Small Cell Lung Cancer Palak Desai MD Natural









































- Slides: 84

Small Cell Lung Cancer Palak Desai, MD



Natural History of SCLC is distinguished from NSCLC by its rapid doubling time, high growth fraction, and the early development of widespread metastases Although considered highly responsive to chemotherapy and radiotherapy, SCLC usually relapses within two years despite treatment Overall, only three to eight percent of all patients with SCLC (10 to 13 percent of those with limited disease) survive beyond five years

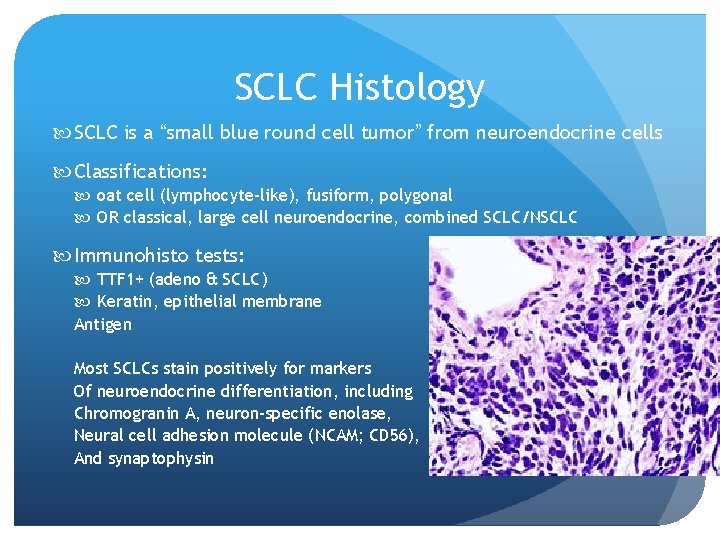
SCLC Histology SCLC is a “small blue round cell tumor” from neuroendocrine cells Classifications: oat cell (lymphocyte-like), fusiform, polygonal OR classical, large cell neuroendocrine, combined SCLC/NSCLC Immunohisto tests: TTF 1+ (adeno & SCLC) Keratin, epithelial membrane Antigen Most SCLCs stain positively for markers Of neuroendocrine differentiation, including Chromogranin A, neuron-specific enolase, Neural cell adhesion molecule (NCAM; CD 56), And synaptophysin

Clinical Presentation of SCLC Smokers (almost exclusively) Cough 75% Hemoptysis in 50% Dyspnea and chest pain 40% Constitutional symptoms 10 to 15% Clubbing 16 to 29% pneumonia, weight loss

SCLC Paraneoplastic Syndromes SIADH ectopic ACTH production- Cushing’s syndrome Eaton-Lambert Myasthenic syndrome proximal muscle weakness that improves on repetition (“facilitation”) Hypercalcemia Peripheral Neuropathy

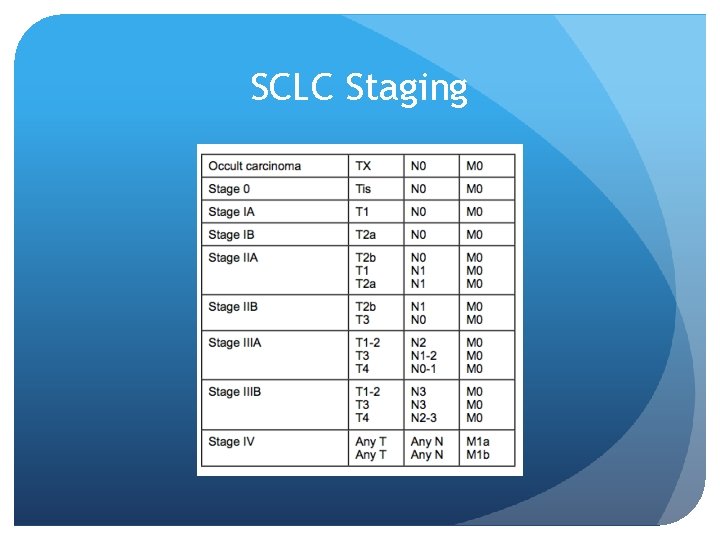
SCLC Staging

SCLC Staging

SCLC Staging

Where does SCLC metastasize to? Brain (30%) Adrenal (20 -40%) Liver (25%) Lung Skeleton (35%)

Prognostic Factors The host factors of poor performance status and weight loss Stage (limited versus extensive). In extensive disease, the number of organ sites involved is inversely related to prognosis Metastatic involvement of the central nervous system, the marrow, or the liver is unfavorable compared to other sites, although these variables are confounded by the number of sites of involvement. In most trials, women fare better than men, although the reasons for this are not known. The presence of paraneoplastic syndromes is generally unfavorable

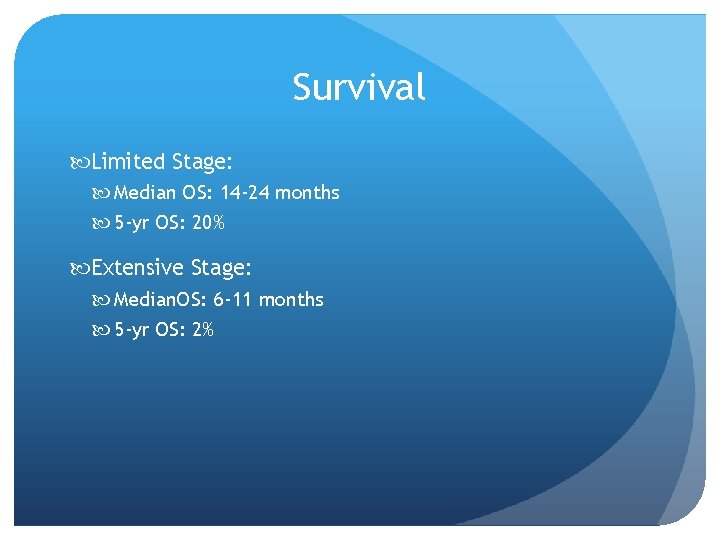
Survival Limited Stage: Median OS: 14 -24 months 5 -yr OS: 20% Extensive Stage: Median. OS: 6 -11 months 5 -yr OS: 2%

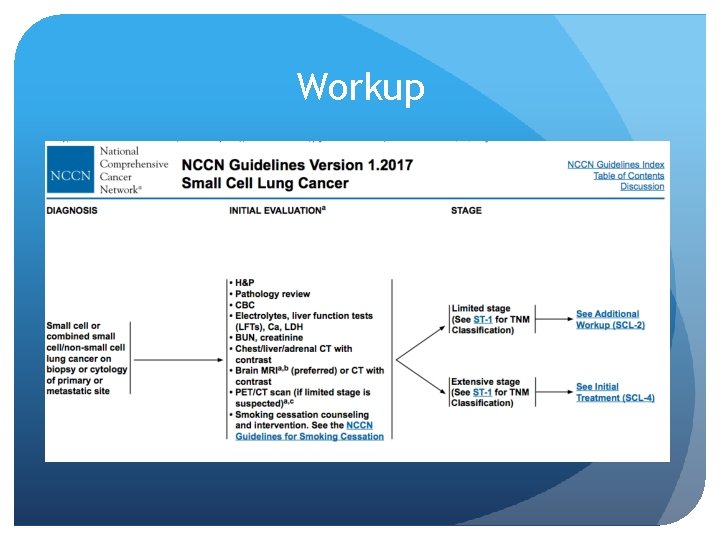
Workup

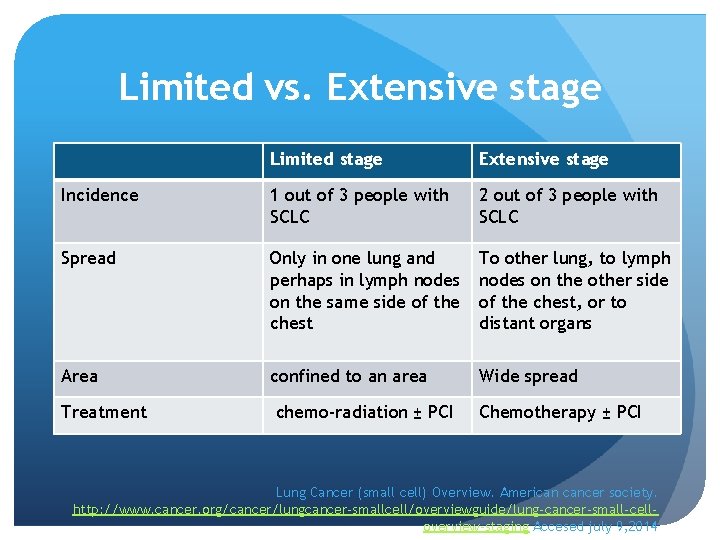
Limited vs. Extensive stage Limited stage Extensive stage Incidence 1 out of 3 people with SCLC 2 out of 3 people with SCLC Spread Only in one lung and perhaps in lymph nodes on the same side of the chest To other lung, to lymph nodes on the other side of the chest, or to distant organs Area confined to an area Wide spread Treatment chemo-radiation ± PCI Chemotherapy ± PCI Lung Cancer (small cell) Overview. American cancer society. http: //www. cancer. org/cancer/lungcancer-smallcell/overviewguide/lung-cancer-small-celloverview-staging Accesed july 9, 2014

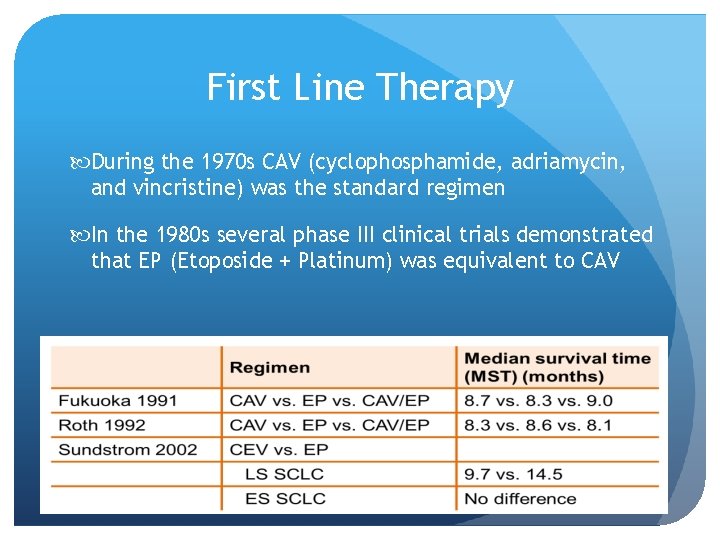
First Line Therapy During the 1970 s CAV (cyclophosphamide, adriamycin, and vincristine) was the standard regimen In the 1980 s several phase III clinical trials demonstrated that EP (Etoposide + Platinum) was equivalent to CAV

CAV vs EP Sundstrom et al, 2002 436 patients were randomized to chemotherapy with EP (n = 218) or CEV (n = 218). Patients were stratified according to extent of disease (limited disease [LD], n = 214; extensive disease [ED], n = 222). The EP group received five courses of etoposide 100 mg/m(2) intravenously (IV) and cisplatin 75 mg/m(2) IV on day 1, followed by oral etoposide 200 mg/m(2) daily on days 2 to 4. The CEV group received five courses of epirubicin 50 mg/m(2), cyclophosphamide 1, 000 mg/m(2), and vincristine 2 mg, all IV on day 1. In addition, LD patients received thoracic radiotherapy concurrent with chemotherapy cycle 3, and those achieving complete remission during the treatment period received prophylactic cranial irradiation. Sundstrom et al, JCO 2002

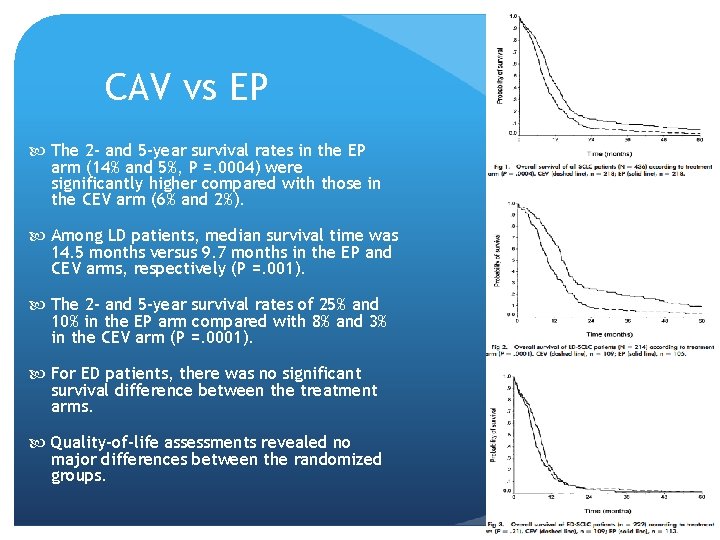
CAV vs EP The 2 - and 5 -year survival rates in the EP arm (14% and 5%, P =. 0004) were significantly higher compared with those in the CEV arm (6% and 2%). Among LD patients, median survival time was 14. 5 months versus 9. 7 months in the EP and CEV arms, respectively (P =. 001). The 2 - and 5 -year survival rates of 25% and 10% in the EP arm compared with 8% and 3% in the CEV arm (P =. 0001). For ED patients, there was no significant survival difference between the treatment arms. Quality-of-life assessments revealed no major differences between the randomized groups.

Carboplatin vs Cisplatin In clinical practice, carboplatin is frequently substituted for cisplatin to reduse the risk of emesis, neuropathy, and nephropathy. The use of carboplatin carries a greater risk of myelosuppression Randomized trials have suggested similar efficacy of cisplatin and carboplatin in patients with small cell lung cancer

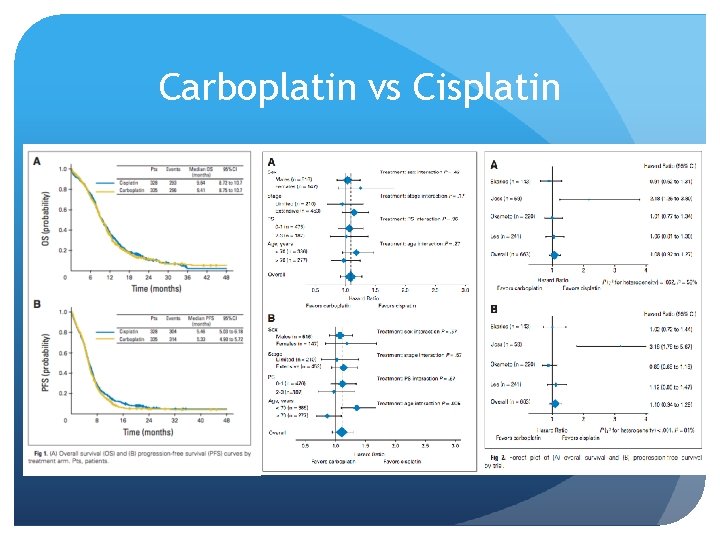
Carboplatin vs Cisplatin Rossi et al, 2012– Meta-analysis Four eligible trials with 663 patients (328 assigned to cisplatin and 335 to carboplatin) were included in the analysis. Median OS was 9. 6 months for cisplatin and 9. 4 months for carboplatin (hazard ratio [HR], 1. 08; 95% CI, 0. 92 to 1. 27; P =. 37). There was no evidence of treatment difference between the cisplatin and carboplatin arms according to sex, stage, performance status, or age. Median PFS was 5. 5 and 5. 3 months for cisplatin and carboplatin, respectively (HR, 1. 10; 95% CI, 0. 94 to 1. 29; P =. 25). ORR was 67. 1% and 66. 0%, respectively (relative risk, 0. 98; 95% CI, 0. 84 to 1. 16; P =. 83). Toxicity profile was significantly different for each of the arms: hematologic toxicity was higher with carboplatin, and nonhematologic toxicity was higher with cisplatin. Rossi et al, JCO 2012

Carboplatin vs Cisplatin

Other Chemotherapy Combinations

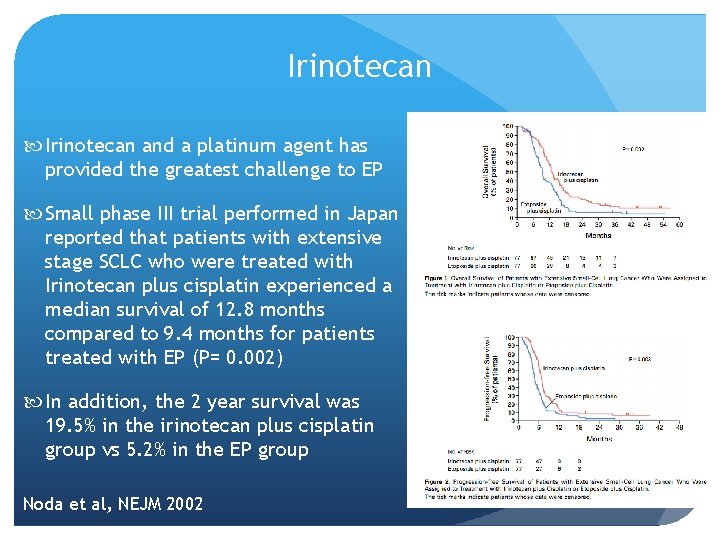
Irinotecan and a platinum agent has provided the greatest challenge to EP Small phase III trial performed in Japan reported that patients with extensive stage SCLC who were treated with Irinotecan plus cisplatin experienced a median survival of 12. 8 months compared to 9. 4 months for patients treated with EP (P= 0. 002) In addition, the 2 year survival was 19. 5% in the irinotecan plus cisplatin group vs 5. 2% in the EP group Noda et al, NEJM 2002

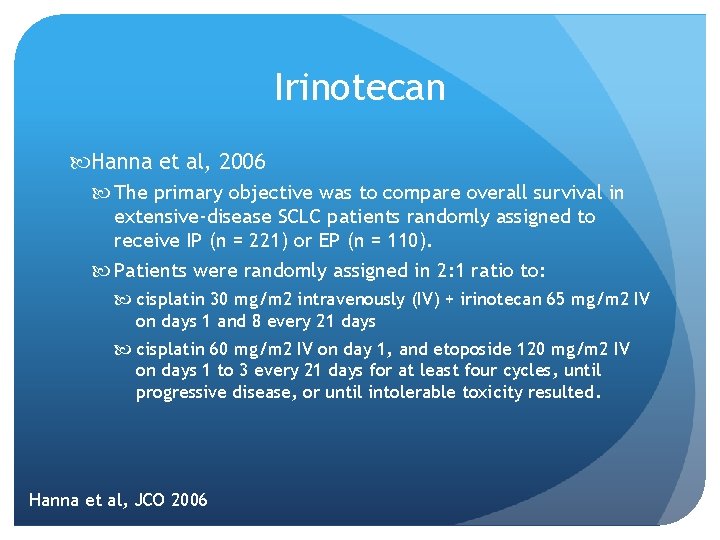
Irinotecan Hanna et al, 2006 The primary objective was to compare overall survival in extensive-disease SCLC patients randomly assigned to receive IP (n = 221) or EP (n = 110). Patients were randomly assigned in 2: 1 ratio to: cisplatin 30 mg/m 2 intravenously (IV) + irinotecan 65 mg/m 2 IV on days 1 and 8 every 21 days cisplatin 60 mg/m 2 IV on day 1, and etoposide 120 mg/m 2 IV on days 1 to 3 every 21 days for at least four cycles, until progressive disease, or until intolerable toxicity resulted. Hanna et al, JCO 2006

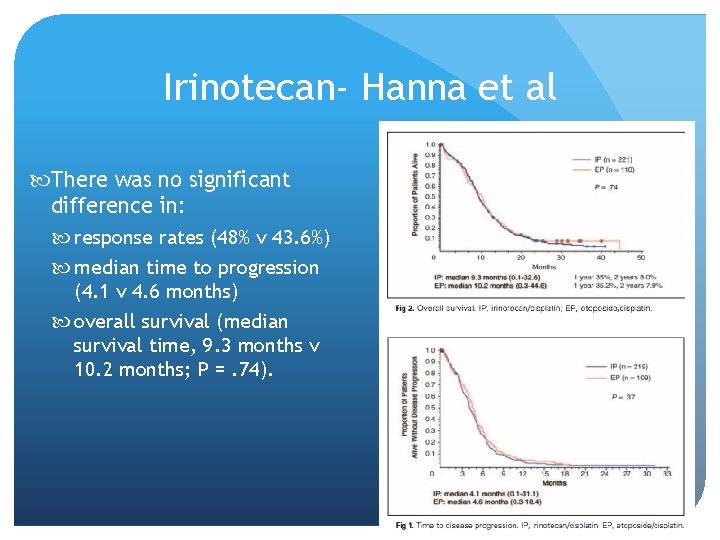
Irinotecan- Hanna et al There was no significant difference in: response rates (48% v 43. 6%) median time to progression (4. 1 v 4. 6 months) overall survival (median survival time, 9. 3 months v 10. 2 months; P =. 74).


Irinotecan SWOG S 0124 North American Trial 651 patients Patients were randomly assigned: IP (irinotecan 60 mg/m(2) on days 1, 8, and 15; cisplatin 60 mg/m(2) day 1, every 4 weeks) EP (etoposide 100 mg/m(2) on days 1 through 3; cisplatin 80 mg/m(2) day 1, every 3 weeks). Lara et al, JCO, 2009

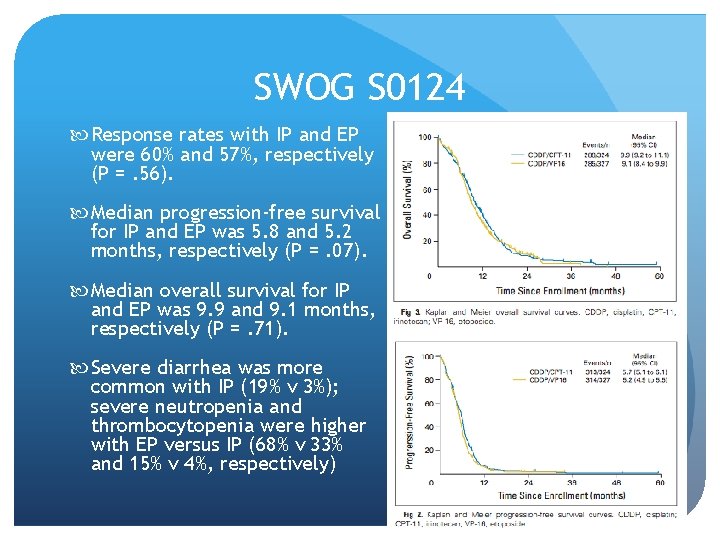
SWOG S 0124 Response rates with IP and EP were 60% and 57%, respectively (P =. 56). Median progression-free survival for IP and EP was 5. 8 and 5. 2 months, respectively (P =. 07). Median overall survival for IP and EP was 9. 9 and 9. 1 months, respectively (P =. 71). Severe diarrhea was more common with IP (19% v 3%); severe neutropenia and thrombocytopenia were higher with EP versus IP (68% v 33% and 15% v 4%, respectively)

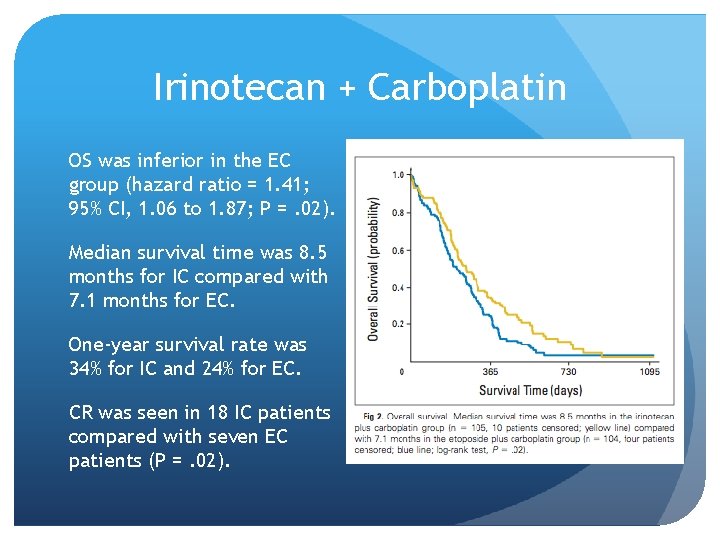
Irinotecan + Carboplatin Hermes et al Randomized phase III trial 209 patients Patients with ED SCLC were randomly assigned to receive either (IC)--carboplatin (AUC= 4) and irinotecan (175 mg/m 2) intravenously both on day 1 (EC) consisted of carboplatin as in IC and etoposide (120 mg/m(2)/d) orally on days 1 through 5. Courses were repeated every 3 weeks with four cycles planned. Doses were reduced by one third in patients with a WHO performance status (PS) of 3 to 4 and/or age older than 70 years. Primary end point was overall survival (OS). Secondary end points were quality of life (QOL) and complete response (CR) rate. Hermes et al, JCO 2008

Irinotecan + Carboplatin OS was inferior in the EC group (hazard ratio = 1. 41; 95% CI, 1. 06 to 1. 87; P =. 02). Median survival time was 8. 5 months for IC compared with 7. 1 months for EC. One-year survival rate was 34% for IC and 24% for EC. CR was seen in 18 IC patients compared with seven EC patients (P =. 02).

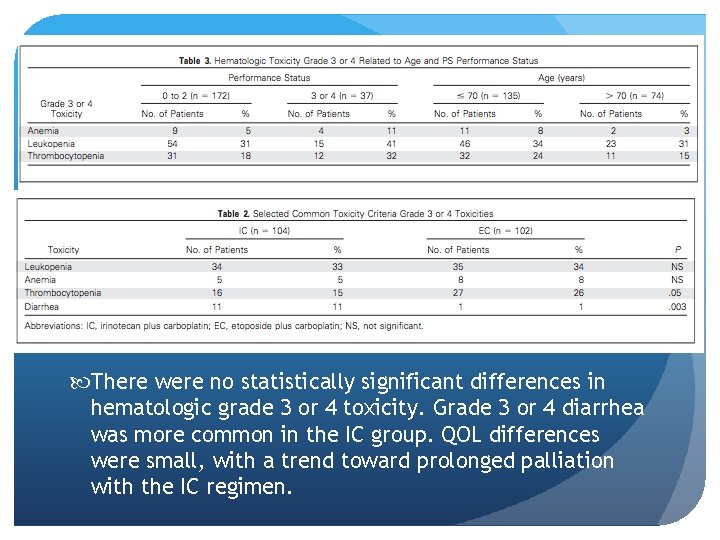
There were no statistically significant differences in hematologic grade 3 or 4 toxicity. Grade 3 or 4 diarrhea was more common in the IC group. QOL differences were small, with a trend toward prolonged palliation with the IC regimen.

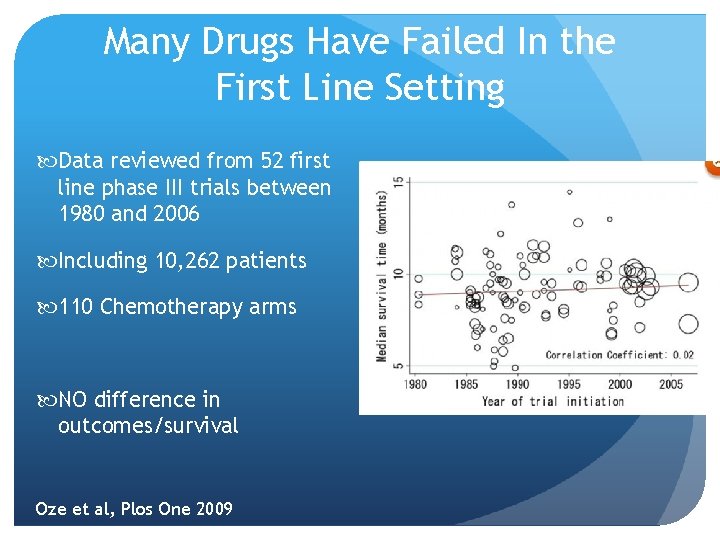
Many Drugs Have Failed In the First Line Setting Data reviewed from 52 first line phase III trials between 1980 and 2006 Including 10, 262 patients 110 Chemotherapy arms NO difference in outcomes/survival Oze et al, Plos One 2009

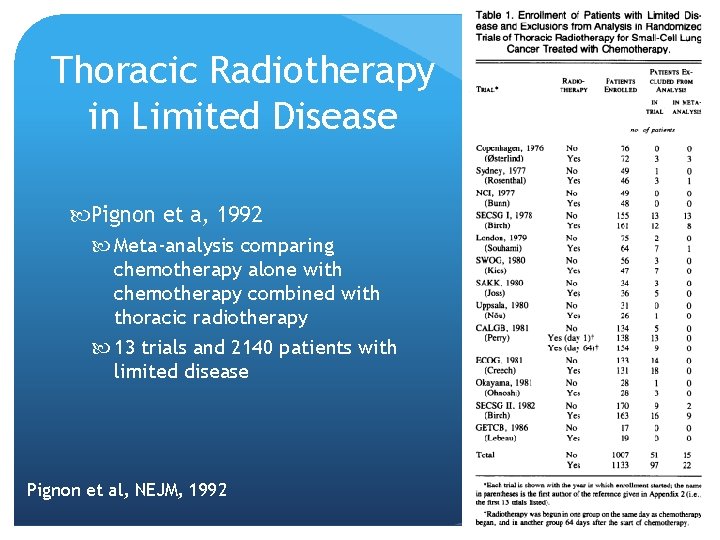
Thoracic Radiotherapy in Limited Disease Pignon et a, 1992 Meta-analysis comparing chemotherapy alone with chemotherapy combined with thoracic radiotherapy 13 trials and 2140 patients with limited disease Pignon et al, NEJM, 1992

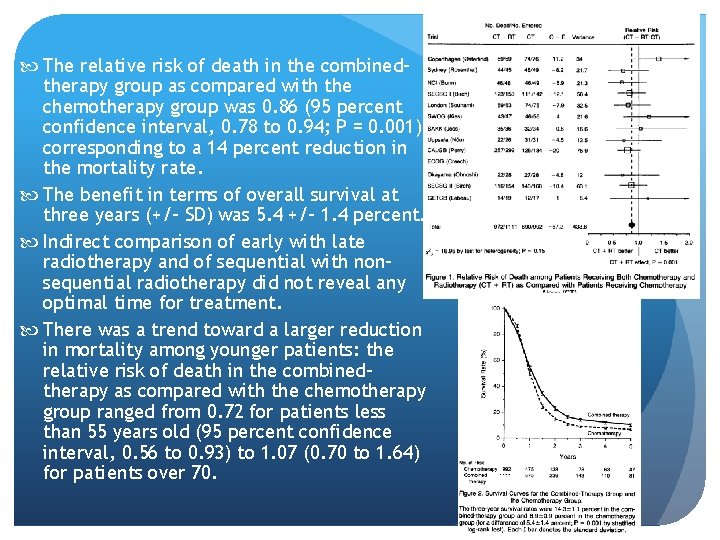
The relative risk of death in the combinedtherapy group as compared with the chemotherapy group was 0. 86 (95 percent confidence interval, 0. 78 to 0. 94; P = 0. 001), corresponding to a 14 percent reduction in the mortality rate. The benefit in terms of overall survival at three years (+/- SD) was 5. 4 +/- 1. 4 percent. Indirect comparison of early with late radiotherapy and of sequential with nonsequential radiotherapy did not reveal any optimal time for treatment. There was a trend toward a larger reduction in mortality among younger patients: the relative risk of death in the combinedtherapy as compared with the chemotherapy group ranged from 0. 72 for patients less than 55 years old (95 percent confidence interval, 0. 56 to 0. 93) to 1. 07 (0. 70 to 1. 64) for patients over 70.

Response Rates In patients with limited stage disease, response rates of 70% to 90% are expected after treatment with EP + thoracic radiotherapy In extensive stage disease, response rates of 60% to 70% can be achieved with chemotherapy alone Unfortunately median survival rates are only 14 to 20 months for limited stage disease and 9 to 11 months for patients with extensive stage disease After appropriate treatment, the 2 year survival rate is approximately 40% for limited stage disease and 5% in those with extensive stage disease Chute et al, JCO 1999

Adding a 3 rd agent? Many strategies have been evaluated in an effort to improve on the standard treatment for extensive stage disease, including the addition of a third agent to standard 2 -drug regimens In 2 trials, the addition of ifosfamide or cyclophosphamide + an anthracycline to EP showed modest survival advantage for patients with extensive disease.

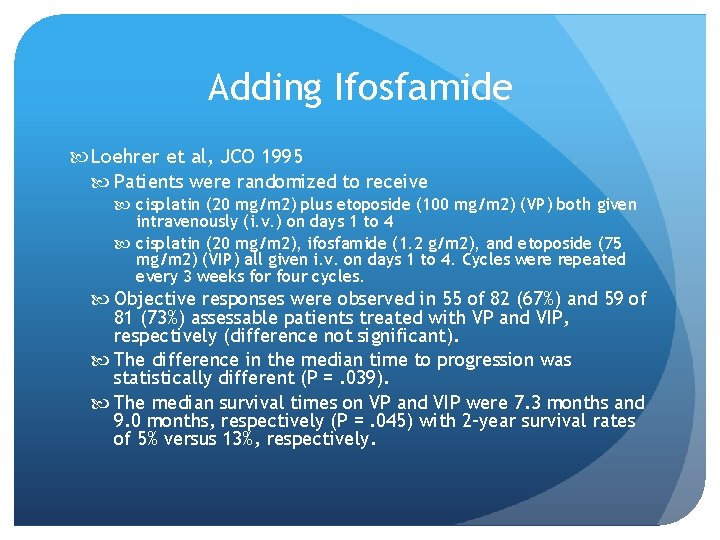
Adding Ifosfamide Loehrer et al, JCO 1995 Patients were randomized to receive cisplatin (20 mg/m 2) plus etoposide (100 mg/m 2) (VP) both given intravenously (i. v. ) on days 1 to 4 cisplatin (20 mg/m 2), ifosfamide (1. 2 g/m 2), and etoposide (75 mg/m 2) (VIP) all given i. v. on days 1 to 4. Cycles were repeated every 3 weeks for four cycles. Objective responses were observed in 55 of 82 (67%) and 59 of 81 (73%) assessable patients treated with VP and VIP, respectively (difference not significant). The difference in the median time to progression was statistically different (P =. 039). The median survival times on VP and VIP were 7. 3 months and 9. 0 months, respectively (P =. 045) with 2 -year survival rates of 5% versus 13%, respectively.

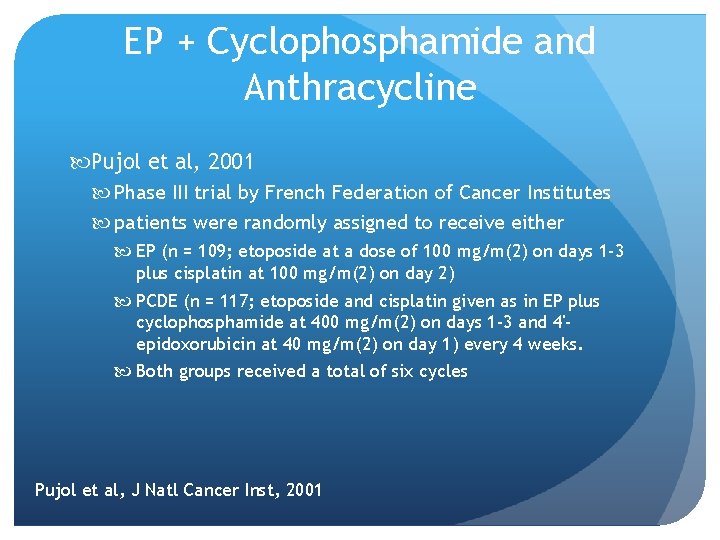
EP + Cyclophosphamide and Anthracycline Pujol et al, 2001 Phase III trial by French Federation of Cancer Institutes patients were randomly assigned to receive either EP (n = 109; etoposide at a dose of 100 mg/m(2) on days 1 -3 plus cisplatin at 100 mg/m(2) on day 2) PCDE (n = 117; etoposide and cisplatin given as in EP plus cyclophosphamide at 400 mg/m(2) on days 1 -3 and 4'epidoxorubicin at 40 mg/m(2) on day 1) every 4 weeks. Both groups received a total of six cycles Pujol et al, J Natl Cancer Inst, 2001

EP + Cyclophosphamide and Anthracycline Pujol et al, 2001 Patients in the PCDE arm had a statistically significant higher frequency of combined complete plus partial responses compared with those in the EP arm (21% plus 55% versus 13% plus 48%, respectively; P =. 02). Patients in the PCDE arm survived longer than those in the EP arm (1 -year survival rate: 40% and 29%, respectively; median survival: 10. 5 and 9. 3 months, respectively; logrank P =. 0067). relative risk of death for patients in the PCDE arm compared with those in the EP arm was 0. 70 (95% confidence interval = 0. 51 to 0. 95); the disease also progressed more slowly in patients in the PCDE arm.

Safety Hematologic toxicity was higher in the PCDE arm (22% with documented infections compared with 8% in the EP arm; P =. 0038), and the toxicity-related death rate was 9% in the PCDE arm versus 5. 5% in the EP arm (P =. 22). The global health status showed similar improvement in both arms during treatment. These findings have not been uniformly observed, and the addition of an alkylating agent, with or without anthracycline, significantly increases hematologic toxicity when compared to EP alone

The addition of paclitaxel to either cisplatin or carboplatin plus etoposide yielded promising results in phase II trials but did not improve overall survival, and was associated with unacceptable toxicity in a subsequent phase III study Maintenance chemotherapy has not been shown to prolong survival as well Neill et al, JCO 2005, Zhou et al, Plos One 2013

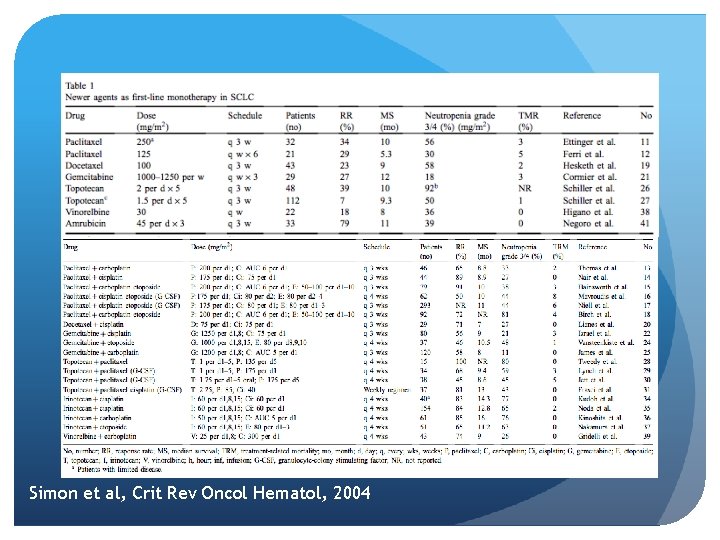
Simon et al, Crit Rev Oncol Hematol, 2004

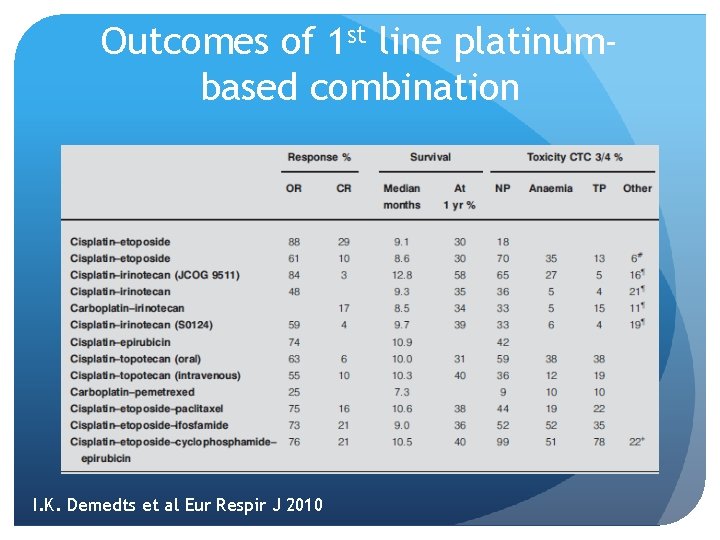
Outcomes of 1 st line platinumbased combination I. K. Demedts et al Eur Respir J 2010

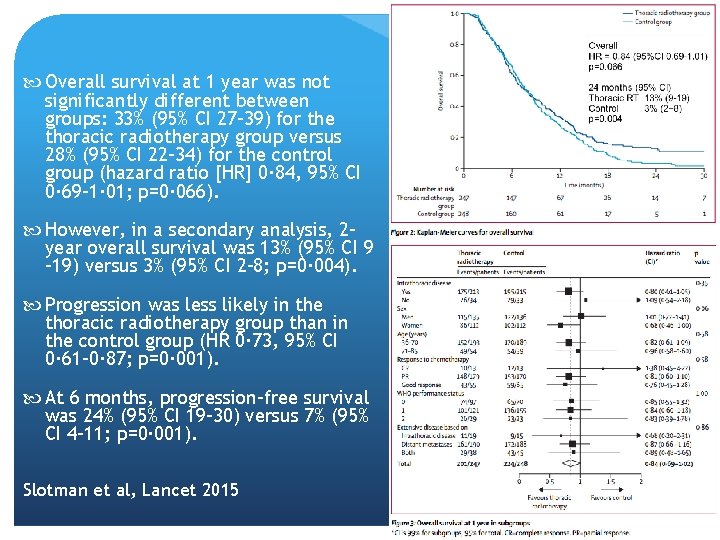
Thoracic Radiotherapy for ES Most patients with extensive stage small-cell lung cancer (ESSCLC) who undergo chemotherapy, and prophylactic cranial irradiation, have persistent intrathoracic disease. Phase 3 randomized controlled trial at 42 hospitals, 498 patients Patients with WHO performance score 0– 2 and confirmed ES-SCLC who responded to chemotherapy. They were randomly assigned (1: 1) to receive either thoracic radiotherapy (30 Gy in ten fractions) or no thoracic radiotherapy. All underwent prophylactic cranial irradiation. The primary endpoint was overall survival at 1 year in the intention-to-treat population. Secondary endpoints included progression-free survival Slotman et al, Lancet 2015

Overall survival at 1 year was not significantly different between groups: 33% (95% CI 27– 39) for the thoracic radiotherapy group versus 28% (95% CI 22– 34) for the control group (hazard ratio [HR] 0· 84, 95% CI 0· 69– 1· 01; p=0· 066). However, in a secondary analysis, 2 year overall survival was 13% (95% CI 9 – 19) versus 3% (95% CI 2– 8; p=0· 004). Progression was less likely in the thoracic radiotherapy group than in the control group (HR 0· 73, 95% CI 0· 61– 0· 87; p=0· 001). At 6 months, progression-free survival was 24% (95% CI 19– 30) versus 7% (95% CI 4– 11; p=0· 001). Slotman et al, Lancet 2015

Second Line Therapy Although SCLC is very responsive to initial treatment, most patients relapse with relatively resistant disease These patients have a median survival of only 4 to 5 months when treated with further chemotherapy Second line and third line chemotherapy provides significant palliation in many patients, although the likelihood of response is highly dependent on the time from initial therapy to relapse If the interval is less than 3 months, response to most agents is poor (<10%) If interval is more than 3 months, then the expected response rates are approximately 25% If patients relapse more than 6 months after first line treatment, then treatment with their original regimen is recommended

Topotecan and cyclophosphamide, doxorubicin, and vincristine (CAV) were evaluated in a randomized, multicenter study of patients with small-cell lung cancer (SCLC) who had relapsed at least 60 days after completion of first-line therapy. Patients received either topotecan (1. 5 mg/m 2) as a 30 -minute infusion daily for 5 days every 21 days (n = 107) CAV (cyclophosphamide 1, 000 mg/m 2, doxorubicin 45 mg/m 2, and vincristine 2 mg) infused on day 1 every 21 days (n = 104). Eligibility included the following: bidimensionally measurable disease ECOG performance status of less than or equal to 2 and adequate marrow, liver, and renal function. Von Pawel et al, JCO 1999

Response rate was: 26 of 107 patients (24. 3%) treated with topotecan 19 of 104 patients (18. 3%) treated with CAV (P =. 285). Median times to progression were 13. 3 weeks (topotecan) and 12. 3 weeks (CAV) (P =. 552). Median survival was 25. 0 weeks for topotecan and 24. 7 weeks for CAV (P =. 795). The proportion of patients who experienced symptom improvement was greater in the topotecan group than in the CAV group for four of eight symptoms evaluated, including dyspnea, anorexia, hoarseness, and fatigue, as well as interference with daily activity (P< or =. 043). Grade 4 neutropenia occurred in 37. 8% of topotecan courses versus 51. 4% of CAV courses (P<. 001). Grade 4 thrombocytopenia and grade 3/4 anemia occurred more frequently with topotecan, occurring in 9. 8% and 17. 7% of topotecan courses versus 1. 4% and 7. 2% of CAV courses, respectively (P<. 001 for both). Nonhematologic toxicities were generally grade 1 to 2 for both regimens. Von Pawel et al, JCO 1999

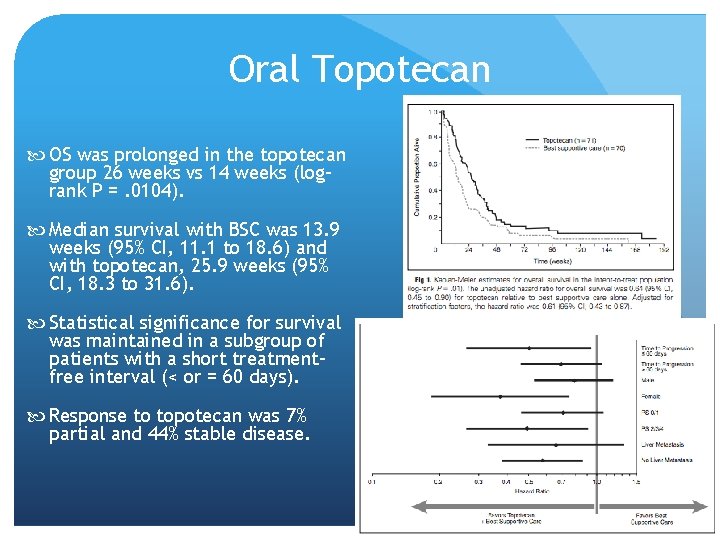
Oral Topotecan O’Brien et al, 2006 randomly assigned patients with relapsed SCLC not considered as candidates for standard intravenous therapy to best supportive care (BSC) alone (n = 70) or oral topotecan (2. 3 mg/m 2/d, days 1 through 5, every 21 days) plus BSC (topotecan; n = 71). Primary end point was overall survival O’Brien et al, JCO 2006

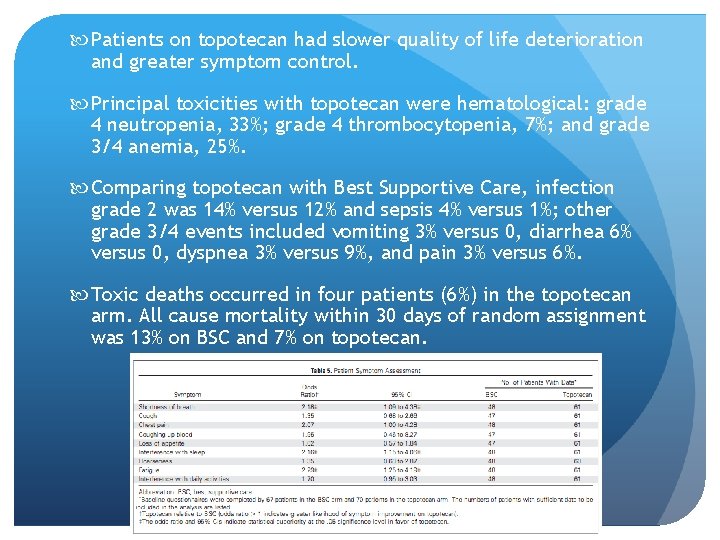
Oral Topotecan OS was prolonged in the topotecan group 26 weeks vs 14 weeks (logrank P =. 0104). Median survival with BSC was 13. 9 weeks (95% CI, 11. 1 to 18. 6) and with topotecan, 25. 9 weeks (95% CI, 18. 3 to 31. 6). Statistical significance for survival was maintained in a subgroup of patients with a short treatmentfree interval (< or = 60 days). Response to topotecan was 7% partial and 44% stable disease.

Patients on topotecan had slower quality of life deterioration and greater symptom control. Principal toxicities with topotecan were hematological: grade 4 neutropenia, 33%; grade 4 thrombocytopenia, 7%; and grade 3/4 anemia, 25%. Comparing topotecan with Best Supportive Care, infection grade 2 was 14% versus 12% and sepsis 4% versus 1%; other grade 3/4 events included vomiting 3% versus 0, diarrhea 6% versus 0, dyspnea 3% versus 9%, and pain 3% versus 6%. Toxic deaths occurred in four patients (6%) in the topotecan arm. All cause mortality within 30 days of random assignment was 13% on BSC and 7% on topotecan.

Prophylactic Cranial Irradiation EORTC Study Patients between the ages of 18 and 75 years with extensive stage small-cell lung cancer were randomly assigned to undergo prophylactic cranial irradiation (irradiation group) or receive no furtherapy (control group). The primary end point was the time to symptomatic brain metastases. CT or MRI of the brain was performed when any predefined key symptom suggestive of brain metastases was present. The two groups (each with 143 patients) were well balanced regarding baseline characteristics. Slotman et al, NEJM 2007

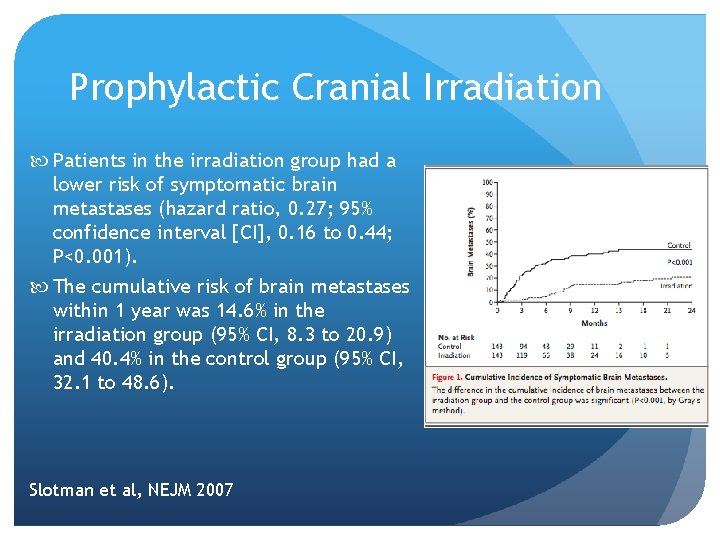
Prophylactic Cranial Irradiation Patients in the irradiation group had a lower risk of symptomatic brain metastases (hazard ratio, 0. 27; 95% confidence interval [CI], 0. 16 to 0. 44; P<0. 001). The cumulative risk of brain metastases within 1 year was 14. 6% in the irradiation group (95% CI, 8. 3 to 20. 9) and 40. 4% in the control group (95% CI, 32. 1 to 48. 6). Slotman et al, NEJM 2007

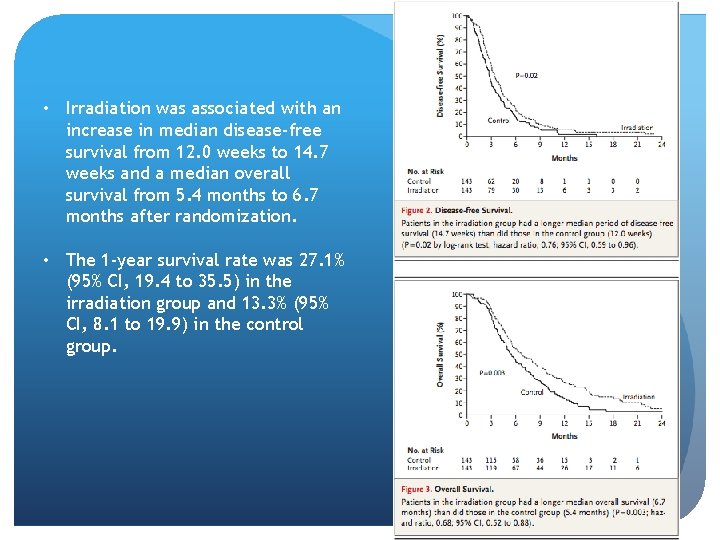
• Irradiation was associated with an increase in median disease-free survival from 12. 0 weeks to 14. 7 weeks and a median overall survival from 5. 4 months to 6. 7 months after randomization. • The 1 -year survival rate was 27. 1% (95% CI, 19. 4 to 35. 5) in the irradiation group and 13. 3% (95% CI, 8. 1 to 19. 9) in the control group.

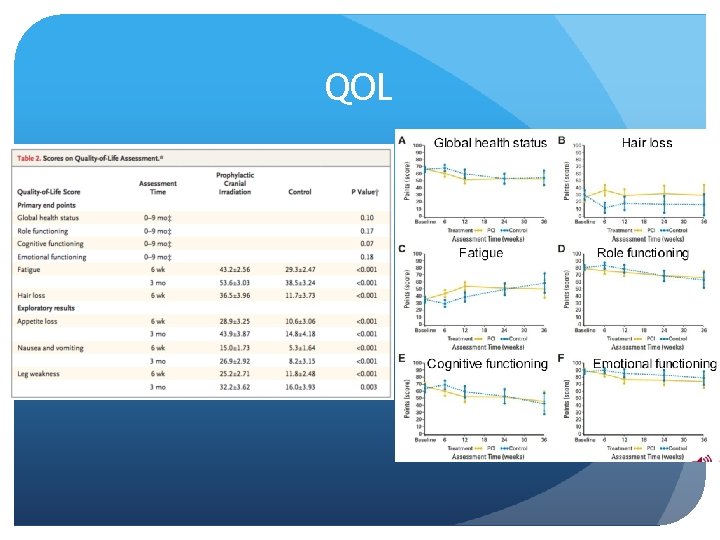
QOL

Future Treatment Strategies

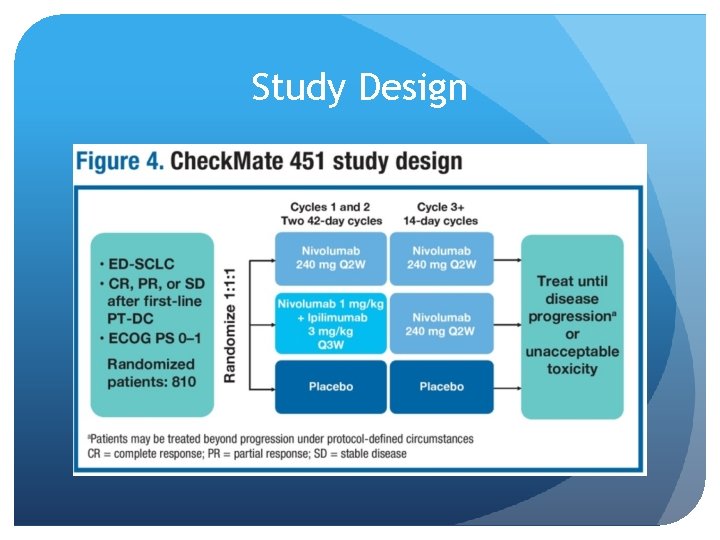
Immunotherapy Checkmate 451 (ASCO 2016) Ipilumumab and Nivolumab as second line and maintenance therapy in patients with extensive stage disease Adult pts with ED-SCLC who achieve stable disease or better after first-line PT-DC and have ECOG performance status 0– 1 are eligible. Pts with active central nervous system metastases, autoimmune disease, or toxicities attributed to prior anticancer therapy not resolved to grade ≤ 1 were ineligible. Primary endpoints are overall survival and progression-free survival.


Study Design

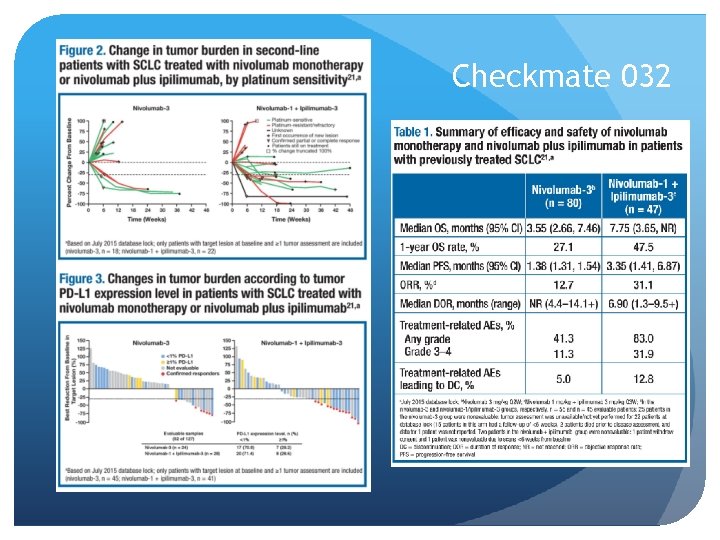
Checkmate 032


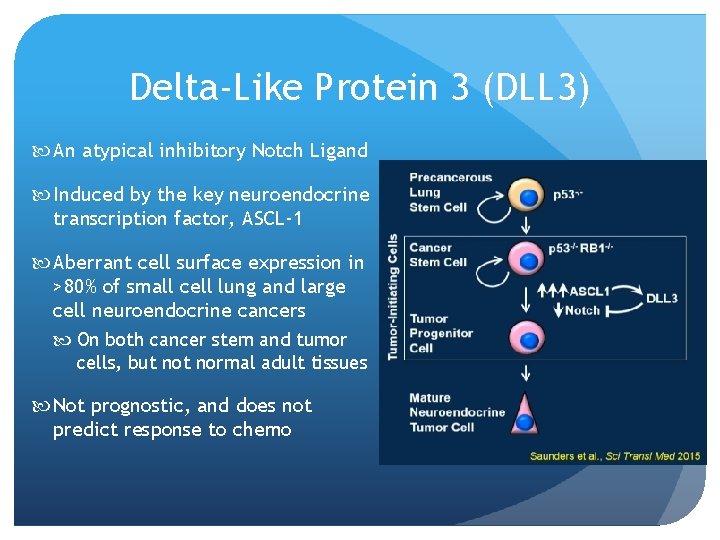
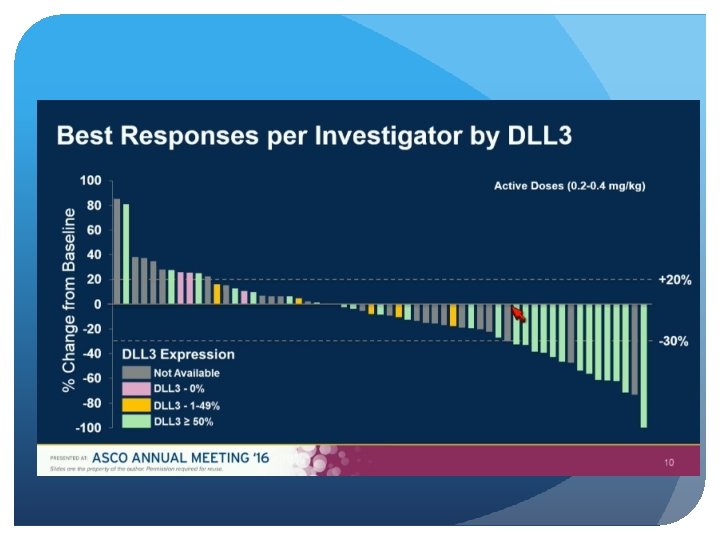
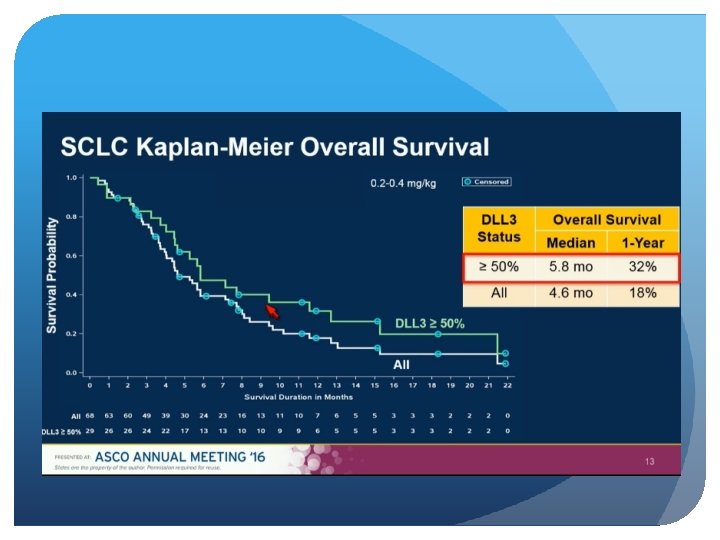
Delta-Like Protein 3 (DLL 3) An atypical inhibitory Notch Ligand Induced by the key neuroendocrine transcription factor, ASCL-1 Aberrant cell surface expression in >80% of small cell lung and large cell neuroendocrine cancers On both cancer stem and tumor cells, but normal adult tissues Not prognostic, and does not predict response to chemo


Patients with progressive SCLC after at least 1 previous systemic therapy were eligible. Efficacy was assessed by the investigator via RECIST and toxicity when available, archived tumor tissue was assessed retrospectively for DLL 3 expression by immunohistochemistry.






Single-agent activity in recurrent/refractory SCLC Comparable responses in second and third line Responses and survival improved vs. historical approved treatments First biomarker-directed therapy in SCLC Manageable safety profile Results justify further clinical development






Summary Most SCLC pts present with ED-SCLC. Initial response rates are high, but disease often rapidly recurs or progresses. While 50 -70% of patients with EDSCLC respond to first line platinum-based doublet chemotherapy, all patients ultimately relapse, most within the first year. Outcomes with second-line treatment are poor. Sadly not much has changed in the past decade in regards to first line therapy, but new treatments such as immunotherapy and antibody-drug conjugates show promising results in the second line setting.





Thank you



 Mds
Mds Palak desai md
Palak desai md Palak choksi
Palak choksi Anne evered
Anne evered Spinach and palak difference
Spinach and palak difference Tnm stage lung cancer
Tnm stage lung cancer Difference between siadh and diabetes insipidus
Difference between siadh and diabetes insipidus Gesundheit central european lung cancer patient network
Gesundheit central european lung cancer patient network Lung cancer location
Lung cancer location Lung cancer screening shared decision making tool
Lung cancer screening shared decision making tool Nursing diagnosis of lung cancer
Nursing diagnosis of lung cancer Dr. amy ford
Dr. amy ford Optimal lung cancer pathway
Optimal lung cancer pathway Lifetime risk of lung cancer
Lifetime risk of lung cancer Dr. shraddha desai
Dr. shraddha desai Dr ankita desai
Dr ankita desai Dr trupti desai
Dr trupti desai Unsend message monica
Unsend message monica Seemal desai
Seemal desai Rummy glle
Rummy glle Heta desai
Heta desai Priyank desai
Priyank desai Dr roopal desai
Dr roopal desai Dr chetna desai
Dr chetna desai Shlok desai
Shlok desai Desai penelitian
Desai penelitian Avinash desai
Avinash desai Scholar and gypsy by anita desai summary
Scholar and gypsy by anita desai summary Dimple desai
Dimple desai Yash desai
Yash desai Mukta desai
Mukta desai Small cell size
Small cell size Natural hazards vs natural disasters
Natural hazards vs natural disasters Natural income
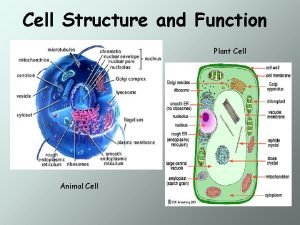
Natural income Cell city analogy project
Cell city analogy project Denuding tower
Denuding tower Prokaryotic cell vs eukaryotic cell
Prokaryotic cell vs eukaryotic cell Prokaryotic cell and eukaryotic cell similarities
Prokaryotic cell and eukaryotic cell similarities Venn diagram of organelles in plant and animal cells
Venn diagram of organelles in plant and animal cells Half cell reaction
Half cell reaction Dry cell vs wet cell
Dry cell vs wet cell Comparing animal and plant cells venn diagram
Comparing animal and plant cells venn diagram Function of cells
Function of cells Plant cell and animal cell diagram
Plant cell and animal cell diagram What is the function of vacuole in the cell
What is the function of vacuole in the cell Cell wall vs cell membrane
Cell wall vs cell membrane Cell strain
Cell strain Finite and continuous cell lines
Finite and continuous cell lines Cell city introduction
Cell city introduction Primary source batteries
Primary source batteries Difference between plant cell and bacterial cell
Difference between plant cell and bacterial cell Cell-cell junction
Cell-cell junction Cell-cell junction
Cell-cell junction What cell organelle is like lysol spray cleaning the cell
What cell organelle is like lysol spray cleaning the cell Cell cycle and cell division
Cell cycle and cell division Life
Life Eukaryotic cell animal cell
Eukaryotic cell animal cell The scientist mathias schleiden studied _______ in ______.
The scientist mathias schleiden studied _______ in ______. Cell structure and function organizer
Cell structure and function organizer Idealized animal cell and plant cell
Idealized animal cell and plant cell Walker cell and hadley cell
Walker cell and hadley cell Prokaryotic versus eukaryotic
Prokaryotic versus eukaryotic Cell cycle and cell division
Cell cycle and cell division Biology.arizona.edu/cell bio/activities/cell cycle/01.html
Biology.arizona.edu/cell bio/activities/cell cycle/01.html Cell division phases
Cell division phases Matlab string builder
Matlab string builder Chapter 21 electrochemistry
Chapter 21 electrochemistry Rigid outer covering of plant cells
Rigid outer covering of plant cells Lung compliance formula
Lung compliance formula Lung lobe
Lung lobe Vocal fremitus
Vocal fremitus Inspect thorax
Inspect thorax Aortic impression lung
Aortic impression lung Jugular notch
Jugular notch Farmer's lung disease
Farmer's lung disease Normal lung compliance
Normal lung compliance Dynamic compliance formula
Dynamic compliance formula Lungs 3 lobes
Lungs 3 lobes 3 lobes of lungs
3 lobes of lungs Lung buds
Lung buds 3 types of lung receptors
3 types of lung receptors Friction rub lung sounds
Friction rub lung sounds J receptors
J receptors Right middle lobe pneumonia
Right middle lobe pneumonia Nurses note example
Nurses note example