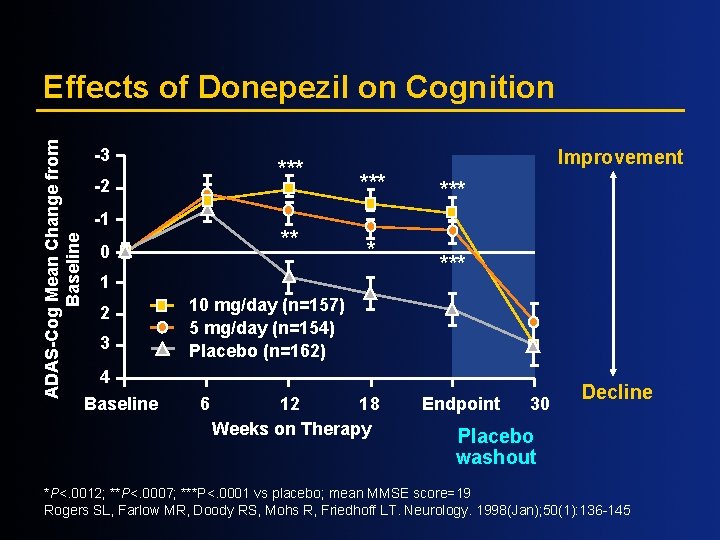
ADAS-Cog Mean Change from Baseline Effects of Donepezil on Cognition -3 *** -2 -1 ** 0 Improvement *** * 1 2 3 *** 10 mg/day (n=157) 5 mg/day (n=154) Placebo (n=162) 4 Baseline 6 12 18 Weeks on Therapy Endpoint 30 Decline Placebo washout *P<. 0012; **P<. 0007; ***P<. 0001 vs placebo; mean MMSE score=19 Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. Neurology. 1998(Jan); 50(1): 136 -145

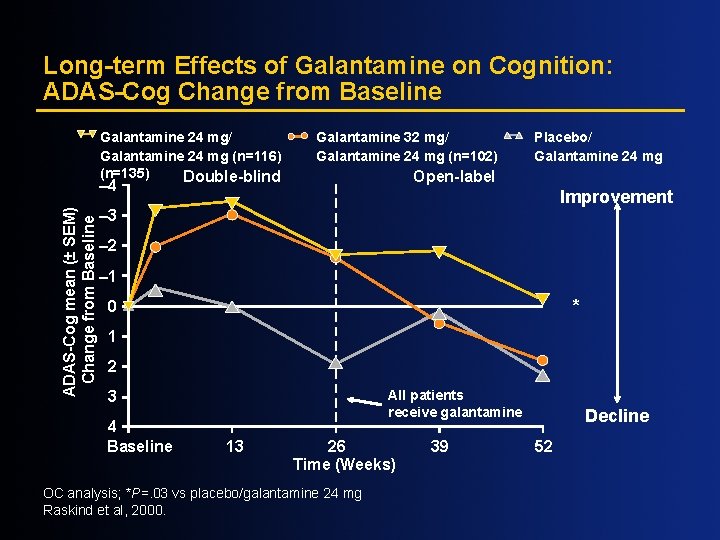
Long-term Effects of Galantamine on Cognition: ADAS-Cog Change from Baseline Galantamine 24 mg/ Galantamine 24 mg (n=116) (n=135) Double-blind Galantamine 32 mg/ Galantamine 24 mg (n=102) Open-label – 4 ADAS-Cog mean (± SEM) Change from Baseline Placebo/ Galantamine 24 mg Improvement – 3 – 2 – 1 * 0 1 2 All patients receive galantamine 3 4 Baseline 13 26 Time (Weeks) OC analysis; *P=. 03 vs placebo/galantamine 24 mg Raskind et al, 2000. 39 Decline 52

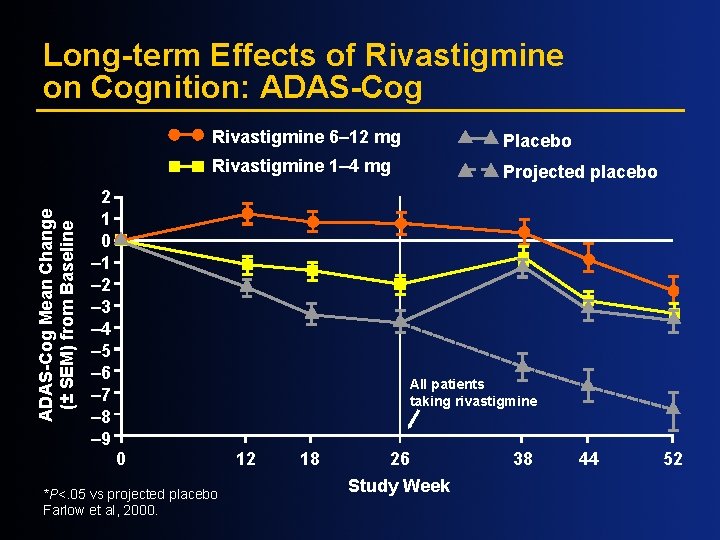
ADAS-Cog Mean Change (± SEM) from Baseline Long-term Effects of Rivastigmine on Cognition: ADAS-Cog 2 1 0 – 1 – 2 – 3 – 4 – 5 – 6 – 7 – 8 – 9 Rivastigmine 6– 12 mg Placebo Rivastigmine 1– 4 mg Projected placebo All patients taking rivastigmine 0 *P<. 05 vs projected placebo Farlow et al, 2000. 12 18 26 Study Week 38 44 52

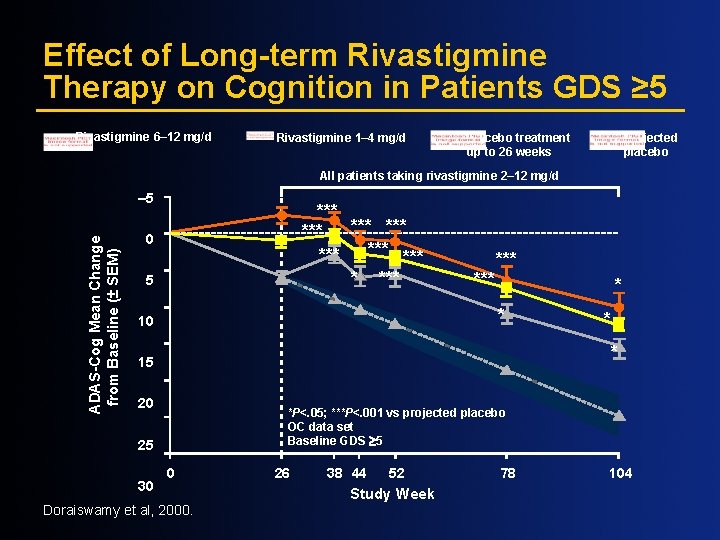
Effect of Long-term Rivastigmine Therapy on Cognition in Patients GDS ≥ 5 Rivastigmine 6– 12 mg/d Rivastigmine 1– 4 mg/d Placebo treatment up to 26 weeks Projected placebo All patients taking rivastigmine 2– 12 mg/d ADAS-Cog Mean Change from Baseline (± SEM) – 5 *** *** * *** 0 5 *** * * 10 * 15 20 *P<. 05; ***P<. 001 vs projected placebo OC data set Baseline GDS ³ 5 25 30 * 0 Doraiswamy et al, 2000. 26 38 44 52 Study Week 78 104

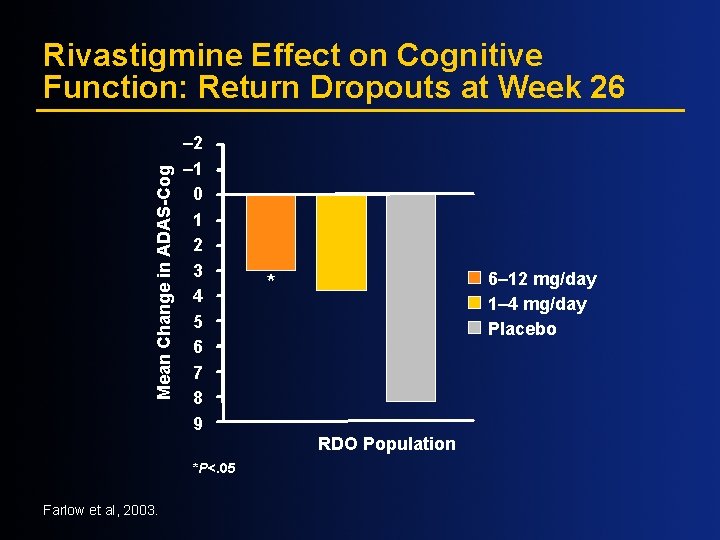
Mean Change in ADAS-Cog Rivastigmine Effect on Cognitive Function: Return Dropouts at Week 26 – 2 – 1 0 1 2 3 4 5 6 7 8 9 *P<. 05 Farlow et al, 2003. 6– 12 mg/day 1– 4 mg/day Placebo * RDO Population

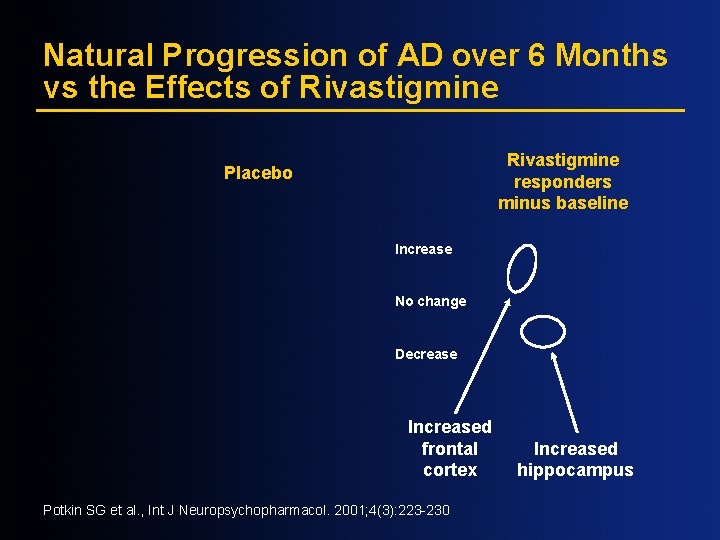
Natural Progression of AD over 6 Months vs the Effects of Rivastigmine responders minus baseline Placebo Increase No change Decrease Increased frontal cortex Potkin SG et al. , Int J Neuropsychopharmacol. 2001; 4(3): 223 -230 Increased hippocampus

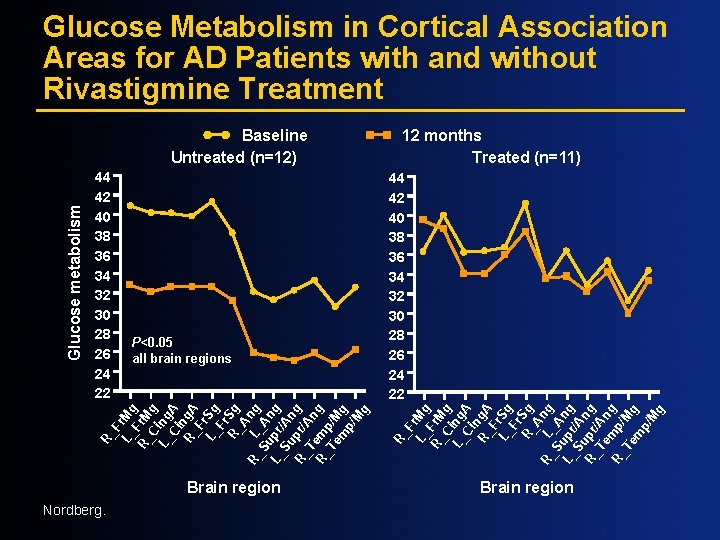
Glucose Metabolism in Cortical Association Areas for AD Patients with and without Rivastigmine Treatment P<0. 05 all brain regions 44 42 40 38 36 34 32 30 28 26 24 22 R_ F L_ r. Mg R_ Fr. M C g L_ ing Ci A n R_ g. A F L_ r. Sg Fr R_ Sg A R_ L ng Su _A L_ pr ng Su /A R_ pr ng Te /An R_ mp/ g Te Mg m p/ M g 44 42 40 38 36 34 32 30 28 26 24 22 12 months Treated (n=11) R_ F L_ r. Mg R_ Fr. M C g L_ ing Ci A n R_ g. A F L_ r. Sg Fr R_ Sg A R_ L ng Su _A L_ pr ng Su /An R_ pr/ g Te An R_ m g Te p/M m g p/ M g Glucose metabolism Baseline Untreated (n=12) Brain region Nordberg.

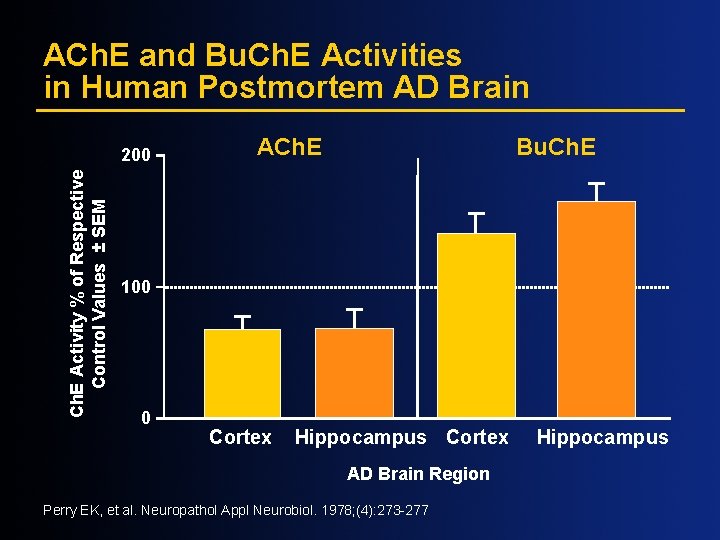
ACh. E and Bu. Ch. E Activities in Human Postmortem AD Brain Ch. E Activity % of Respective Control Values ± SEM 200 ACh. E Bu. Ch. E 100 0 Cortex Hippocampus Cortex AD Brain Region Perry EK, et al. Neuropathol Appl Neurobiol. 1978; (4): 273 -277 Hippocampus

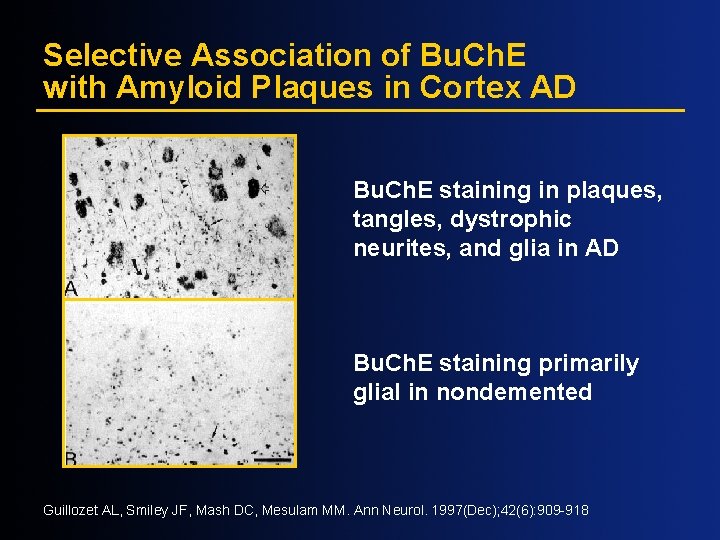
Selective Association of Bu. Ch. E with Amyloid Plaques in Cortex AD Bu. Ch. E staining in plaques, tangles, dystrophic neurites, and glia in AD Bu. Ch. E staining primarily glial in nondemented Guillozet AL, Smiley JF, Mash DC, Mesulam MM. Ann Neurol. 1997(Dec); 42(6): 909 -918

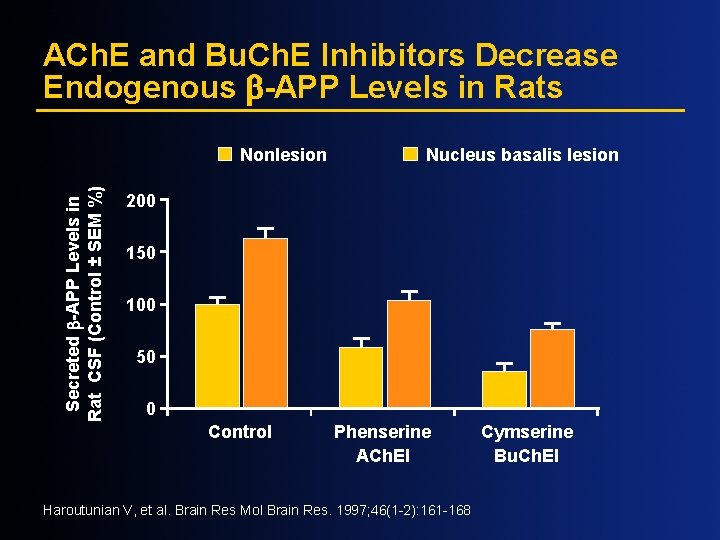
ACh. E and Bu. Ch. E Inhibitors Decrease Endogenous -APP Levels in Rats Secreted -APP Levels in Rat CSF (Control ± SEM %) Nonlesion Nucleus basalis lesion 200 150 100 50 0 Control Phenserine ACh. EI Haroutunian V, et al. Brain Res Mol Brain Res. 1997; 46(1 -2): 161 -168 Cymserine Bu. Ch. EI

Disease Progression: Rate of Cognitive Decline in DLB Correlated with Bu. Ch. E Levels at Autopsy MMSE Decline/Year 12 10 8 6 4 2 R=0. 88 P<. 002 0 – 2 0 0. 7 0. 8 0. 9 Bu. Ch. E Level in Medial Temporal Cortex N=9 Perry et al, in press. 1. 0
![Correlation between CSF Phosphotau and MMSE Decrease in MCI Annual MMSE Decrease 10 0 Correlation between CSF [Phospho-tau] and MMSE Decrease in MCI Annual MMSE Decrease 10 0](https://slidetodoc.com/presentation_image_h/bcfa456fd29dff1e3eddc2f55ff24c75/image-42.jpg)
Correlation between CSF [Phospho-tau] and MMSE Decrease in MCI Annual MMSE Decrease 10 0 – 10 – 20 0 RHO=-. 30 P<. 01 Buerger et al, 2002. 300 600 900 1, 200 1, 500 CSF p-tau 231 (pg/m. L) 1, 800 2, 100

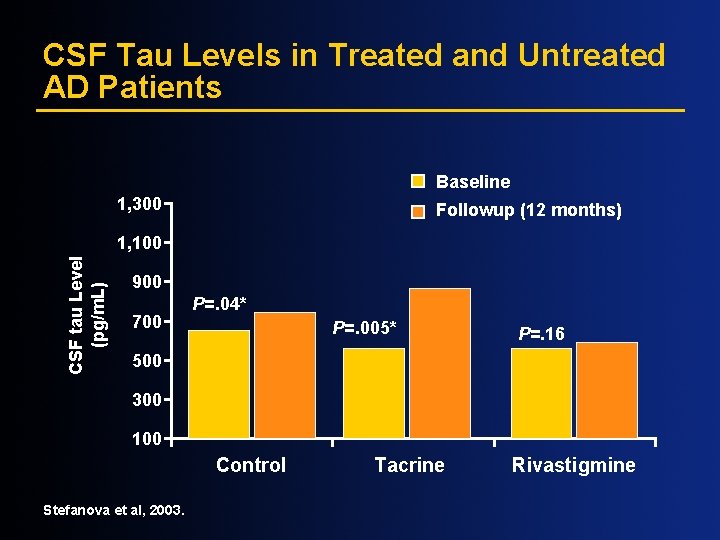
CSF Tau Levels in Treated and Untreated AD Patients Baseline 1, 300 Followup (12 months) CSF tau Level (pg/m. L) 1, 100 900 700 P=. 04* P=. 005* P=. 16 500 300 100 Control Stefanova et al, 2003. Tacrine Rivastigmine

Greater Awareness May Lead to Earlier Diagnosis and Treatment Physician would have been consulted sooner if caregiver or others had known about early signs Physician would have been consulted sooner if caregiver or others had known about prescription medication n 50% cases of moderate AD remain undiagnosed n 70% cases of mild AD remain undiagnosed Harris Interactive Inc. , 2001.

Summary n Both ACh. E and Bu. Ch. E can contribute to the loss of ACh, which underlies many of the symptoms in AD n Inhibition of CSF ACh. E and Bu. Ch. E with rivastigmine correlates with clinical benefits n Bu. Ch. E increases with severity of AD n Bu. Ch. E may play a role in plaque maturation and APP processing n Both ACh. E and Bu. Ch. E are pharmacological targets n Lack of upregulation with rivastigmine implies that cognitive benefits are sustained during long-term treatment n Increased ACh. E protein following galantamine and donepezil is of uncertain clinical importance

Summary (cont’d) n AD is an expensive illness in human and economic terms for patient and care givers n Diagnosis is often not made, especially in early and mild AD n Frequently treatment is not initiated or sustained n Early treatment pays off; delaying treatment has long-term consequences n Functional imaging can aid in early diagnosis of dementia n Moderately ill patients can robustly respond to tx n Cholinesterase inhibitors attenuate symptomatic decline and may modify disease progression
 The integration of eye, hand, and foot movements
The integration of eye, hand, and foot movements Health related fitness and skill related fitness
Health related fitness and skill related fitness Bharathi viswanathan
Bharathi viswanathan Georgia alzheimers planning
Georgia alzheimers planning Alzheimers fast score
Alzheimers fast score Vaskulär demens
Vaskulär demens Alzheimers nz conference 2020
Alzheimers nz conference 2020 Site:slidetodoc.com
Site:slidetodoc.com Alzheimers society contented dementia
Alzheimers society contented dementia Alzheimer's eye test joke
Alzheimer's eye test joke Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Chụp tư thế worms-breton
Chụp tư thế worms-breton Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 101012 bằng
101012 bằng Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế
Cái miệng nó xinh thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thứ tự các dấu thăng giáng ở hóa biểu
Thứ tự các dấu thăng giáng ở hóa biểu Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phối cảnh
Phối cảnh Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dot
Dot Số.nguyên tố
Số.nguyên tố Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì