Solutions Types of Mixtures Types of Aqueous Solutions














![[H 3 O+] versus [OH-] Acidic Solutions: [H 3 O+] > [OH-] This means [H 3 O+] versus [OH-] Acidic Solutions: [H 3 O+] > [OH-] This means](https://slidetodoc.com/presentation_image_h2/e925371532b28791a2a88426ed227e6d/image-16.jpg)




















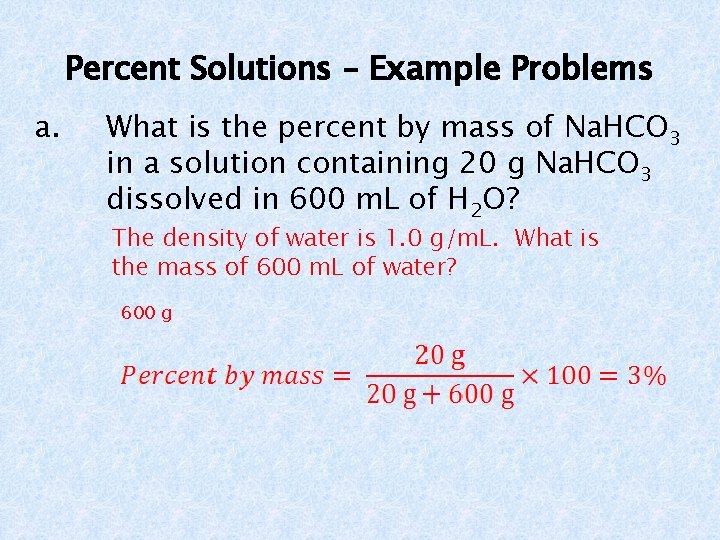






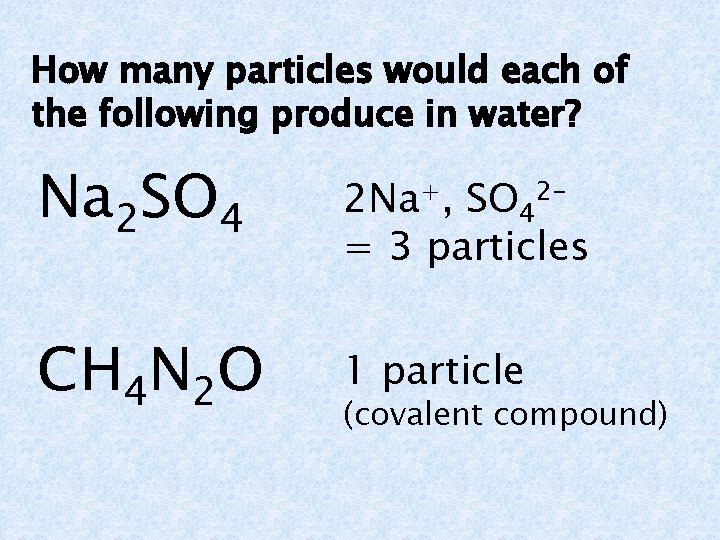


- Slides: 83

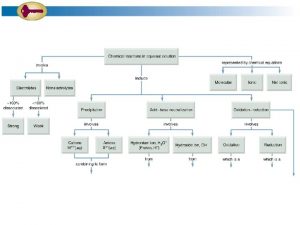
Solutions • • • Types of Mixtures Types of Aqueous Solutions Solubility Concentration Colligative Properties of Solutions

Types of Mixtures can be classified as solutions, suspensions, or colloids based upon the following properties: Uniformity – Is the mixture homogeneous or heterogeneous? Composition – What are the parts of the mixture? Particle Size – How big are the particles? Effect of Light – Do the particles exhibit the Tyndall effect? Effect of Gravity – Do the particles settle upon standing? Filtration – Can the particles be filtered?

Solutions A solution is a homogeneous mixture in which one substance is dissolved in another substance. Solutions may exist as mixtures of gases, liquids, or solids. Examples of solutions include: Air Soda Alloys (ex. gold jewelry, bronze) Saltwater

Parts of a Solution A solution consists of two parts. Solute – the substance that is being dissolved. Solvent – the substance that is doing the dissolving.

Types of Solutions An aqueous solution is one in which water is the solvent. A tincture is one in which alcohol is the solvent. Identify the solute and solvent in each of the following: Salt water Solvent: water Solute: salt 0. 5 M Lead(II) nitrate Solute: Lead(II) nitrate Solvent: water

Properties of Solutions The particles making up a solution are too small to be seen (0. 01 to 1 nm in diameter). They are too small to be filtered. The particles do not settle when the solution is allowed to stand. The particles do not exhibit the Tyndall effect. The Tyndall effect is the scattering of a light beam as it hits larger particles. An example would be the headlights of a car on a foggy day.

Suspensions A suspension is a heterogeneous mixture. The particles are large enough to be seen (over 100 nm in diameter). The particles in a suspension are so large that they settle out unless the mixture is constantly stirred or agitated. The particles in a suspension can be filtered. The particles in a suspension exhibit the Tyndall effect.

Examples of Suspensions Examples of suspensions include Vinegar and oil dressing Muddy water Many children’s medicines

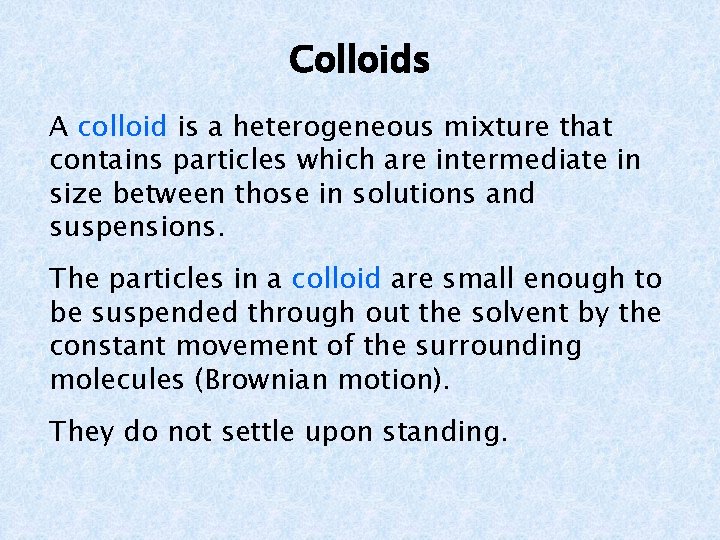
Colloids A colloid is a heterogeneous mixture that contains particles which are intermediate in size between those in solutions and suspensions. The particles in a colloid are small enough to be suspended through out the solvent by the constant movement of the surrounding molecules (Brownian motion). They do not settle upon standing.

Colloids There are two parts of a colloid. Dispersed phase – the colloidal particles Dispersion medium – this can be a solid, liquid, or a gas The particles in a colloid cannot be separated by filtration. The particles in a colloid exhibit the Tyndall effect.

Examples of Colloids Examples of colloids include Whipped cream (gas in a liquid) Marshmallow (gas in a solid) Jelly (solid in a liquid)

Types of Aqueous Solutions There are several different ways in which aqueous solutions can be further classified. Solutions can be classified as acidic, basic, or neutral. What do you know about acidic solutions? basic solutions? neutral solutions?

Types of Aqueous Solutions can also be classified as electrolytic or nonelectrolytic. What do you know about acidic electrolytes? nonelectrolytes?

Acidic, Basic and Neutral Solutions All aqueous solutions contain hydronium ions (H 3 O+)* and hydroxide ions (OH-). *The hydronium ion and the hydrogen ion (H ) are often used + interchangeably.

Acidic, Basic and Neutral Solutions A solution is classified as acidic, basic or neutral by comparing the number of hydronium ions in solution with the number of hydroxide ions, by determining the p. H of the solution, or by using indicators. How do you think an indicator works?
![H 3 O versus OH Acidic Solutions H 3 O OH This means [H 3 O+] versus [OH-] Acidic Solutions: [H 3 O+] > [OH-] This means](https://slidetodoc.com/presentation_image_h2/e925371532b28791a2a88426ed227e6d/image-16.jpg)
[H 3 O+] versus [OH-] Acidic Solutions: [H 3 O+] > [OH-] This means that the [H 3 O+] is greater than 10 -7 M. Basic Solutions: [H 3 O+] < [OH-] This means that the [OH-] is greater than 10 -7 M. Neutral Solutions: [H 3 O+] = [OH-] This means that both the [H 3 O+] and the [OH-] are equal to 10 -7 M?

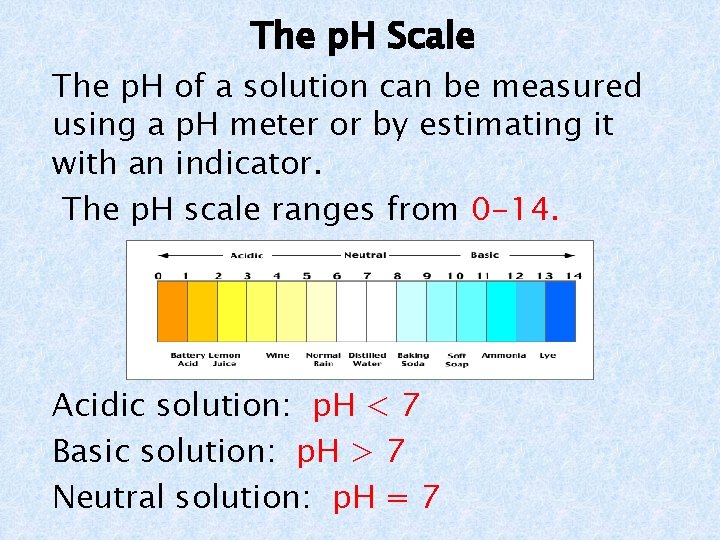
The p. H Scale The p. H of a solution can be measured using a p. H meter or by estimating it with an indicator. The p. H scale ranges from 0 -14. Acidic solution: p. H < 7 Basic solution: p. H > 7 Neutral solution: p. H = 7

Indicators can also be used to classify solutions as acidic, basic or neutral. Indicators change color in acids and bases. One common indicator is litmus paper. Blue litmus paper turns red in acidic solutions. Red litmus paper turns blue in basic solutions.

Acidic, Basic & Neutral Solutions Which of the following substances is the most acidic? Most basic? Ammonia, p. H = 11 Milk, p. H = 6. 6 Vinegar, p. H = 2. 2 Vinegar is the most acidic. Ammonia is the most basic.

Electrolytes An electrolyte is a substance that dissolves in water to produce a solution that conducts an electric current. Acids, bases, and salts (ionic compounds) are electrolytes. A conductivity tester can be used to determine if a solute is an electrolyte or nonelectrolyte.

Strong Electrolytes A strong electrolyte is a substance that completely ionizes in water. A strong electrolyte will cause the bulb of a conductivity tester to glow brightly. Soluble Ionic compounds, strong acids, and strong bases are strong electrolytes. Strong acids include: HCl. O 4, HCl. O 3, HCl, HBr, HI, HNO 3, H 2 SO 4 Strong bases include: Li. OH, Na. OH, KOH, Rb. OH, Cs. OH, Ca(OH)2, Sr(OH)2, and Ba(OH)2)

Weak Electrolytes A weak electrolyte is a substance that only partially ionizes in water. A weak electrolyte will cause the bulb of a conductivity tester to glow dimly. Weak acids and bases are examples of weak electrolytes.

Nonelectrolytes A nonelectrolyte is a substance that dissolves in water to produce a solution that does not conduct an electric current. A nonelectrolyte will not cause the bulb of a conductivity tester to glow. Many molecular (covalent) compounds such as sugar are nonelectrolytes. They do not ionize in water.

Classify each of the following substances as an electrolyte or nonelectrolyte. Explain your answer. Lithium chloride Electrolyte, it is an ionic compound. Glucose, C 6 H 12 O 6 Nonelectrolyte, it is a covalent compound. Sulfuric Acid, H 2 SO 4 Electrolyte, it is an acid. Sodium hydroxide, Na. OH Electrolyte, it is a base. Propanol, C 3 H 7 OH Nonelectrolyte, it is a covalent compound called an alcohol.

Electrolyte is a "medical/scientific" term for salts, specifically ions. The term electrolyte means that this ion is electricallycharged and moves to either a negative (cathode) or positive (anode) electrode: ions that move to the cathode (cations) are positively charged ions that move to the anode (anions) are negatively charged For example, your body fluids -- blood, plasma, interstitial fluid (fluid between cells) -- are like seawater and have a high concentration of sodium chloride (table salt, or Na. Cl). The electrolytes in sodium chloride are: sodium ion (Na+) - cation chloride ion (Cl-) – anion

Electrolytes are important because they are what your cells (especially nerve, heart, muscle) use to maintain voltages across their cell membranes and to carry electrical impulses (nerve impulses, muscle contractions) across themselves and to other cells. Your kidneys work to keep the electrolyte concentrations in your blood constant despite changes in your body. For example, when you exercise heavily, you lose electrolytes in your sweat, particularly sodium and potassium. These electrolytes must be replaced to keep the electrolyte concentrations of your body fluids constant.

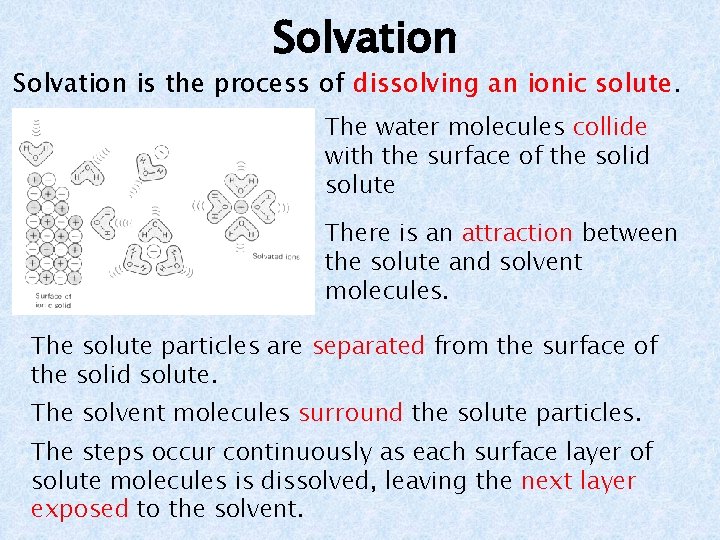
Solvation is the process of dissolving an ionic solute. The water molecules collide with the surface of the solid solute There is an attraction between the solute and solvent molecules. The solute particles are separated from the surface of the solid solute. The solvent molecules surround the solute particles. The steps occur continuously as each surface layer of solute molecules is dissolved, leaving the next layer exposed to the solvent.

Factors Affecting the Rate of Solution Formation Stirring (Agitation) or shaking the solution brings more particles of the solute in contact with the solvent sooner causing the solute to dissolve at a faster rate.

Factors Affecting the Rate of Solution Formation Powdering the solid solute increases the amount of surface area so that more solute particles can come into contact with more of the solvent.

Factors Affecting the Rate of Solution Formation If heat is applied to a solution, the molecules move faster and farther apart causing more collisions between the solute and solvent.

Solubility is a measure of how much of a solute can be dissolved in a given amount of solvent at a given temperature. The units for solubility are g/100 m. L H 2 O, g/100 g H 2 O, and g/L (for a gas).

The Universal Solvent Water is known as the universal solvent because of its ability to dissolve so many substances. Water has the ability to dissolve so many substances because it is a polar molecule.

“Like Dissolves Like” The general rule for whether or not a substance with dissolve in another substance is “Like Dissolves Like” Polar solvents, like water, can dissolve polar and ionic solutes. The charged ions or polar solute molecules are attracted to the polar ends of the water molecules, holding each other together in solution.

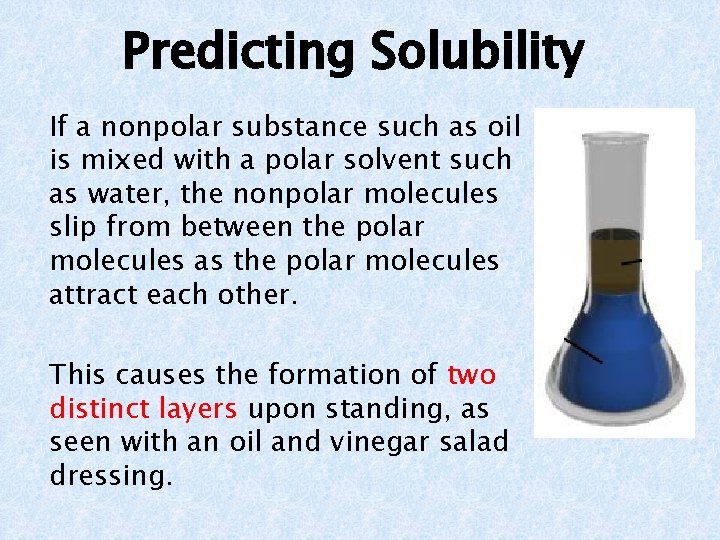
Predicting Solubility Nonpolar solvents can dissolve nonpolar solutes. A nonpolar molecule has an even charge distribution so there is no attraction to the polar water molecules.

Predicting Solubility If a nonpolar substance such as oil is mixed with a polar solvent such as water, the nonpolar molecules slip from between the polar molecules as the polar molecules attract each other. This causes the formation of two distinct layers upon standing, as seen with an oil and vinegar salad dressing.

Classify each of the following as soluble or insoluble in water. Justify your answer. Li. Cl Soluble, Li. Cl is an ionic compound. NH 3 Soluble, NH 3 is a polar covalent compound. C 6 H 6 Insoluble, C 6 H 6 is a nonpolar covalent compound.

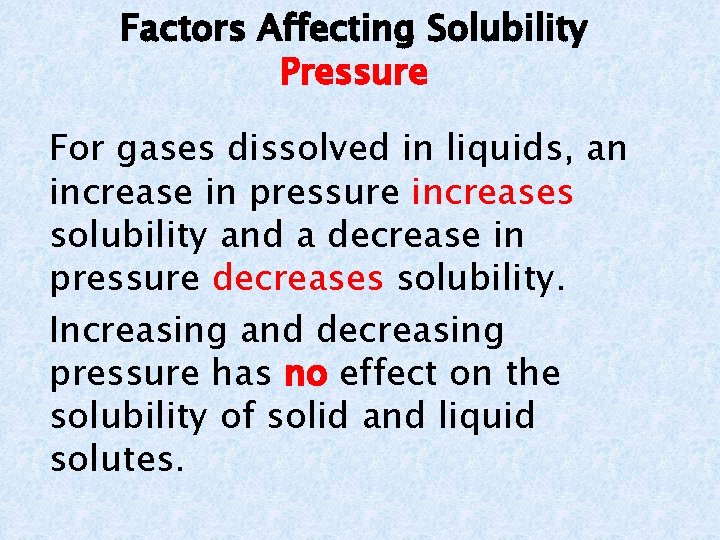
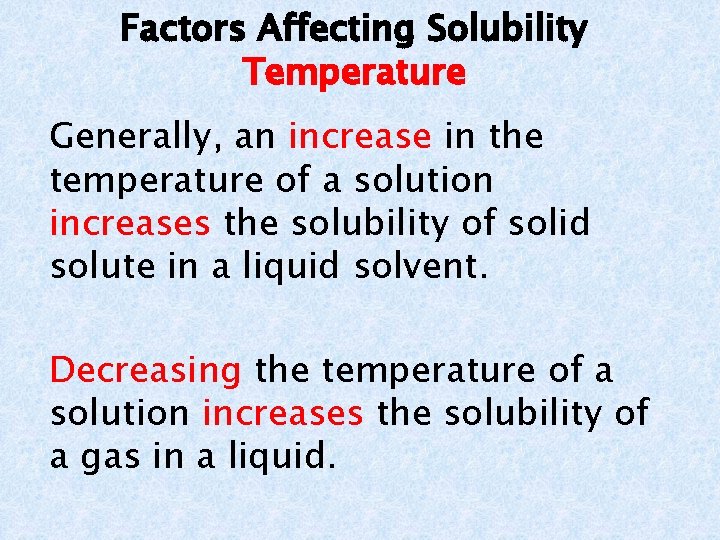
Factors Affecting Solubility Pressure For gases dissolved in liquids, an increase in pressure increases solubility and a decrease in pressure decreases solubility. Increasing and decreasing pressure has no effect on the solubility of solid and liquid solutes.

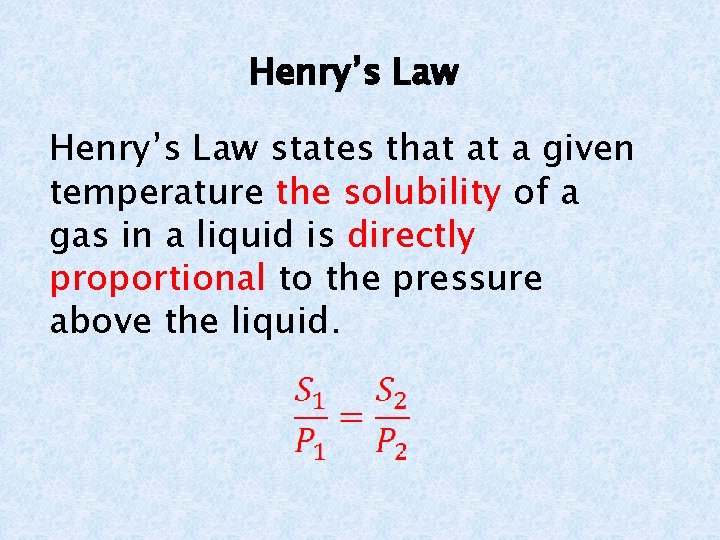
Henry’s Law states that at a given temperature the solubility of a gas in a liquid is directly proportional to the pressure above the liquid.

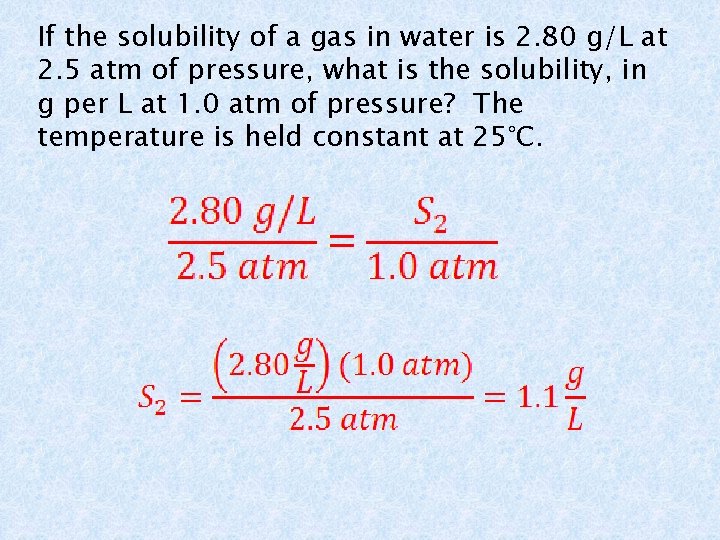
If the solubility of a gas in water is 2. 80 g/L at 2. 5 atm of pressure, what is the solubility, in g per L at 1. 0 atm of pressure? The temperature is held constant at 25°C.

Factors Affecting Solubility Temperature Generally, an increase in the temperature of a solution increases the solubility of solid solute in a liquid solvent. Decreasing the temperature of a solution increases the solubility of a gas in a liquid.

Solubility Solid solute/ Liquid solvent Increase Pressure Decrease Increase Temperature Decrease Liquid solute/ liquid solvent Gas solute/ liquid solvent

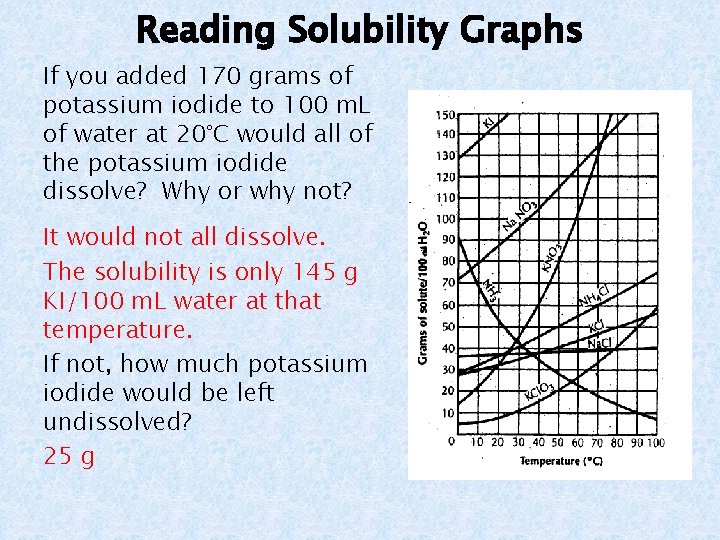
Reading Solubility Graphs A solubility graph indicates the amount of solute that will dissolve in 100 m. L (100 g) of water.

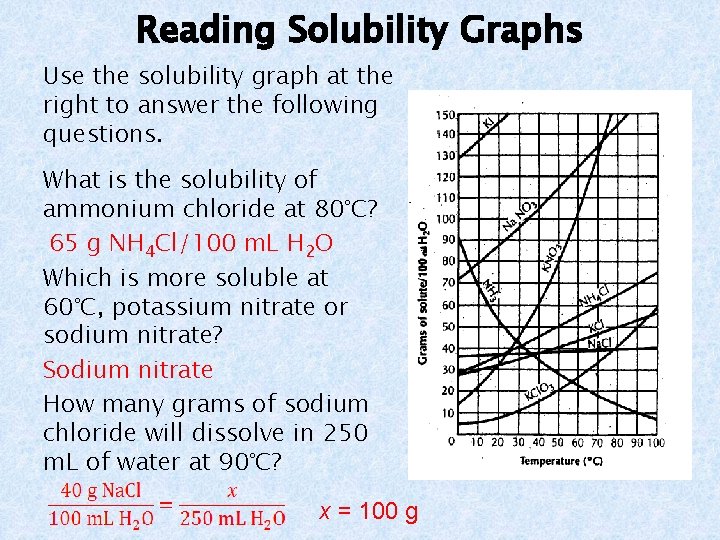
Reading Solubility Graphs Use the solubility graph at the right to answer the following questions. What is the solubility of ammonium chloride at 80°C? 65 g NH 4 Cl/100 m. L H 2 O Which is more soluble at 60°C, potassium nitrate or sodium nitrate? Sodium nitrate How many grams of sodium chloride will dissolve in 250 m. L of water at 90°C? x = 100 g

Reading Solubility Graphs If you added 170 grams of potassium iodide to 100 m. L of water at 20°C would all of the potassium iodide dissolve? Why or why not? It would not all dissolve. The solubility is only 145 g KI/100 m. L water at that temperature. If not, how much potassium iodide would be left undissolved? 25 g

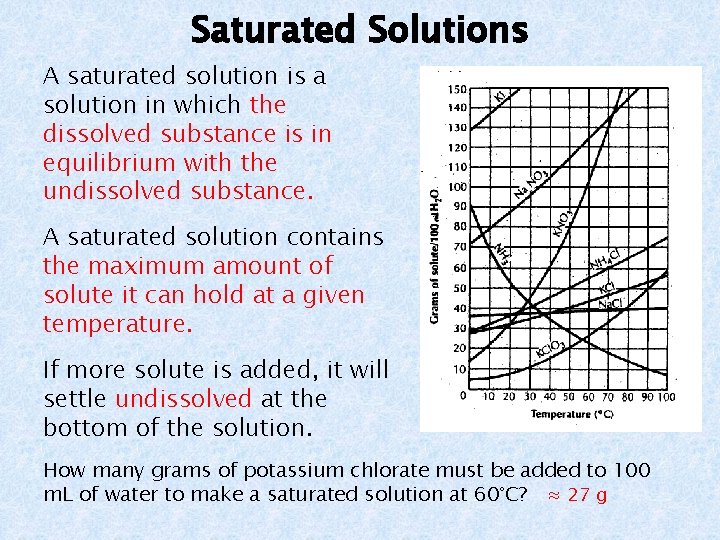
Saturated Solutions A saturated solution is a solution in which the dissolved substance is in equilibrium with the undissolved substance. A saturated solution contains the maximum amount of solute it can hold at a given temperature. If more solute is added, it will settle undissolved at the bottom of the solution. How many grams of potassium chlorate must be added to 100 m. L of water to make a saturated solution at 60°C? ≈ 27 g

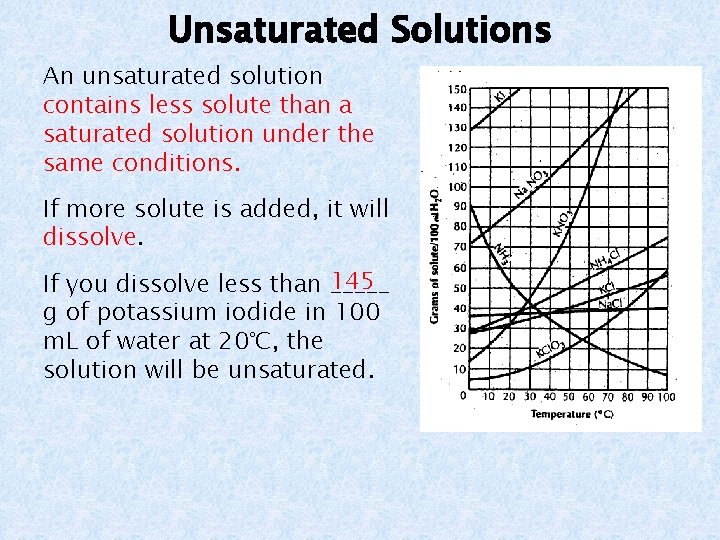
Unsaturated Solutions An unsaturated solution contains less solute than a saturated solution under the same conditions. If more solute is added, it will dissolve. 145 If you dissolve less than _____ g of potassium iodide in 100 m. L of water at 20°C, the solution will be unsaturated.

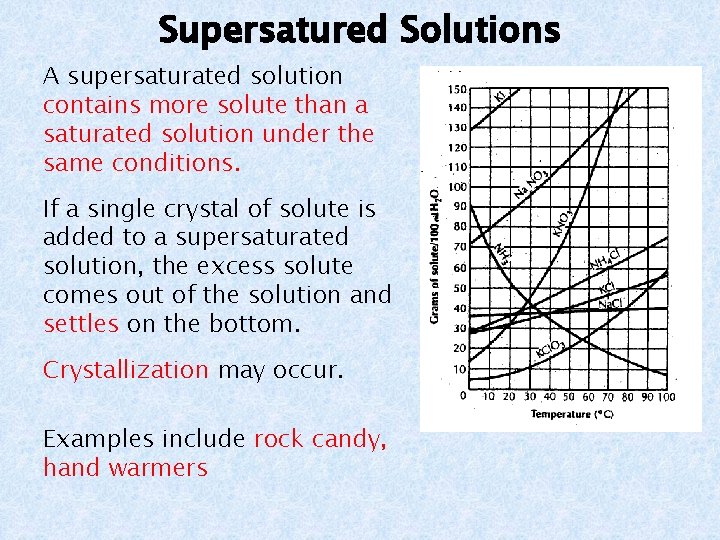
Supersatured Solutions A supersaturated solution contains more solute than a saturated solution under the same conditions. If a single crystal of solute is added to a supersaturated solution, the excess solute comes out of the solution and settles on the bottom. Crystallization may occur. Examples include rock candy, hand warmers

Supersatured Solutions How could a student make a supersaturated solution of sugar water in order to make rock candy? The student would make a saturated solution at a higher temperature and then allow it to cool.

Saturated, Unsaturated and Supersatured Solutions How could you experimentally determine if a solution is saturated, unsaturated, or supersaturated? Add more solute. If it dissolves, the original solution was unsaturated. If it crystallizes or causes more solute to come out of solution, it was supersaturated. If it sinks to the bottom, it was saturated.

Concentration of Solutions The concentration of a solution refers to the amount of solute dissolved in a given amount of solvent or solution. Concentrated Solution - contains a relatively large amount of solute in a solvent Dilute Solution – contains a relatively small amount of solute in a solvent

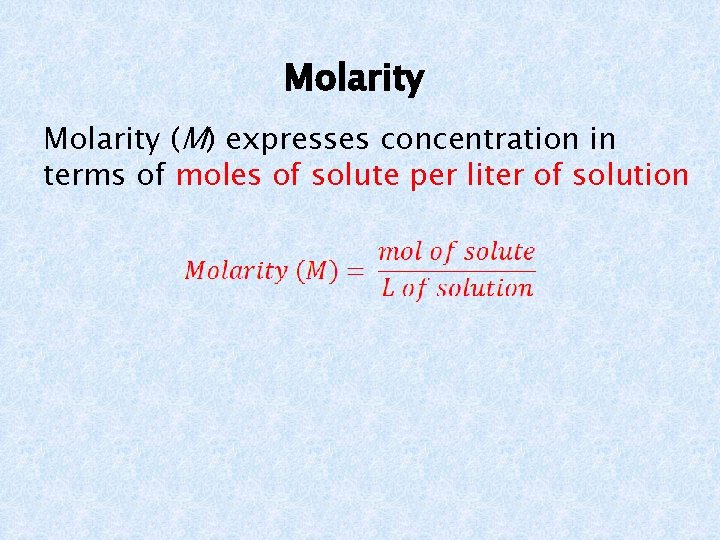
Molarity (M) expresses concentration in terms of moles of solute per liter of solution

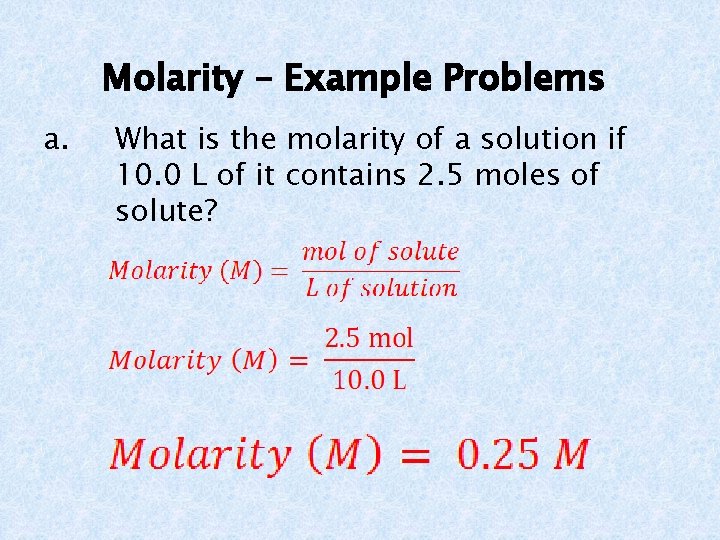
Molarity – Example Problems a. What is the molarity of a solution if 10. 0 L of it contains 2. 5 moles of solute?

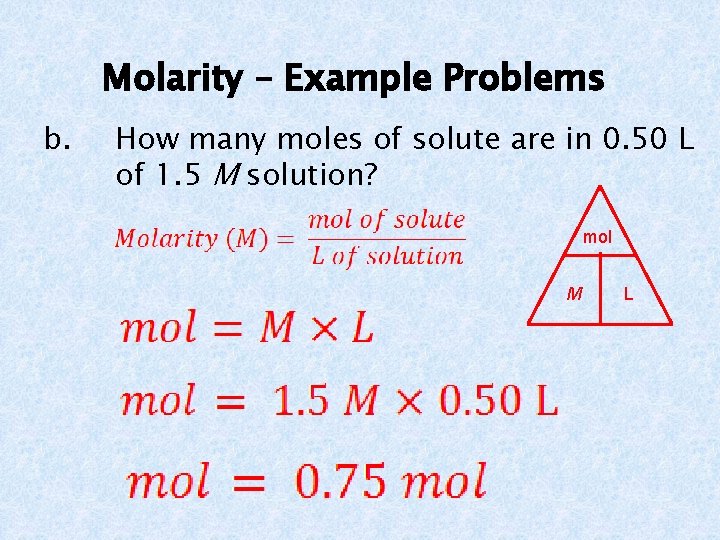
Molarity – Example Problems b. How many moles of solute are in 0. 50 L of 1. 5 M solution? mol M L

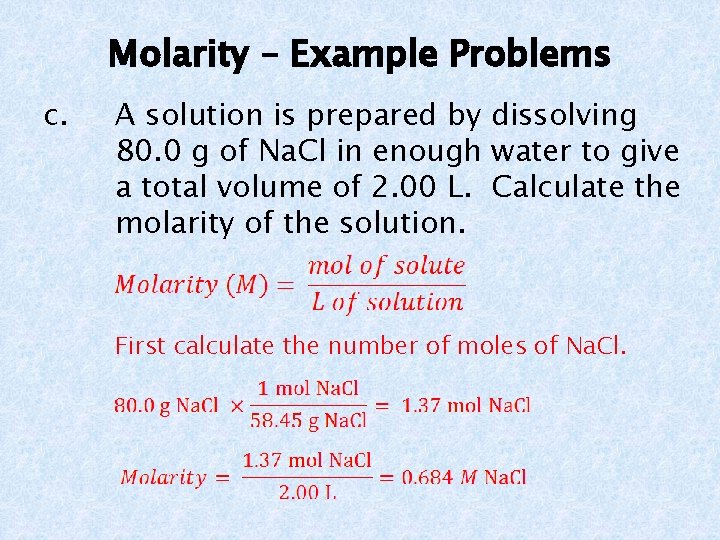
Molarity – Example Problems c. A solution is prepared by dissolving 80. 0 g of Na. Cl in enough water to give a total volume of 2. 00 L. Calculate the molarity of the solution. First calculate the number of moles of Na. Cl.

How would you prepare 1. 0 L of a 0. 500 M solution of copper(II) Sulfate Pentahydrate?

How would you prepare 1. 0 L of a 0. 500 M solution of copper(II) Sulfate Pentahydrate?

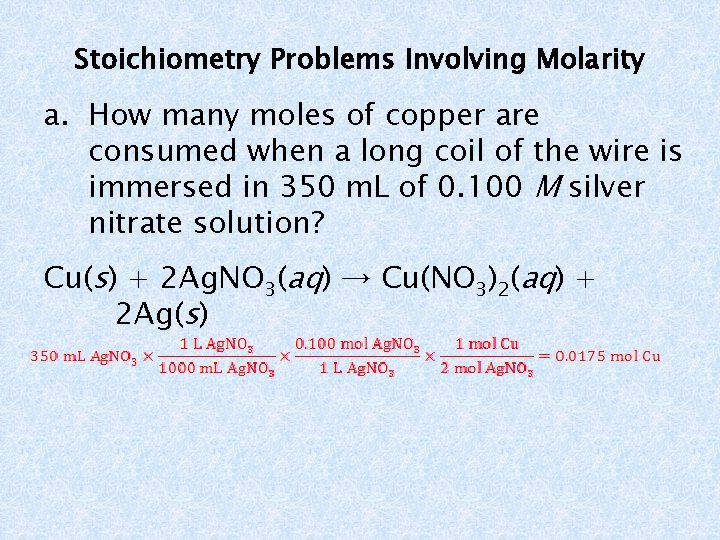
Stoichiometry Problems Involving Molarity a. How many moles of copper are consumed when a long coil of the wire is immersed in 350 m. L of 0. 100 M silver nitrate solution? Cu(s) + 2 Ag. NO 3(aq) → Cu(NO 3)2(aq) + 2 Ag(s)

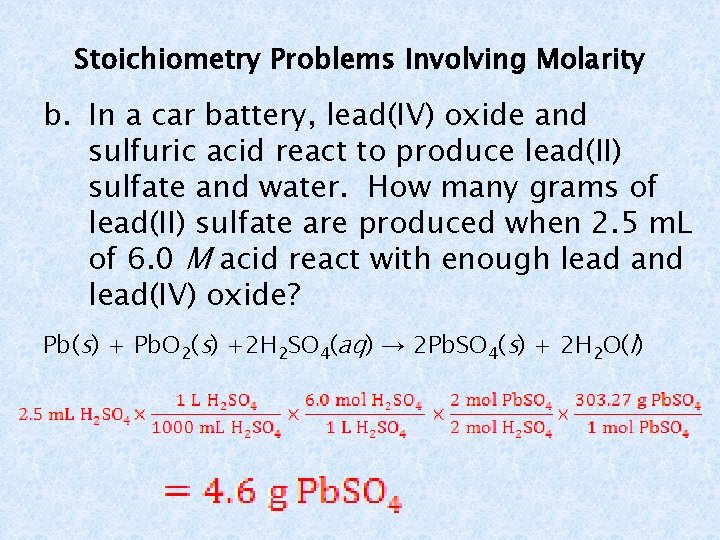
Stoichiometry Problems Involving Molarity b. In a car battery, lead(IV) oxide and sulfuric acid react to produce lead(II) sulfate and water. How many grams of lead(II) sulfate are produced when 2. 5 m. L of 6. 0 M acid react with enough lead and lead(IV) oxide? Pb(s) + Pb. O 2(s) +2 H 2 SO 4(aq) → 2 Pb. SO 4(s) + 2 H 2 O(l)

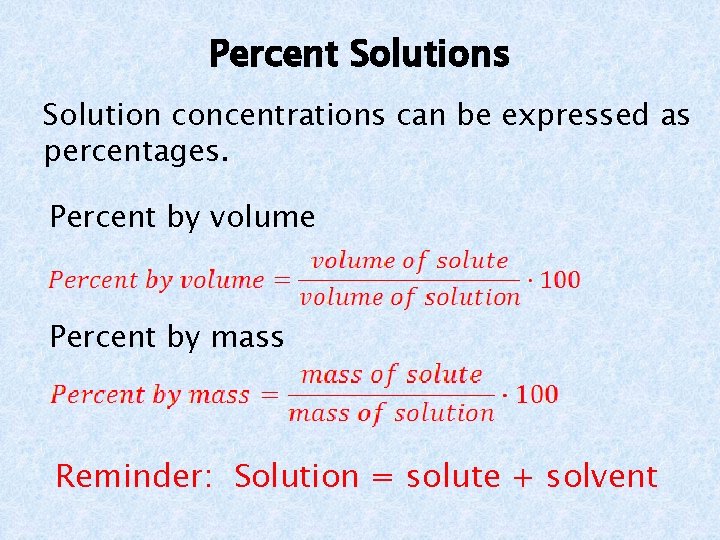
Percent Solutions Solution concentrations can be expressed as percentages. Percent by volume Percent by mass Reminder: Solution = solute + solvent

Percent Solutions What are some examples of solutions that are sold by percent concentration? hydrogen peroxide rubbing alcohol
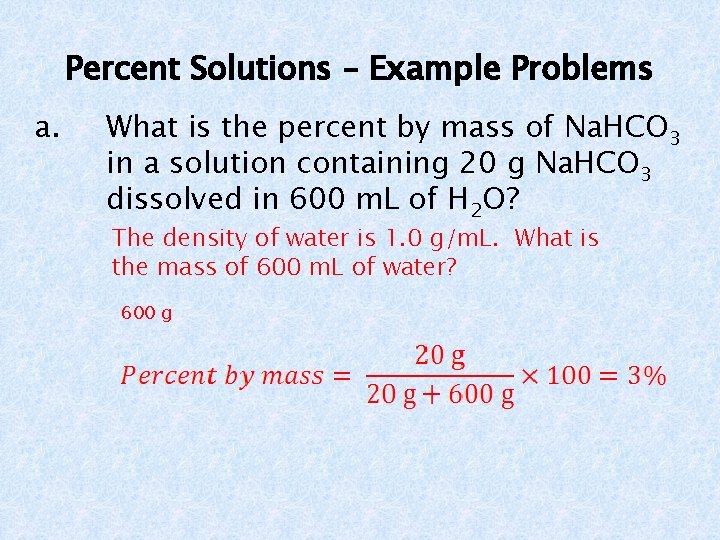
Percent Solutions – Example Problems a. What is the percent by mass of Na. HCO 3 in a solution containing 20 g Na. HCO 3 dissolved in 600 m. L of H 2 O? The density of water is 1. 0 g/m. L. What is the mass of 600 m. L of water? 600 g

Percent Solutions – Example Problems b. If you have 150. 0 m. L of a 30. % aqueous solution of ethanol, what volumes of ethanol and water are in the solution?

Dilutions Many substances used in the laboratory are purchased as concentrated solutions and it is often necessary to dilute them in order to obtain the desired concentration. M 1 V 1=M 2 V 2

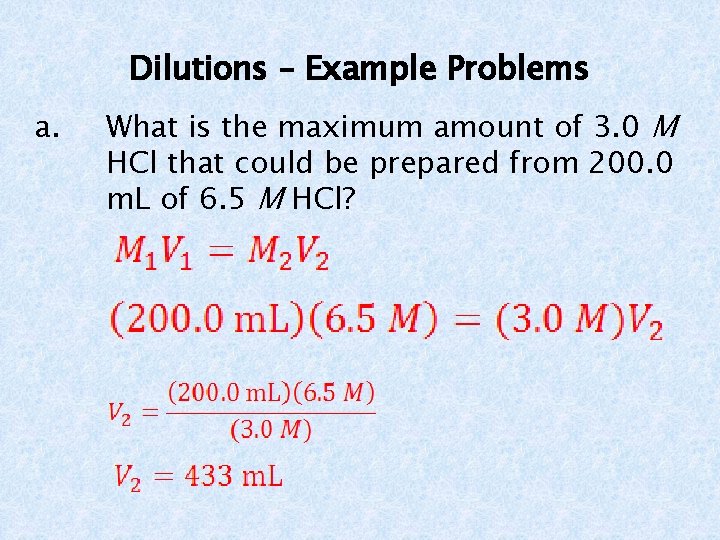
Dilutions – Example Problems a. What is the maximum amount of 3. 0 M HCl that could be prepared from 200. 0 m. L of 6. 5 M HCl?

Dilutions – Example Problems b. How would you prepare 2. 8 L of 0. 20 M Na. OH solution from a 6. 0 M solution? Add 0. 093 L of 6. 0 M Na. OH to enough water to make 2. 8 L of solution.

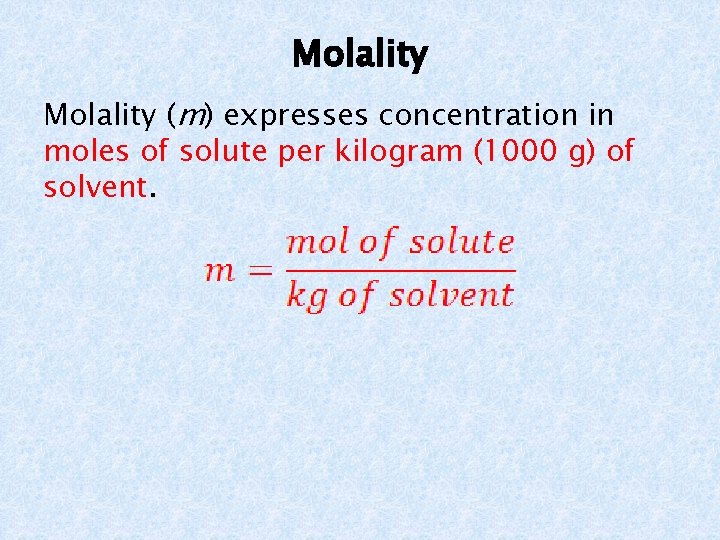
Molality (m) expresses concentration in moles of solute per kilogram (1000 g) of solvent.

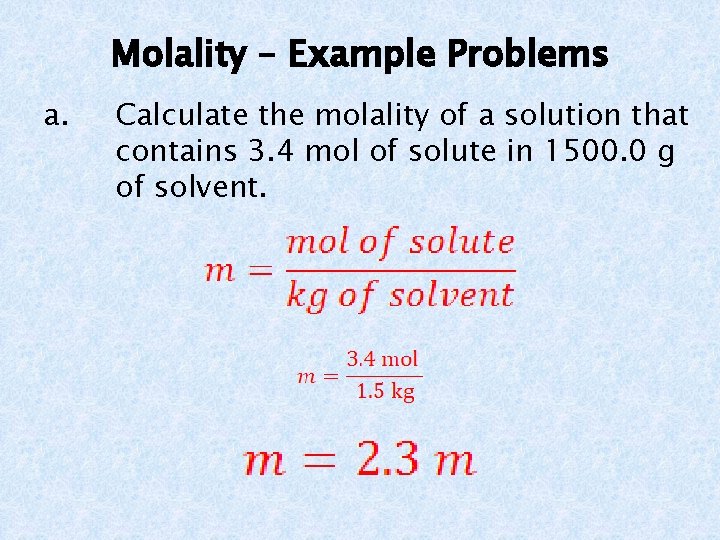
Molality – Example Problems a. Calculate the molality of a solution that contains 3. 4 mol of solute in 1500. 0 g of solvent.

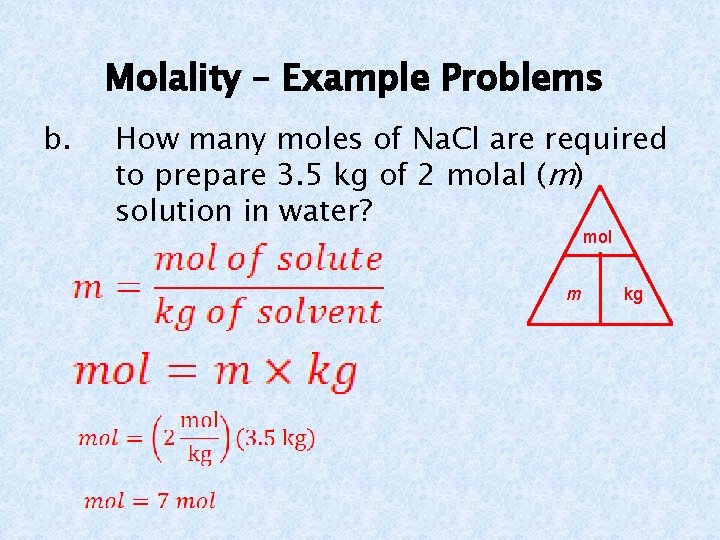
Molality – Example Problems b. How many moles of Na. Cl are required to prepare 3. 5 kg of 2 molal (m) solution in water? mol m kg

Colligative Properties of Solutions When a solute is added to a solvent, the physical properties of the resulting solution are different from that of the pure solvent. Colligative properties are the properties of a solvent that depend primarily on the concentration of solute particles and not the nature (identity) of the particles. Some examples of colligative properties include vapor pressure lowering, boiling point elevation, freezing point depression and osmotic pressure.

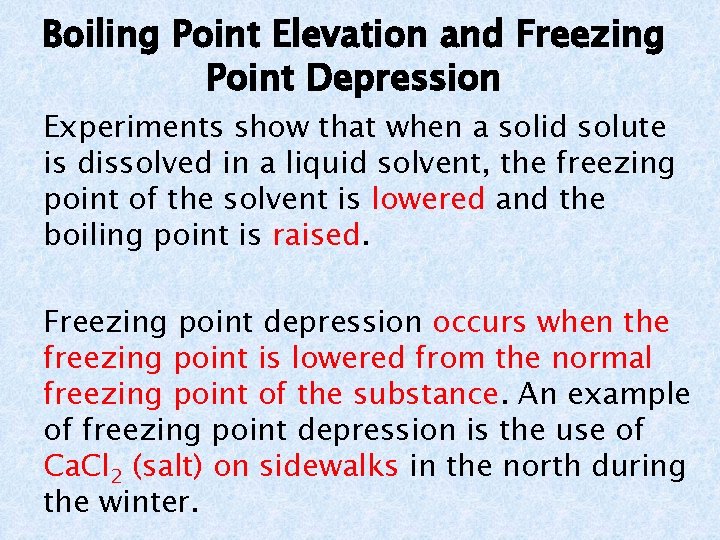
Boiling Point Elevation and Freezing Point Depression Experiments show that when a solid solute is dissolved in a liquid solvent, the freezing point of the solvent is lowered and the boiling point is raised. Freezing point depression occurs when the freezing point is lowered from the normal freezing point of the substance. An example of freezing point depression is the use of Ca. Cl 2 (salt) on sidewalks in the north during the winter.

Boiling Point Elevation and Freezing Point Depression Boiling point elevation occurs when the boiling point is raised from the normal boiling point of the substance. An example of boiling point elevation is the use of antifreeze in cars to keep them from overheating in the summer.

Number of Solute Particles in Solution Boiling point and freezing point changes are proportional to the number of solute particles in solution. The greater the number of solute particles the greater the change.

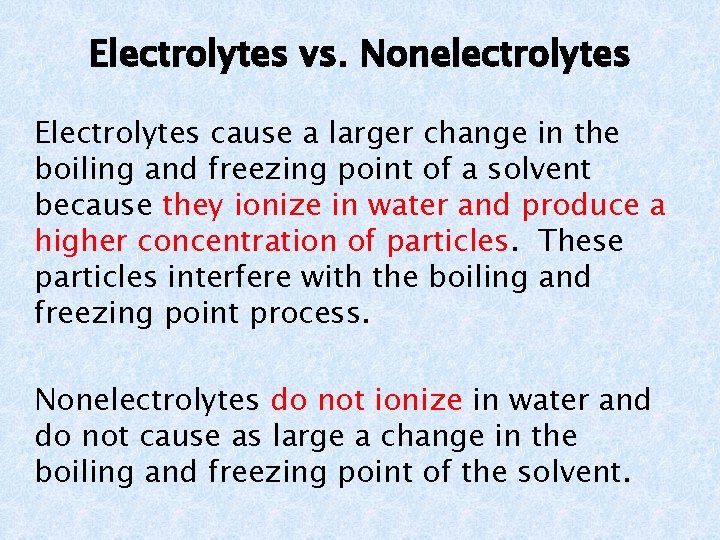
Electrolytes vs. Nonelectrolytes Electrolytes cause a larger change in the boiling and freezing point of a solvent because they ionize in water and produce a higher concentration of particles. These particles interfere with the boiling and freezing point process. Nonelectrolytes do not ionize in water and do not cause as large a change in the boiling and freezing point of the solvent.

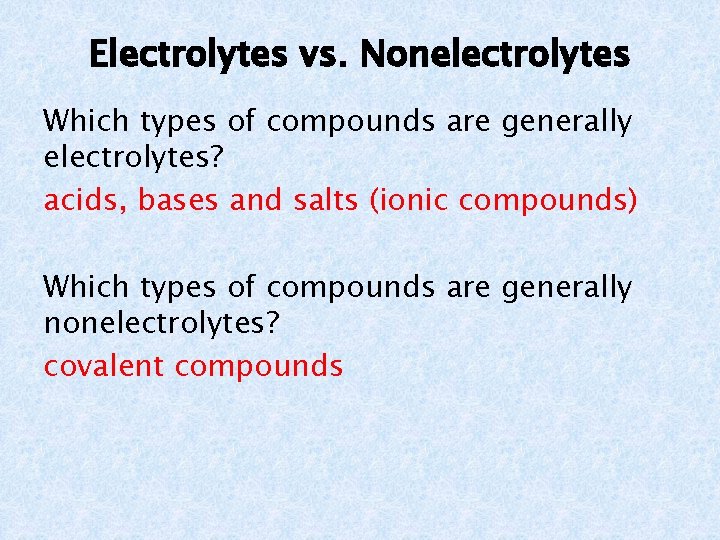
Electrolytes vs. Nonelectrolytes Which types of compounds are generally electrolytes? acids, bases and salts (ionic compounds) Which types of compounds are generally nonelectrolytes? covalent compounds
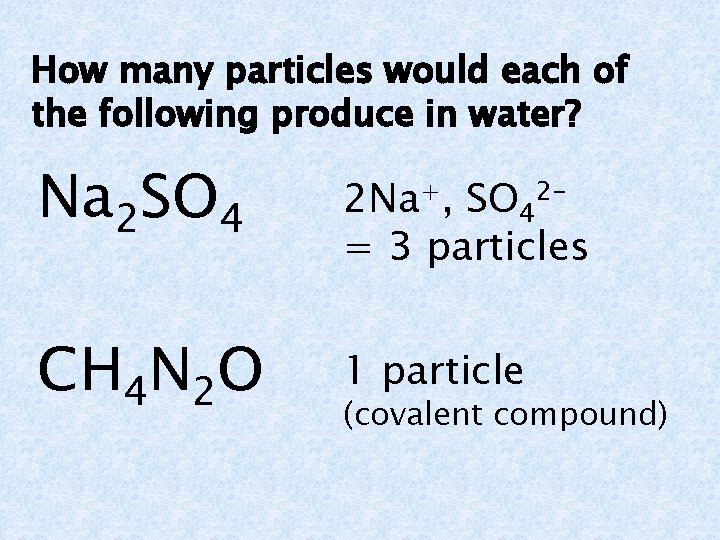
How many particles would each of the following produce in water? Na 2 SO 4 2 Na+, SO 42= 3 particles CH 4 N 2 O 1 particle (covalent compound)

For each of the following pairs indicate which one will affect the freezing/boiling point more. Give a reason for your answer. a. 1 L of 1 M Na. Cl or 1 L of 1 M C 12 H 22 O 11 b. 1 L of 1 M Na. Cl; Salt is ionic and will produce more particles (2). Sugar is covalent (1 particle). 1 L of 1 M Na. Cl or 1 L of 1 M Ba. Cl 2; Na. Cl = 2 particles, Ba. Cl 2 = 3 particles c. 1 L of 1 M Na. Cl or 1 L of 2 M Na. Cl; Higher concentration (equal volumes) = more particles.

Calculating Boiling Point Elevation and Freezing Point Depression The formula for calculating the change in the boiling point of water is: Formula for calculating the change in the freezing point of water: The change in boiling and freezing point can be calculated for solvents other than water.

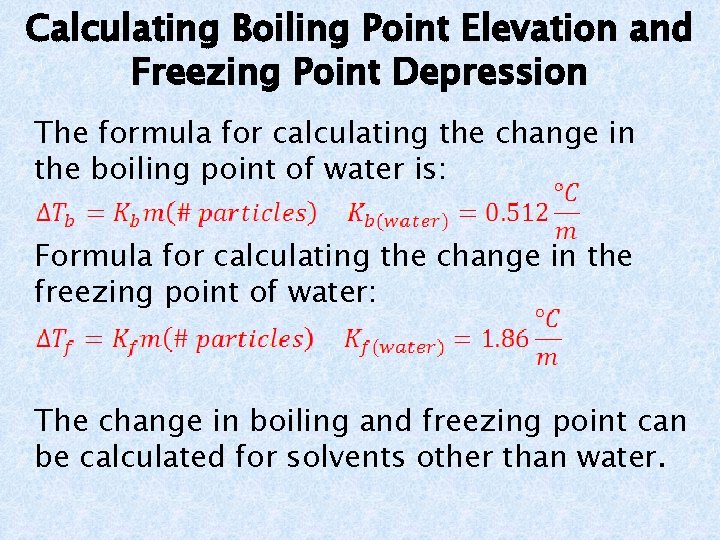
Example Problems a. What is the boiling point of a solution that consists of 1. 0 mol of sucrose (a nonelectrolyte) dissolved in 1. 5 kg of water? The new boiling point = 100°C + 0. 34°C = 100. 34°C

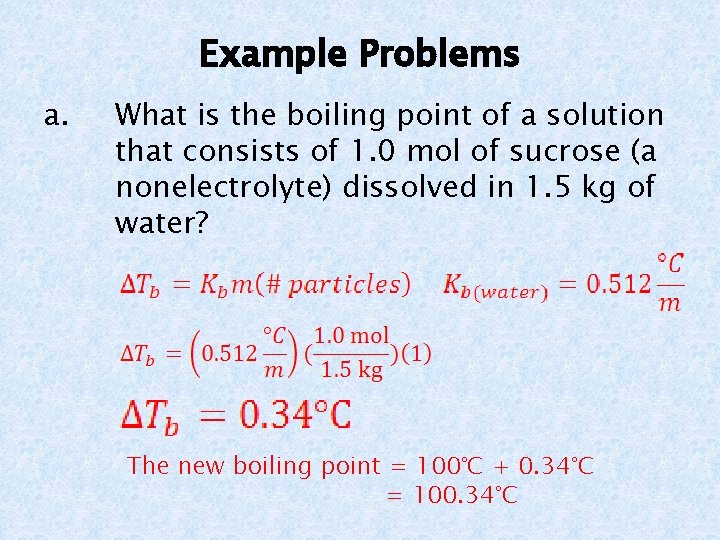
b. Example Problems What is the freezing point of a solution that contains 315 g of Ba. Cl 2 in 2000 g of water? First calculate the number of moles of Ba. Cl 2. Next determine the number of particles. Ba. Cl 2 = 3 particles; 1 Ba 2+, 2 Cl- The new freezing point = 0°C – 4. 22°C = -4. 22°C

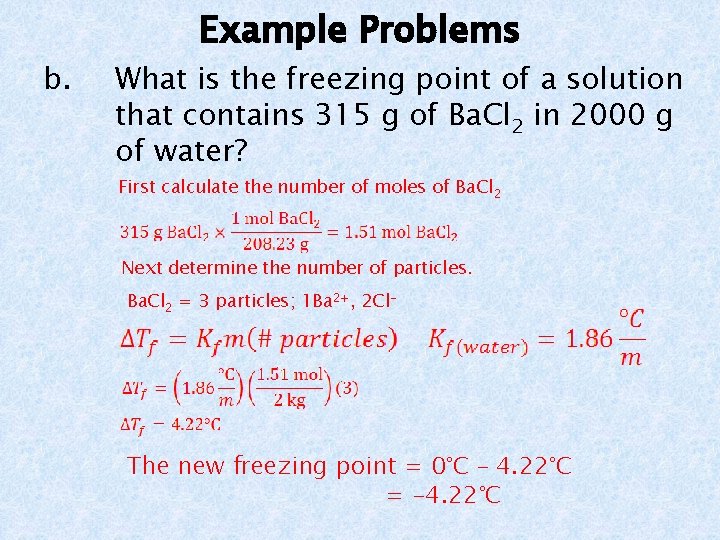
c. Example Problems How many grams of barium nitrate, Ba(NO 3)2, are needed to dissolve in 1 kg of water to make a solution that freezes at -8. 5°C?

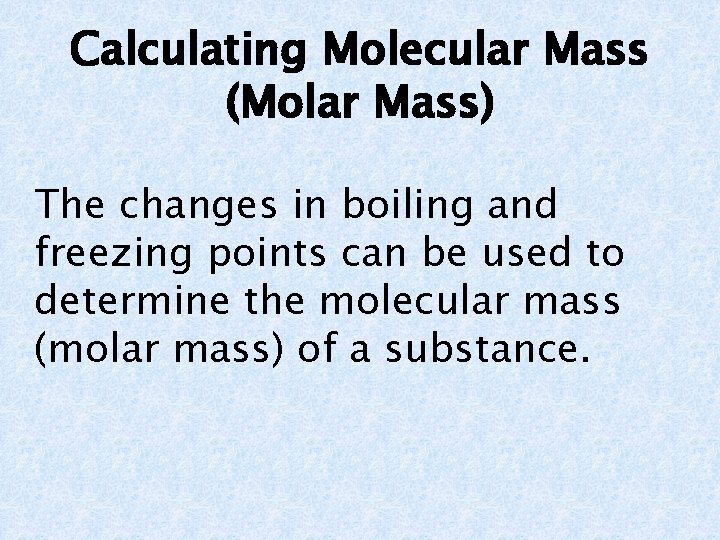
Calculating Molecular Mass (Molar Mass) The changes in boiling and freezing points can be used to determine the molecular mass (molar mass) of a substance.

Example Problem The freezing point for water is lowered to -0. 390°C when 3. 90 g of a nonvolatile molecular solute is dissolved in 475 g of water. Calculate the molecular mass (molar mass) of the solute.

Solutions Lab Carefully, pour the concentrated solution in the volumetric flask. Mix a small amount of water with the measured amount of solute in a beaker. **You must know the volume of all water added, so keep track! Add more water and swirl to mix. Use the dropper to add water to the line.
 Are aqueous solutions homogeneous mixtures
Are aqueous solutions homogeneous mixtures Are aqueous solutions homogeneous mixtures
Are aqueous solutions homogeneous mixtures Reactions in aqueous solutions
Reactions in aqueous solutions Balancing redox reactions in acidic solution
Balancing redox reactions in acidic solution Modern chemistry chapter 13 review answers
Modern chemistry chapter 13 review answers Aqueous solutions module
Aqueous solutions module Types of reactions
Types of reactions Freezing point chapter 13
Freezing point chapter 13 General properties of aqueous solutions
General properties of aqueous solutions Aqueous solution practice problems
Aqueous solution practice problems Electrical conductivity of aqueous solutions
Electrical conductivity of aqueous solutions Chapter 4 reactions in aqueous solutions
Chapter 4 reactions in aqueous solutions Solubility guidelines for aqueous solutions
Solubility guidelines for aqueous solutions Chapter 4 reactions in aqueous solutions
Chapter 4 reactions in aqueous solutions Chapter 4 reactions in aqueous solutions worksheet answers
Chapter 4 reactions in aqueous solutions worksheet answers Chapter 4 reactions in aqueous solutions
Chapter 4 reactions in aqueous solutions Are all solutions homogeneous mixtures
Are all solutions homogeneous mixtures Compare and contrast mixtures and solutions.
Compare and contrast mixtures and solutions. Chapter 14 mixtures and solutions
Chapter 14 mixtures and solutions Chapter 13 mixtures and solutions answers
Chapter 13 mixtures and solutions answers Colloids mixture
Colloids mixture Mixtures solubility and acid/base solutions answer key
Mixtures solubility and acid/base solutions answer key Compounds mixtures and elements worksheet
Compounds mixtures and elements worksheet Separating mixtures simulation
Separating mixtures simulation Types of mixtures
Types of mixtures Mixtures and solutions quiz
Mixtures and solutions quiz Solutions are homogeneous mixtures
Solutions are homogeneous mixtures Matter and materials grade 6
Matter and materials grade 6 Solutions are heterogenous mixtures
Solutions are heterogenous mixtures Types of reaction in non aqueous solvents
Types of reaction in non aqueous solvents Pelarut non air
Pelarut non air What are protonic and non protonic solvents
What are protonic and non protonic solvents 6 types of mixtures
6 types of mixtures Different types of mixtures
Different types of mixtures Types of matter elements compounds and mixtures
Types of matter elements compounds and mixtures Mixture
Mixture Clarity of aqueous extract test
Clarity of aqueous extract test Mnvii
Mnvii Abbreviation for solid in chemistry
Abbreviation for solid in chemistry Additional aspects of aqueous equilibria
Additional aspects of aqueous equilibria Tyndall effect
Tyndall effect Chapter 15 water and aqueous systems answer key
Chapter 15 water and aqueous systems answer key Do pickles conduct electricity
Do pickles conduct electricity Additional aspects of aqueous equilibria
Additional aspects of aqueous equilibria Aqueous reactions and solution stoichiometry
Aqueous reactions and solution stoichiometry An artificial cell consisting of an aqueous solution
An artificial cell consisting of an aqueous solution Aqueous acid base titration
Aqueous acid base titration Suspension vs solution
Suspension vs solution Homogeneous aqueous systems
Homogeneous aqueous systems Is water aqueous
Is water aqueous Manipulation of impression compound
Manipulation of impression compound Dr noland naidoo
Dr noland naidoo Chemsheets reactions of alkenes 2 answers
Chemsheets reactions of alkenes 2 answers Aqueous equilibria
Aqueous equilibria Halogenoalkane to alcohol mechanism
Halogenoalkane to alcohol mechanism Precipitating agent
Precipitating agent Brf3 is protic solvent
Brf3 is protic solvent Non aqueous media
Non aqueous media Macular scar
Macular scar Aqueous humor
Aqueous humor Aqueous ammonia
Aqueous ammonia Eye diagram pearson
Eye diagram pearson Uveitis definition
Uveitis definition What is aqueous
What is aqueous Figure 8.3
Figure 8.3 Which of the following is not monophasic liquid dosage form
Which of the following is not monophasic liquid dosage form Benzalacetone
Benzalacetone Chapter 15 water and aqueous systems
Chapter 15 water and aqueous systems Watery humour
Watery humour Assume that an aqueous solution of a cation
Assume that an aqueous solution of a cation Chapter 15 water and aqueous systems
Chapter 15 water and aqueous systems Red and blue marbles of the same size and mass
Red and blue marbles of the same size and mass Decanting
Decanting Separating mixtures grade 7
Separating mixtures grade 7 Filtration is used to separate
Filtration is used to separate Separation by evaporation
Separation by evaporation Magnetism in separating mixtures
Magnetism in separating mixtures Mixtures separated by distillation
Mixtures separated by distillation Are compounds pure substances
Are compounds pure substances Which are pure substances
Which are pure substances Are compounds pure substances
Are compounds pure substances Allegation method
Allegation method Properties of materials grade 7 powerpoint
Properties of materials grade 7 powerpoint Pictures of mixtures
Pictures of mixtures