Lung Cancer R Zenhusern Lung cancer Epidemiology n













- Slides: 48

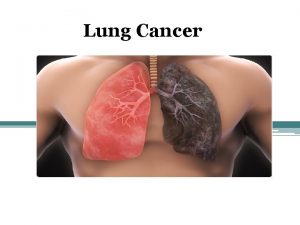
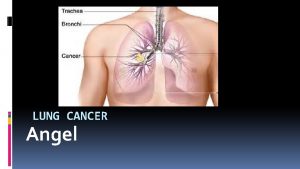
Lung Cancer R. Zenhäusern

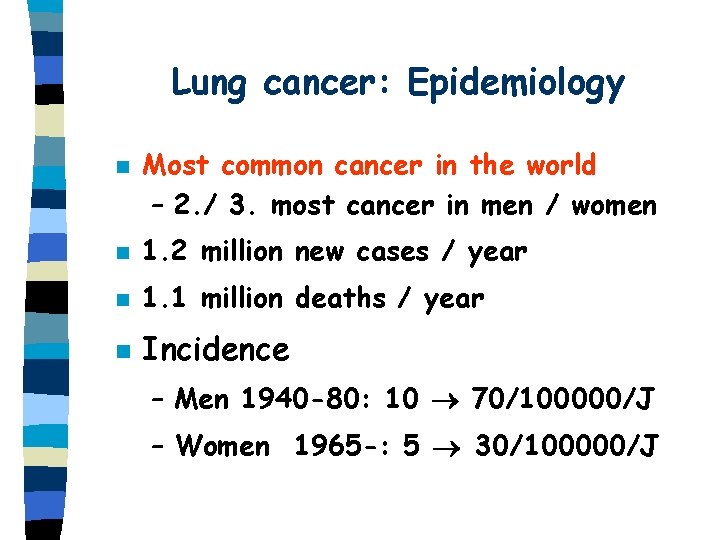
Lung cancer: Epidemiology n Most common cancer in the world – 2. / 3. most cancer in men / women n 1. 2 million new cases / year n 1. 1 million deaths / year n Incidence – Men 1940 -80: 10 70/100000/J – Women 1965 -: 5 30/100000/J

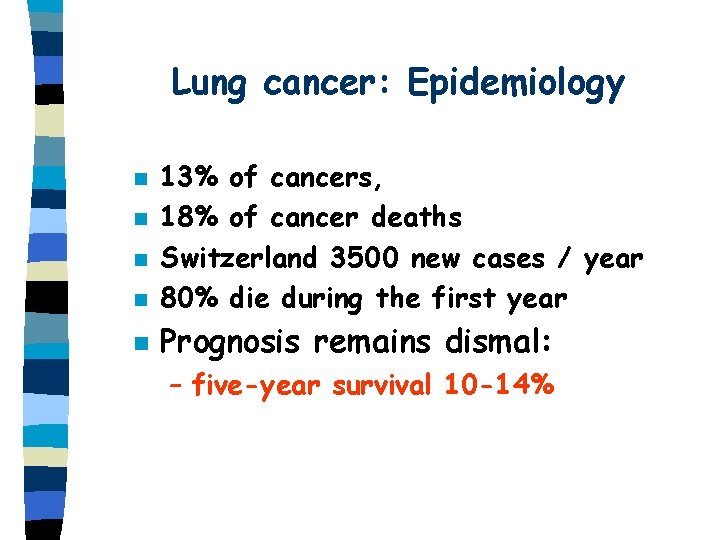
Lung cancer: Epidemiology n 13% of cancers, 18% of cancer deaths Switzerland 3500 new cases / year 80% die during the first year n Prognosis remains dismal: n n n – five-year survival 10 -14%



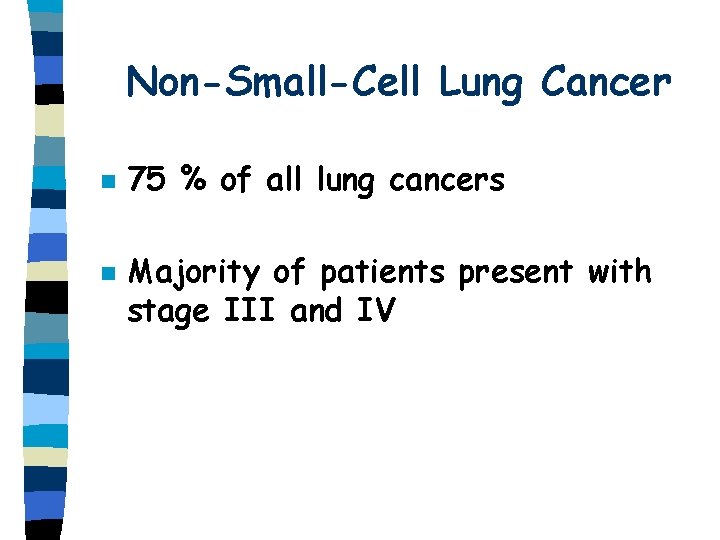
Non-Small-Cell Lung Cancer n n 75 % of all lung cancers Majority of patients present with stage III and IV

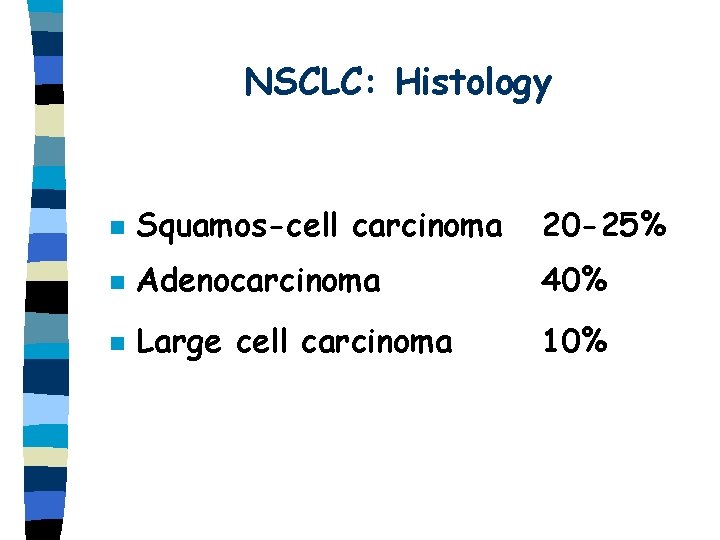
NSCLC: Histology n Squamos-cell carcinoma 20 -25% n Adenocarcinoma 40% n Large cell carcinoma 10%


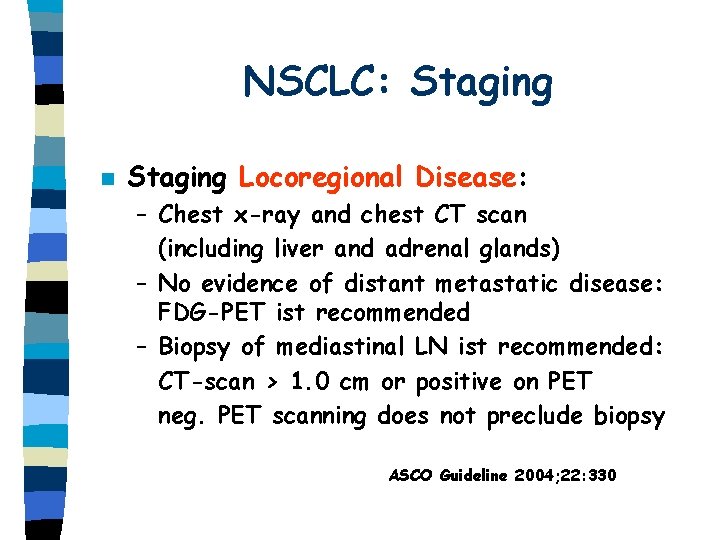
NSCLC: Staging n Staging Locoregional Disease: – Chest x-ray and chest CT scan (including liver and adrenal glands) – No evidence of distant metastatic disease: FDG-PET ist recommended – Biopsy of mediastinal LN ist recommended: CT-scan > 1. 0 cm or positive on PET neg. PET scanning does not preclude biopsy ASCO Guideline 2004; 22: 330

NSCLC: Staging n Staging Distant Metastatic Disease: – No evidence of distant metastatic disease on CT scan of the chest: PET ist recommended – A bone scan is optional – Resectable primary lung lesion and bone lesion on PET/bone scan: MRI/CT and biopsy – Brain: CT or MRI if symptoms, patients with stage III considered for aggressive local Th. – Isolated adrenal mass: biopsy – Isolated liver mass: biopsy ASCO Guideline 2004; 22: 330

Staging of Lung Cancer

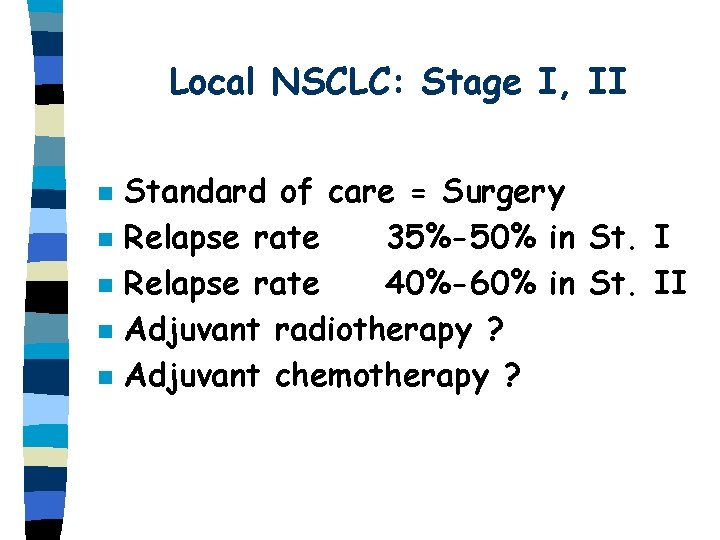
Local NSCLC: Stage I, II n n n Standard of care = Surgery Relapse rate 35%-50% in St. I Relapse rate 40%-60% in St. II Adjuvant radiotherapy ? Adjuvant chemotherapy ?

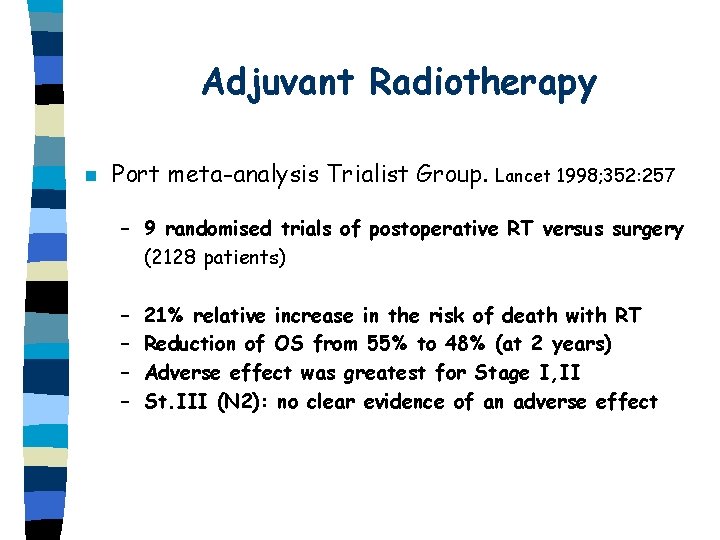
Adjuvant Radiotherapy n Port meta-analysis Trialist Group. Lancet 1998; 352: 257 – 9 randomised trials of postoperative RT versus surgery (2128 patients) – – 21% relative increase in the risk of death with RT Reduction of OS from 55% to 48% (at 2 years) Adverse effect was greatest for Stage I, II St. III (N 2): no clear evidence of an adverse effect

Adjuvant Radiotherapy n Conclusion – Postoperative RT should not be used outside of a clinical trial in Stage I, II lung cancer, unless surgical margins are positive and repeated resection is not feasible.

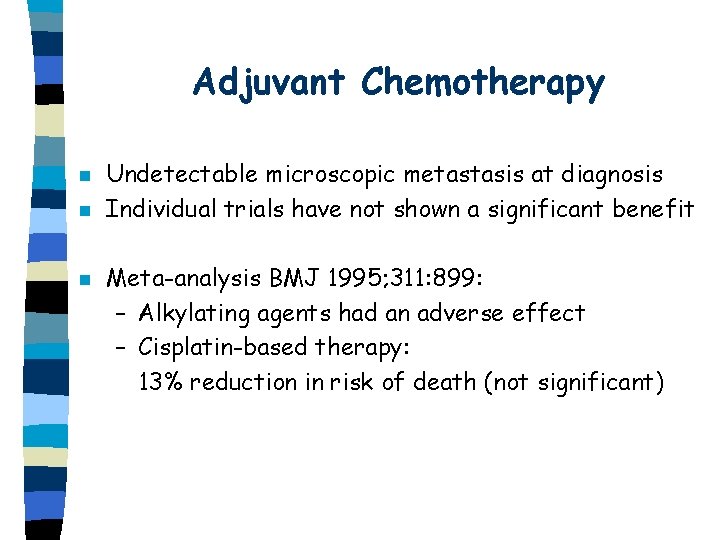
Adjuvant Chemotherapy n n n Undetectable microscopic metastasis at diagnosis Individual trials have not shown a significant benefit Meta-analysis BMJ 1995; 311: 899: – Alkylating agents had an adverse effect – Cisplatin-based therapy: 13% reduction in risk of death (not significant)

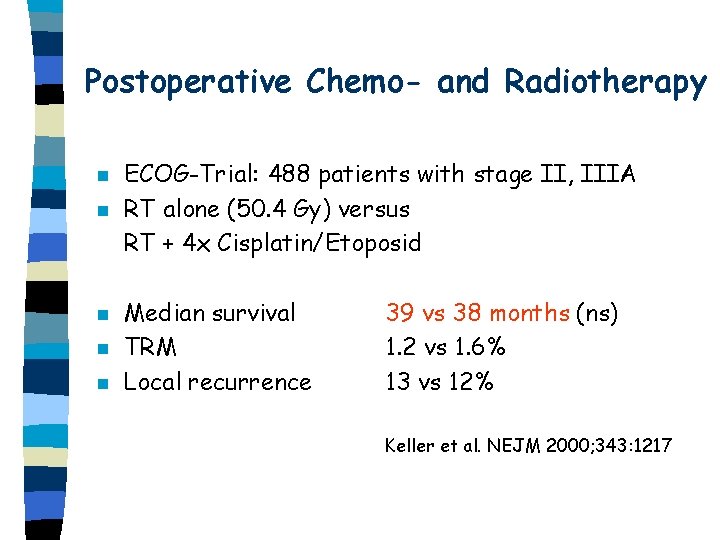
Postoperative Chemo- and Radiotherapy n n n ECOG-Trial: 488 patients with stage II, IIIA RT alone (50. 4 Gy) versus RT + 4 x Cisplatin/Etoposid Median survival TRM Local recurrence 39 vs 38 months (ns) 1. 2 vs 1. 6% 13 vs 12% Keller et al. NEJM 2000; 343: 1217

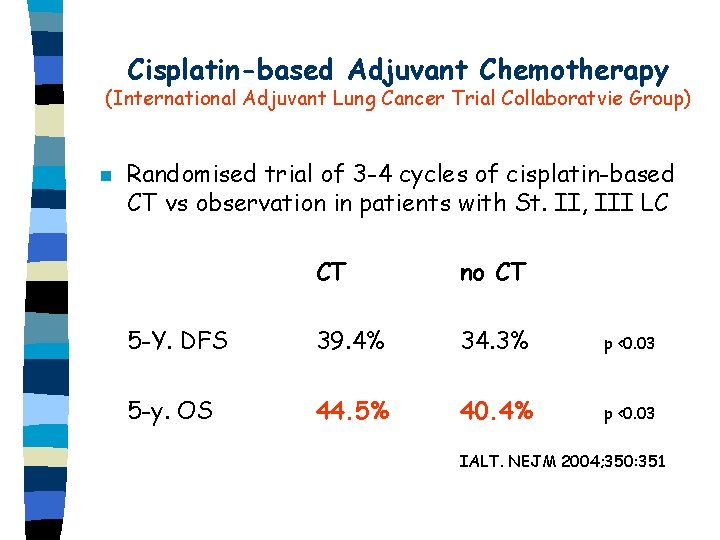
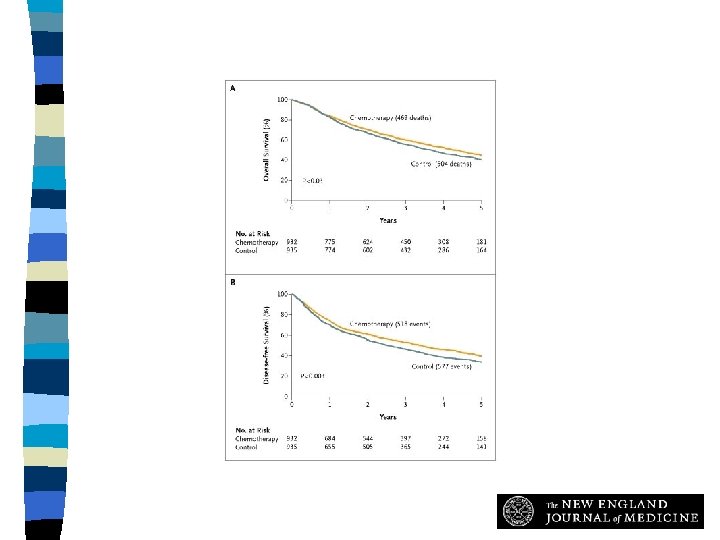
Cisplatin-based Adjuvant Chemotherapy (International Adjuvant Lung Cancer Trial Collaboratvie Group) n Randomised trial of 3 -4 cycles of cisplatin-based CT vs observation in patients with St. II, III LC CT no CT 5 -Y. DFS 39. 4% 34. 3% p <0. 03 5 -y. OS 44. 5% 40. 4% p <0. 03 IALT. NEJM 2004; 350: 351

Overall Survival (Panel A) and Disease-free Survival (Panel B) The International Adjuvant Lung Cancer Trial Collaborative Group, N Engl J Med 2004; 350: 351360

Adjuvant Chemotherapy n Conclusion: – One should consider the use of adjuvant platinum-based chemotherapy in patients with stage I, II or IIA NSCLC

Locally advanced NSCLC n n Thoracic irradiation is the mainstay of treatment for inoperable stage III disease Its curative potential is extremely poor 5 -year survival rates 3 -5%

Locally advanced NSCLC n A meta-analysis of 22 randomised studies showed a beneficial effect of CT added to RT – 10% reduction in risk of death per year – Small absolute survival benefit: 4% after 2 years 2% after 5 years NSCLC Collaborative Group. BMJ 1995; 311: 899

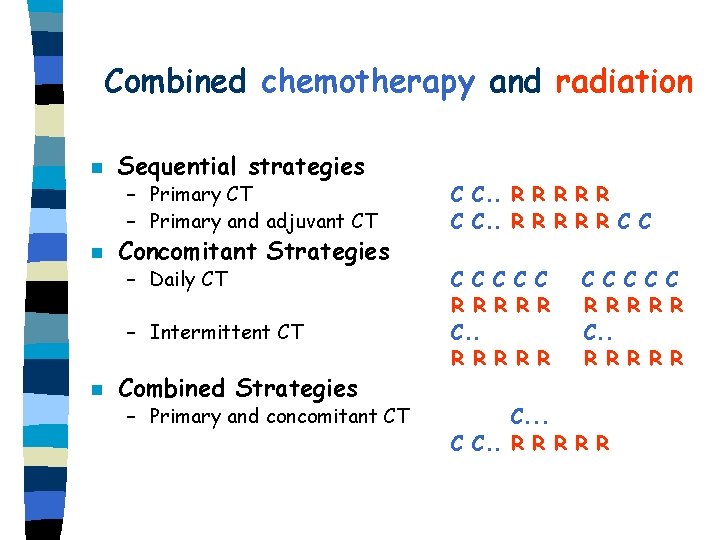
Combined chemotherapy and radiation n n Sequential strategies – Primary CT – Primary and adjuvant CT C C. . R R R R R C C – Daily CT C C C R R R C. . R R R Concomitant Strategies – Intermittent CT n Combined Strategies – Primary and concomitant CT C C C R R R R R C. . . C C. . R R R

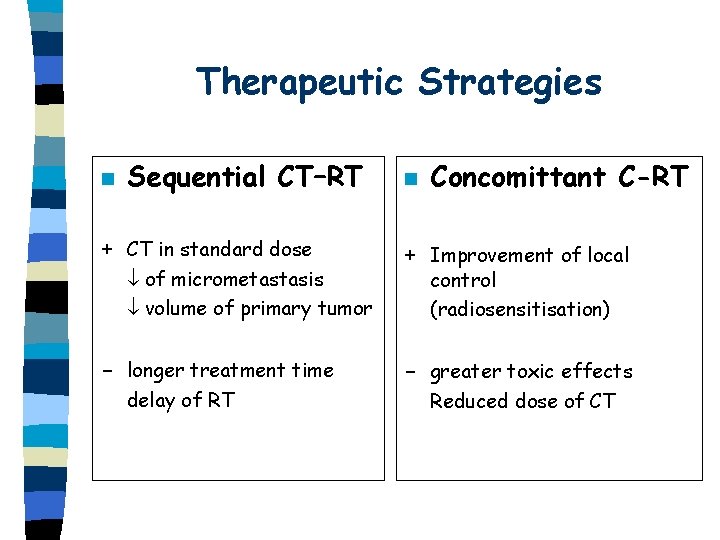
Therapeutic Strategies n Sequential CT–RT + CT in standard dose of micrometastasis volume of primary tumor - longer treatment time delay of RT n Concomittant C-RT + Improvement of local control (radiosensitisation) - greater toxic effects Reduced dose of CT

Sequential chemo- and radiotherapy n n Studies performed in the 1980 s did not show an advantage Three large phase III trials gave pos. Results – Dillman etal. NEJM 1990; 329: 940 – Sause et al. JNCI 1995; 87: 198 – Le Chevalier et al. JNCI 1992; 8: 58

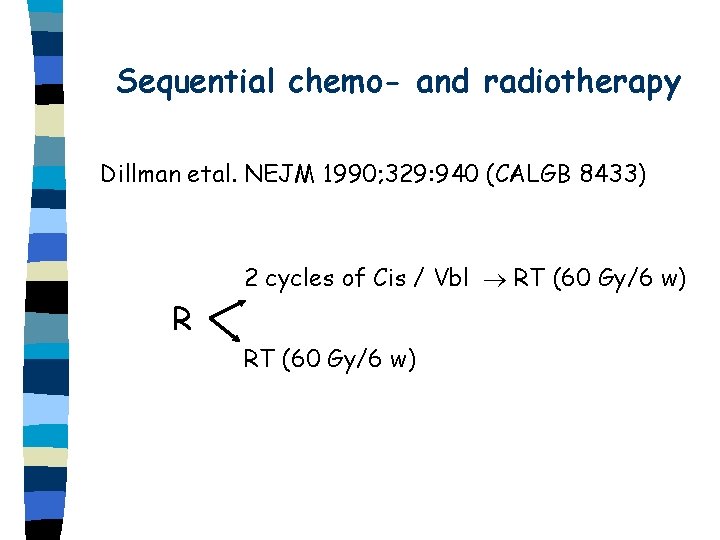
Sequential chemo- and radiotherapy Dillman etal. NEJM 1990; 329: 940 (CALGB 8433) 2 cycles of Cis / Vbl RT (60 Gy/6 w) R RT (60 Gy/6 w)

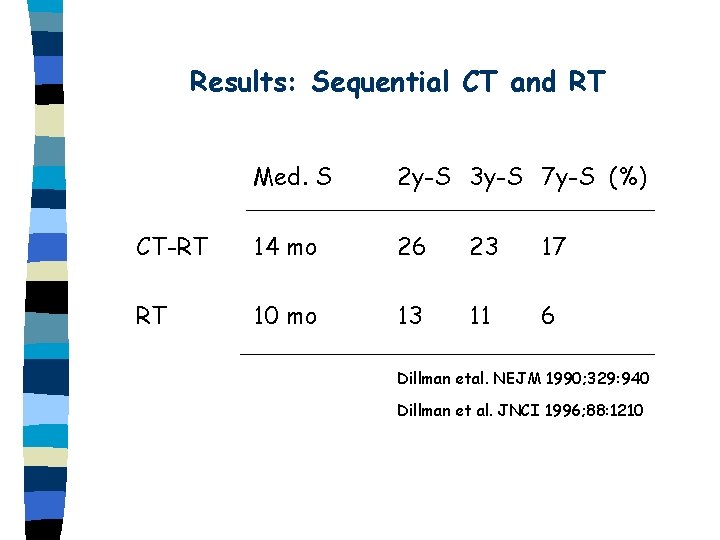
Results: Sequential CT and RT Med. S 2 y-S 3 y-S 7 y-S (%) CT-RT 14 mo 26 23 17 RT 10 mo 13 11 6 Dillman etal. NEJM 1990; 329: 940 Dillman et al. JNCI 1996; 88: 1210

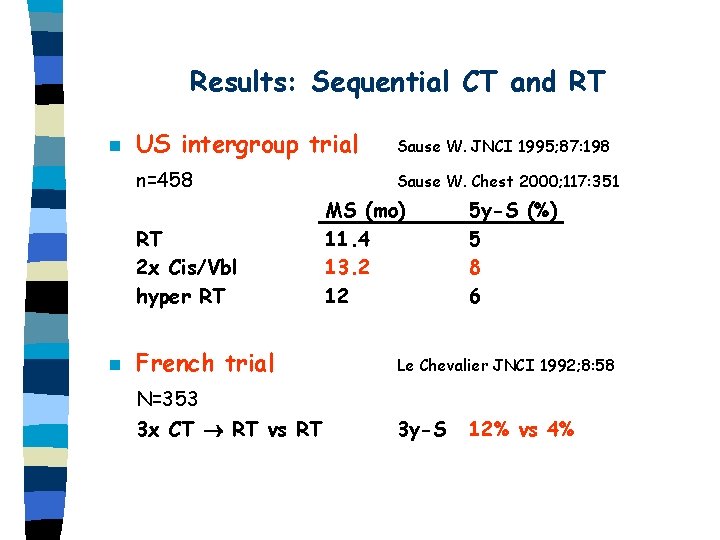
Results: Sequential CT and RT n US intergroup trial Sause W. JNCI 1995; 87: 198 n=458 Sause W. Chest 2000; 117: 351 RT 2 x Cis/Vbl hyper RT n MS (mo) 11. 4 13. 2 12 5 y-S (%) 5 8 6 French trial Le Chevalier JNCI 1992; 8: 58 N=353 3 x CT RT vs RT 3 y-S 12% vs 4%

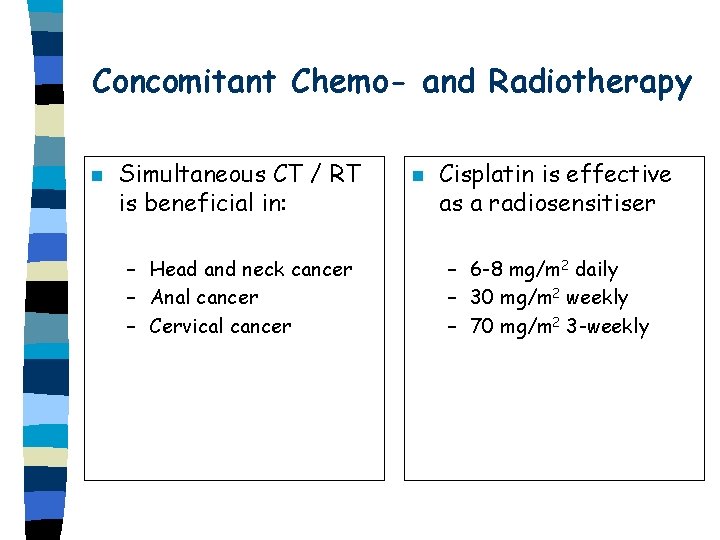
Concomitant Chemo- and Radiotherapy n Simultaneous CT / RT is beneficial in: – Head and neck cancer – Anal cancer – Cervical cancer n Cisplatin is effective as a radiosensitiser – 6 -8 mg/m 2 daily – 30 mg/m 2 weekly – 70 mg/m 2 3 -weekly

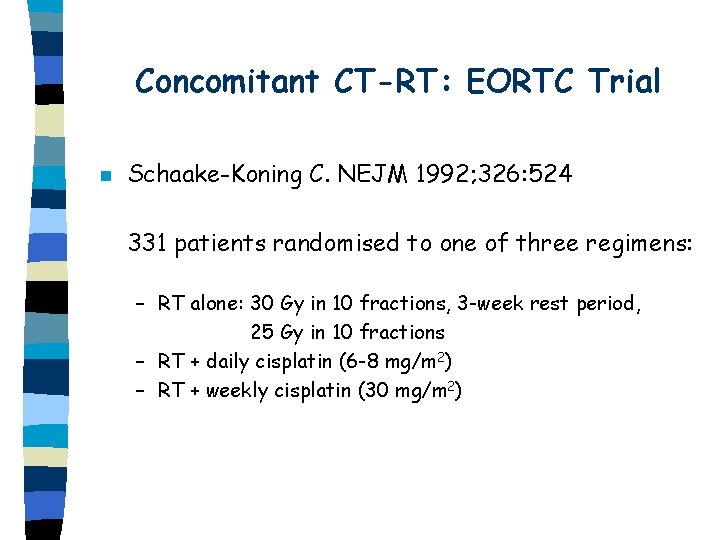
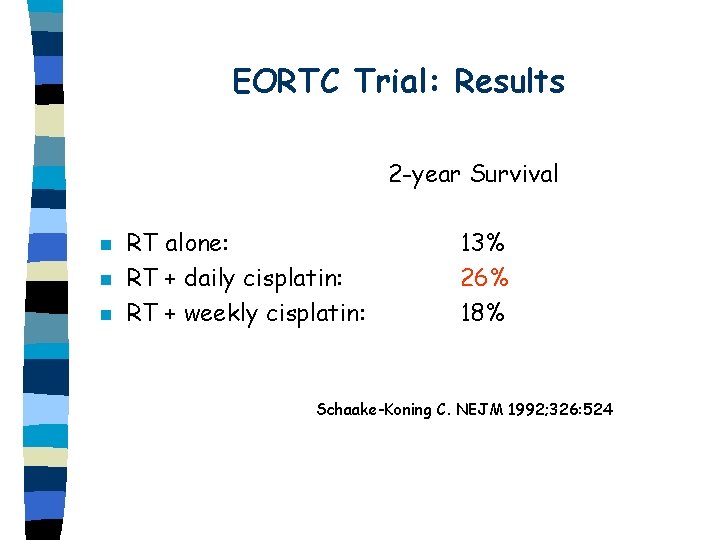
Concomitant CT-RT: EORTC Trial n Schaake-Koning C. NEJM 1992; 326: 524 331 patients randomised to one of three regimens: – RT alone: 30 Gy in 10 fractions, 3 -week rest period, 25 Gy in 10 fractions – RT + daily cisplatin (6 -8 mg/m 2) – RT + weekly cisplatin (30 mg/m 2)

EORTC Trial: Results 2 -year Survival n n n RT alone: RT + daily cisplatin: RT + weekly cisplatin: 13% 26% 18% Schaake-Koning C. NEJM 1992; 326: 524


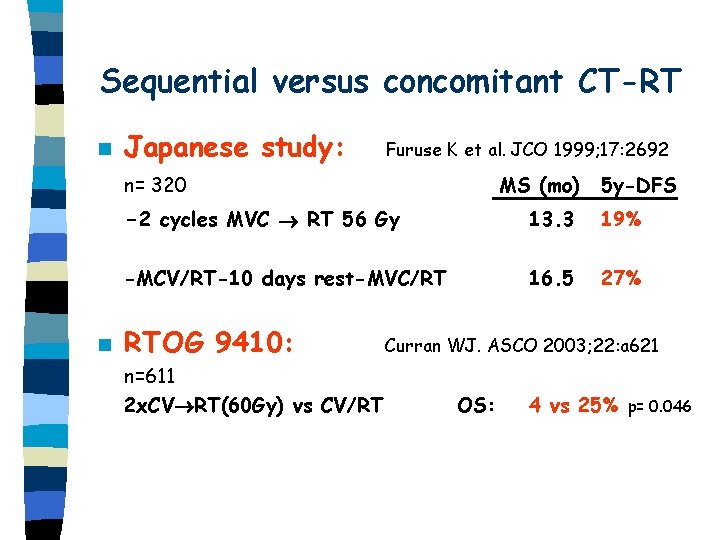
Sequential versus concomitant CT-RT n Japanese study: Furuse K et al. JCO 1999; 17: 2692 n= 320 n MS (mo) 5 y-DFS -2 cycles MVC RT 56 Gy 13. 3 19% -MCV/RT-10 days rest-MVC/RT 16. 5 27% RTOG 9410: n=611 2 x. CV RT(60 Gy) vs CV/RT Curran WJ. ASCO 2003; 22: a 621 OS: 4 vs 25% p= 0. 046

Neoadjuvant Therapy n Pancoast`s tumor, vertebral invasion – Combined neoadjuvant CT-RT should be considered n Tumors with ipsilateral mediastinal spread (N 2) – Poor survival with surgery alone – 2 small randomised trials showed a benefit of neoadjuvant combined CT-RT – Roth et al. JNCI 1994; 86: 673 – Phase II trials report good results of neoadjuvant CT§

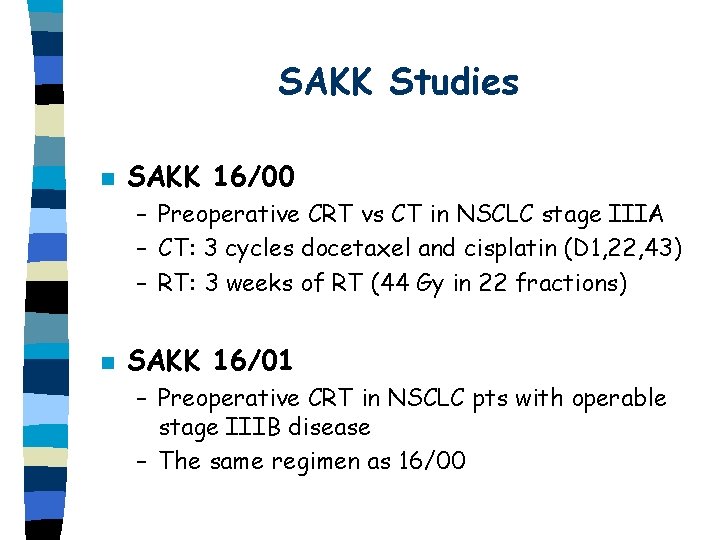
SAKK Studies n SAKK 16/00 – Preoperative CRT vs CT in NSCLC stage IIIA – CT: 3 cycles docetaxel and cisplatin (D 1, 22, 43) – RT: 3 weeks of RT (44 Gy in 22 fractions) n SAKK 16/01 – Preoperative CRT in NSCLC pts with operable stage IIIB disease – The same regimen as 16/00

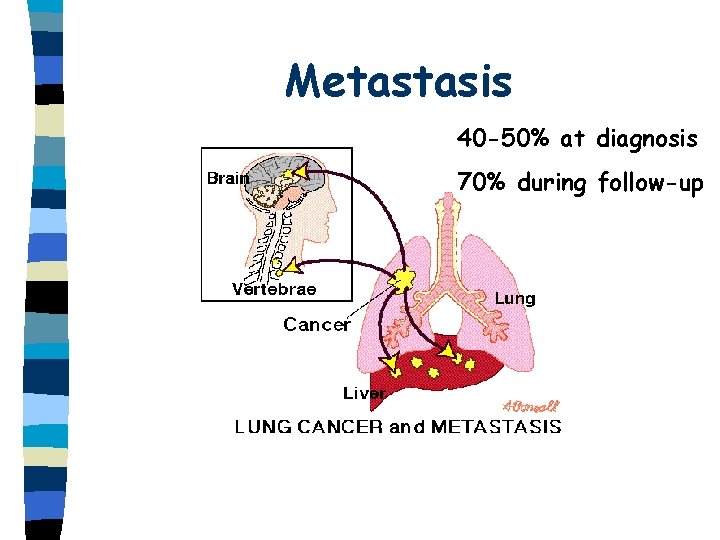
Metastasis 40 -50% at diagnosis 70% during follow-up

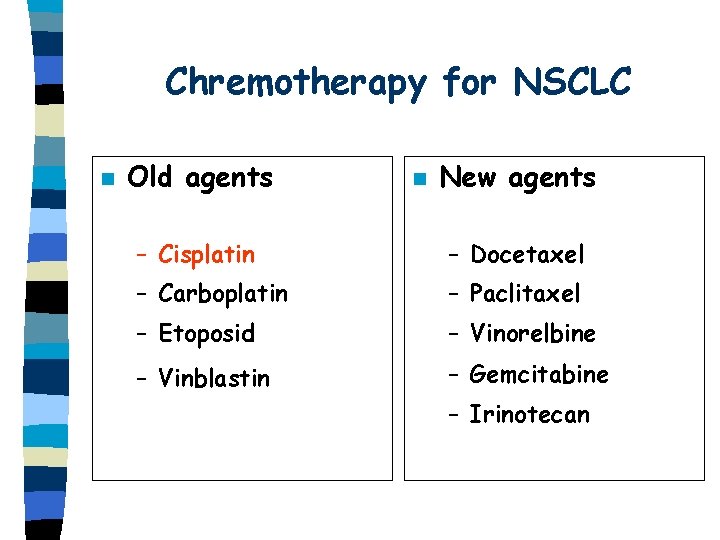
Chremotherapy for NSCLC n Old agents n New agents – Cisplatin – Docetaxel – Carboplatin – Paclitaxel – Etoposid – Vinorelbine – Vinblastin – Gemcitabine – Irinotecan

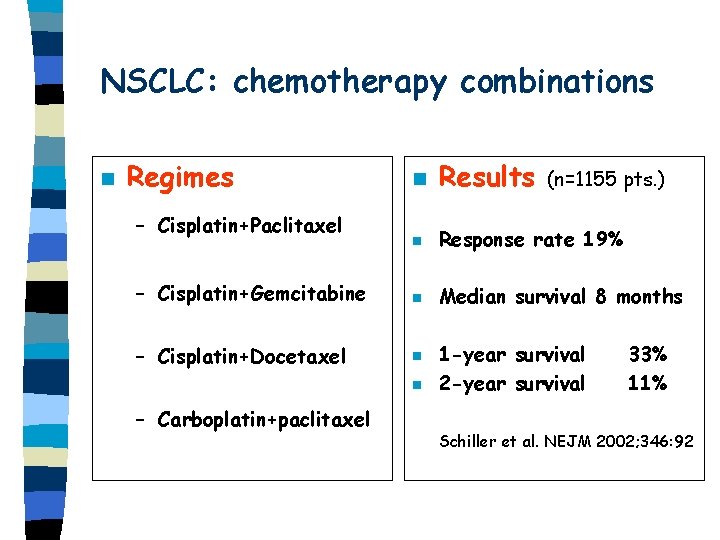
NSCLC: chemotherapy combinations n Regimes n Results n Response rate 19% – Cisplatin+Gemcitabine n Median survival 8 months – Cisplatin+Docetaxel n – Cisplatin+Paclitaxel n – Carboplatin+paclitaxel (n=1155 pts. ) 1 -year survival 2 -year survival 33% 11% Schiller et al. NEJM 2002; 346: 92

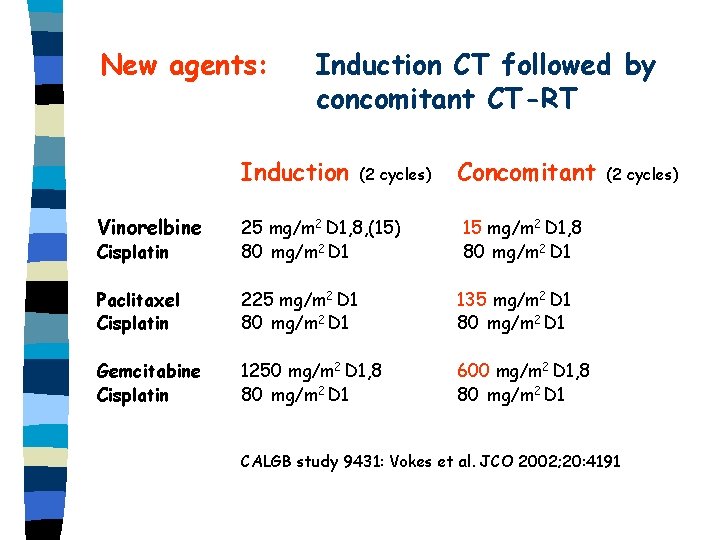
New agents: Induction CT followed by concomitant CT-RT Induction (2 cycles) Concomitant Vinorelbine Cisplatin 25 mg/m 2 D 1, 8, (15) 80 mg/m 2 D 1 15 mg/m 2 D 1, 8 80 mg/m 2 D 1 Paclitaxel Cisplatin 225 mg/m 2 D 1 80 mg/m 2 D 1 135 mg/m 2 D 1 80 mg/m 2 D 1 Gemcitabine Cisplatin 1250 mg/m 2 D 1, 8 80 mg/m 2 D 1 600 mg/m 2 D 1, 8 80 mg/m 2 D 1 (2 cycles) CALGB study 9431: Vokes et al. JCO 2002; 20: 4191

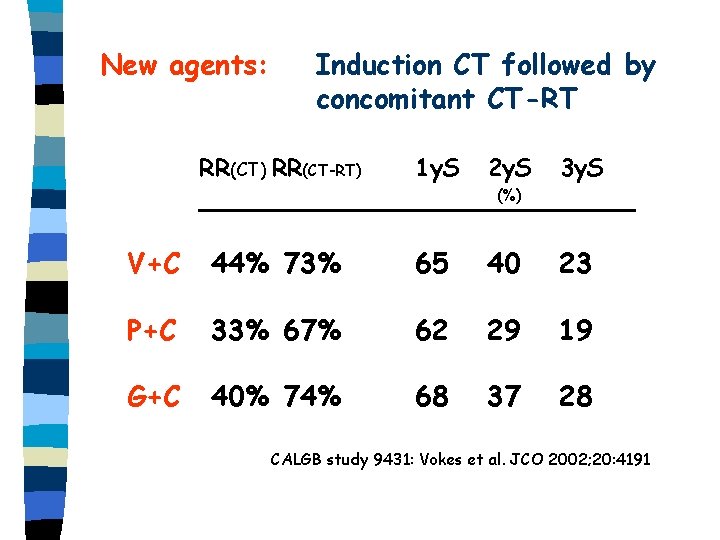
New agents: Induction CT followed by concomitant CT-RT RR(CT) RR(CT-RT) 1 y. S 2 y. S 3 y. S (%) V+C 44% 73% 65 40 23 P+C 33% 67% 62 29 19 G+C 40% 74% 68 37 28 CALGB study 9431: Vokes et al. JCO 2002; 20: 4191

Conclusion: Combined-Modality Therapy for Stage III Disease n n Adding CT to radiation therapy improves survival and alters the course of this disease Phase III studies suggest improvement in both local control and survival with concomitant CT-RT n Combined CT-RT should be the standard of care of patients with good PS and minimal weight loss n The absolute gain from combined CT-RT is still modest n The role of surgery following induction CT-RT is for patients with unresectable Cancer is being explored

Small-cell Lung Cancer (SCLC) n 15 -20% of all lung cancer n Incidence: 15/100000/year n Men : women = 5 : 1

SCLC n n n Rapid local and metastatic spread Mediastinal lymph node metastasis in most cases Median Survival in untreated patients 2 -3 months Superior vena caval obstruction and paraneoplastic syndromes (SIADH, Cushing) Association with smoking

SCLC Staging n Limited Disease Confined to: – One hemithorax – Mediastinum – Ipislateral hilar and supraclavicular nodes n Extensive Disease – Malignant pleura and pericard effusion – Contralateral hilar and supraclavicular nodes

SCLC Therapy n No surgery; SCLC is a systemic disease n Chemotherapy is the standard of care – Cisplatin+Etoposid n Limited stage SCLC: Bimodality therapy with chemotherapy and radiotherapy

SCLC Therapy n The addition of thoracic RT significantly improves survival in patients with LS-SCLC – Meta-analysis. Pignon et al. NEJM 1992; 327: 1618 – 14% reduction in the mortality rate – 5. 4% benefit in terms of OS at 3 years n Early use of RT with CT improves cure rates

SCLC Therapy n n The actuarial risk of CNS metastasis developing 2 years after CR of SCLC is 35%-60% Prophylactic cranial Irradiation is recommended for pts. With LS-SCLC in CR – Meta-analysis: Auperin et al. NEJM; 1999: 341: 475 – PCI: 5. 4% greater absolute survival at 3 years

SCLC Results n Limited Disease: – – – Remission rate CR Median Survival 2 -year Survival 5 -year Survival 80 -90% 50 -60% 18 -20 months 40% 15 -25%

SCLC Results n Extensive Disease: – – Remission rate CR Median Survival 2 -year Survival 70 -80% 20 -30% 8 -10 months < 10%
 Lung cancer screening shared decision making tool
Lung cancer screening shared decision making tool Lifetime risk of lung cancer
Lifetime risk of lung cancer Diabetes insipidus siadh cerebral salt wasting
Diabetes insipidus siadh cerebral salt wasting Seeking consensual validation
Seeking consensual validation Gesundheit central european lung cancer patient network
Gesundheit central european lung cancer patient network Optimal lung cancer pathway
Optimal lung cancer pathway Servier medical art
Servier medical art Optimal lung cancer pathway
Optimal lung cancer pathway Tnm staging lung cancer
Tnm staging lung cancer Wheel of disease causation
Wheel of disease causation Epidemiology person place time
Epidemiology person place time Seven uses of epidemiology
Seven uses of epidemiology Attack rate
Attack rate Distribution in epidemiology
Distribution in epidemiology What is descriptive study in epidemiology
What is descriptive study in epidemiology Epidemiology
Epidemiology Recall bias example
Recall bias example How dr. wafaa elsadr epidemiology professor
How dr. wafaa elsadr epidemiology professor Formula for attack rate
Formula for attack rate Diabetic ketoacidosis epidemiology
Diabetic ketoacidosis epidemiology Defination of epidemiology
Defination of epidemiology Descriptive vs analytical epidemiology
Descriptive vs analytical epidemiology Epidemiological triad
Epidemiological triad Ramboman
Ramboman Spurious association
Spurious association Perbedaan or rr dan pr
Perbedaan or rr dan pr Certification board of infection control and epidemiology
Certification board of infection control and epidemiology Attack rate calculation
Attack rate calculation Epi
Epi How dr. wafaa elsadr epidemiology professor
How dr. wafaa elsadr epidemiology professor Classification of epidemiological studies
Classification of epidemiological studies Distribution in epidemiology
Distribution in epidemiology Defination of epidemiology
Defination of epidemiology Prevalence vs incidence
Prevalence vs incidence John snow epidemiology
John snow epidemiology Meaning of epidermiology
Meaning of epidermiology Gordon nichols
Gordon nichols Field epidemiology ppt
Field epidemiology ppt Nutritional epidemiology definition
Nutritional epidemiology definition Ramboman acronym
Ramboman acronym Difference between descriptive and analytical epidemiology
Difference between descriptive and analytical epidemiology Mp test for malaria
Mp test for malaria Epidemiology definition
Epidemiology definition Attack rate calculation
Attack rate calculation Effect modification epidemiology
Effect modification epidemiology Distribution in epidemiology
Distribution in epidemiology Pros and cons of cross sectional study
Pros and cons of cross sectional study Epidemiology made easy
Epidemiology made easy Prevalence definition epidemiology
Prevalence definition epidemiology