Septic Shock Current Management and New Therapeutic Frontiers










































- Slides: 77

Septic Shock: Current Management and New Therapeutic Frontiers R. Phillip Dellinger, MD Professor of Medicine Robert Wood Johnson Medical School/UMDNJ Director Critical Care Medicine Cooper University Hospital Camden, New Jersey

Personal Financial Disclosures • None relevant to presentation today

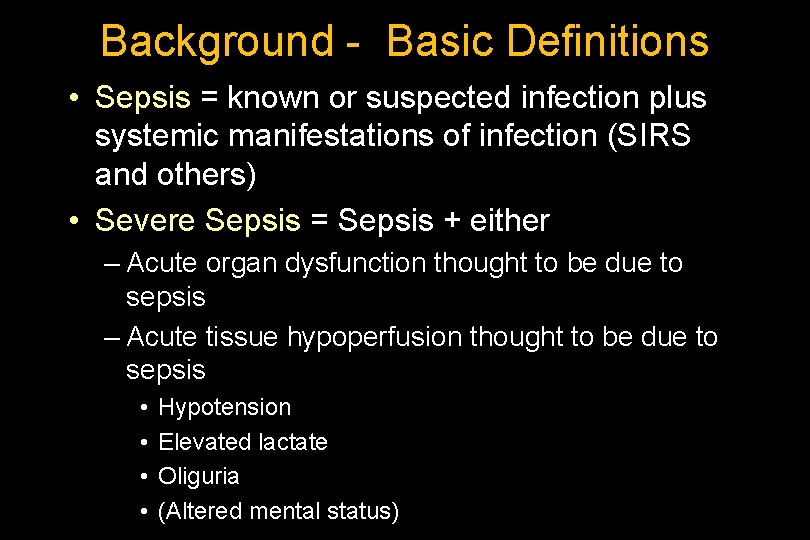
Background - Basic Definitions • Sepsis = known or suspected infection plus systemic manifestations of infection (SIRS and others) • Severe Sepsis = Sepsis + either – Acute organ dysfunction thought to be due to sepsis – Acute tissue hypoperfusion thought to be due to sepsis • • Hypotension Elevated lactate Oliguria (Altered mental status)

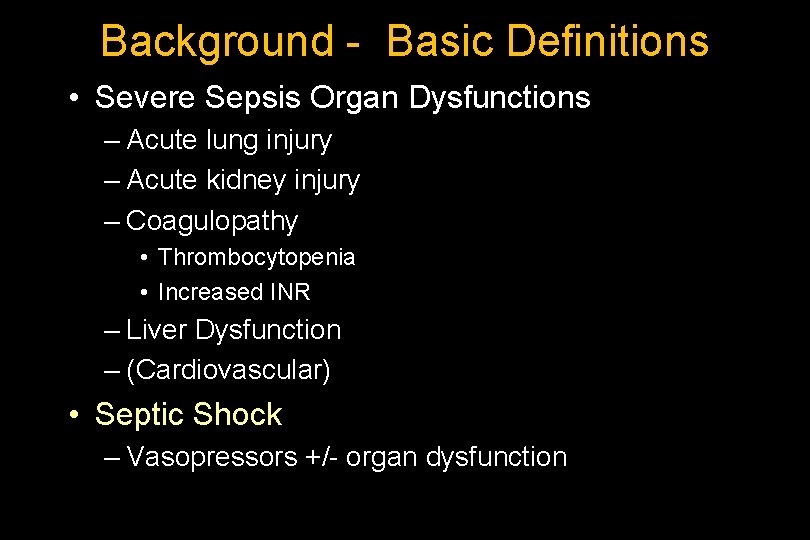
Background - Basic Definitions • Severe Sepsis Organ Dysfunctions – Acute lung injury – Acute kidney injury – Coagulopathy • Thrombocytopenia • Increased INR – Liver Dysfunction – (Cardiovascular) • Septic Shock – Vasopressors +/- organ dysfunction

Surviving Sepsis Campaign (SSC) guidelines for management of severe sepsis and septic shock Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, Ramsay G, Zimmerman JL, Vincent JL, Levy MM and the SSC Management Guidelines Committee Crit Care Med 2004; 32: 858 -873 Intensive Care Med 2004; 30: 536 -555

Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008 Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL. Crit Care Med 2008; 36: 296 -327 Intensive Care Med 2008; 30: 536 -555

Sponsoring Organizations 2008 Guidelines • American Association of Critical Care Nurses • American College of Chest Physicians • American College of Emergency Physicians • Canadian Critical Care Society • European Respiratory Society • European Society of Clinical Microbiology and Infectious Diseases • European Society of Intensive Care Medicine • Indian Society of Critical Care Medicine • International Sepsis Forum • Japanese Society of Intensive Care Medicine • Japanese Association of Acute Medicine • Society of Hospital Medicine • Society of Critical Care Medicine • Surgical Infection Society • World Federation of Critical Care Nurses • World Federation of Societies of Intensive and Critical Care Medicine

SSC Update 2010/2011

Current Surviving Sepsis Campaign Guideline Sponsors (2010/11 Update) • • • American Association of Critical-Care Nurses American College of Chest Physicians American College of Emergency Physicians Australian and New Zealand Intensive Care Society Asia Pacific Association of Critical Care Medicine American Thoracic Society Brazilian Society of Critical Care(AIMB) Canadian Critical Care Society European Respiratory Society European Society of Clinical Microbiology and Infectious Diseases European Society of Intensive Care Medicine European Society of Pediatric and Neonatal Intensive Care • • • • German Sepsis Society Infectious Diseases Society of America Indian Society of Critical Care Medicine Japanese Association for Acute Medicine Japanese Society of Intensive Care Medicine Latin American Sepsis Institute Pan Arab Critical Care Medicine Society Pediatric Acute Lung Injury and Sepsis Investigators Society Academic Emergency Medicine Society of Critical Care Medicine Society of Hospital Medicine Surgical Infection Society World Federation of Critical Care Nurses World Federation of Societies of Intensive and Critical Care Medicine

Evidence Based Medicine Grading System



Grades of Recommendation, Assessment, Development and Evaluation (GRADE)

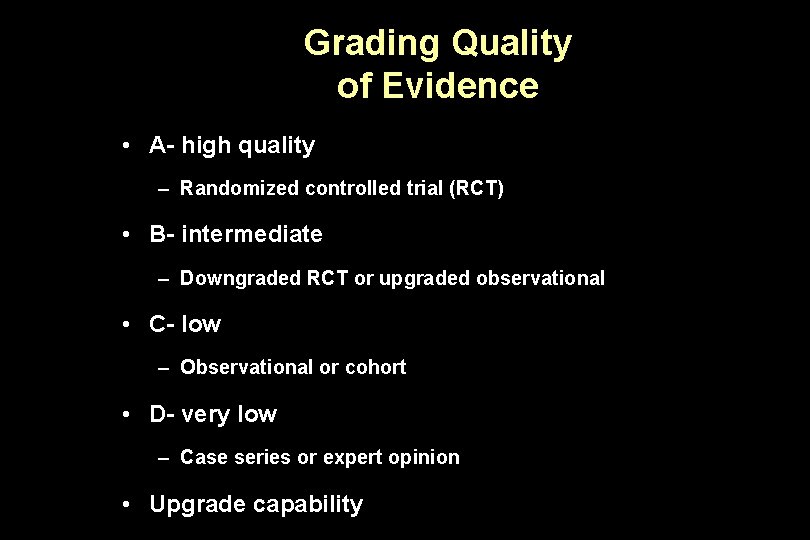
Grading Quality of Evidence • A- high quality – Randomized controlled trial (RCT) • B- intermediate – Downgraded RCT or upgraded observational • C- low – Observational or cohort • D- very low – Case series or expert opinion • Upgrade capability

Grading Strength of Recommendation • 1 - strong recommendation – Do it – We recommend • 2 - weak recommendation – Probably do it – We suggest • Determinants of strength – – Quality of evidence Relative importance of outcomes Risks and costs Absolute magnitude and precision of effect

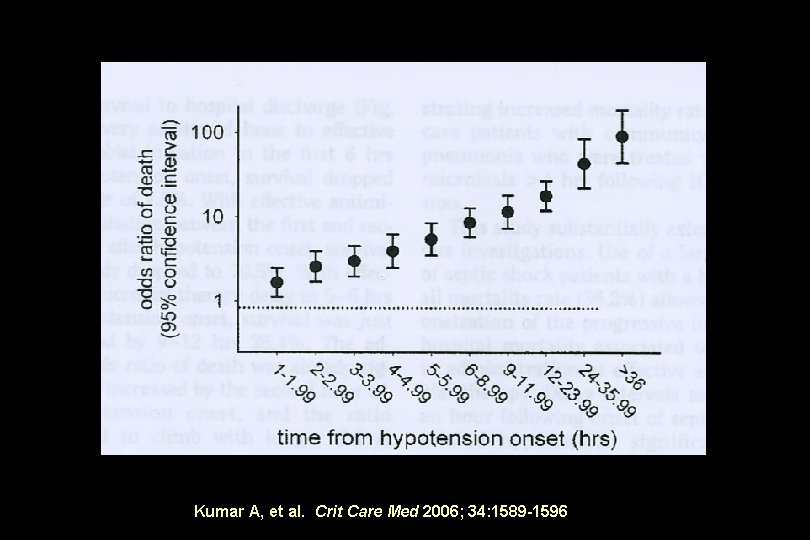
Kumar A, et al. Crit Care Med 2006; 34: 1589 -1596

Antibiotic Therapy Ø We recommend beginning intravenous antibiotics within first hour of recognition of severe sepsis 1 B

• Broad antibiotic coverage initially • Narrow coverage after return of cultures • Source control as soon as possible and within 6 hours



Initial Resuscitation

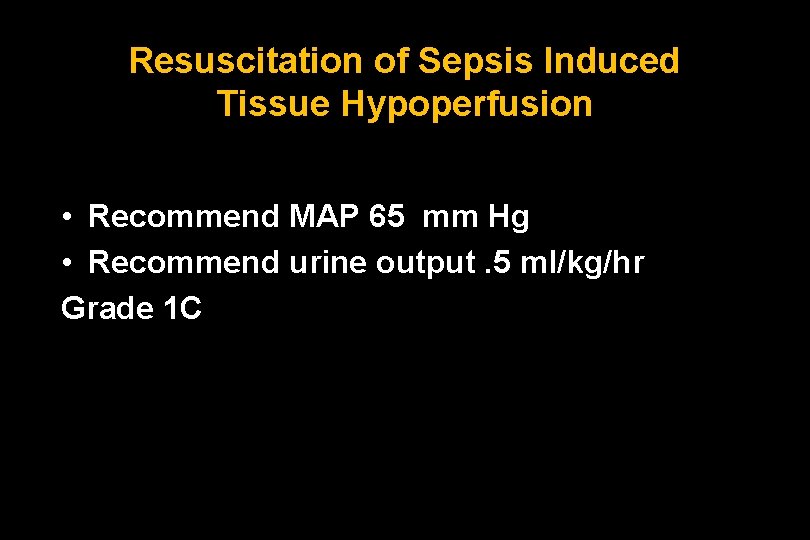
Resuscitation of Sepsis Induced Tissue Hypoperfusion • Recommend MAP 65 mm Hg • Recommend urine output. 5 ml/kg/hr Grade 1 C

Fluid Therapy • Recommend fluid resuscitation may consist of natural or artificial colloids or crystalloids. Grade 1 B

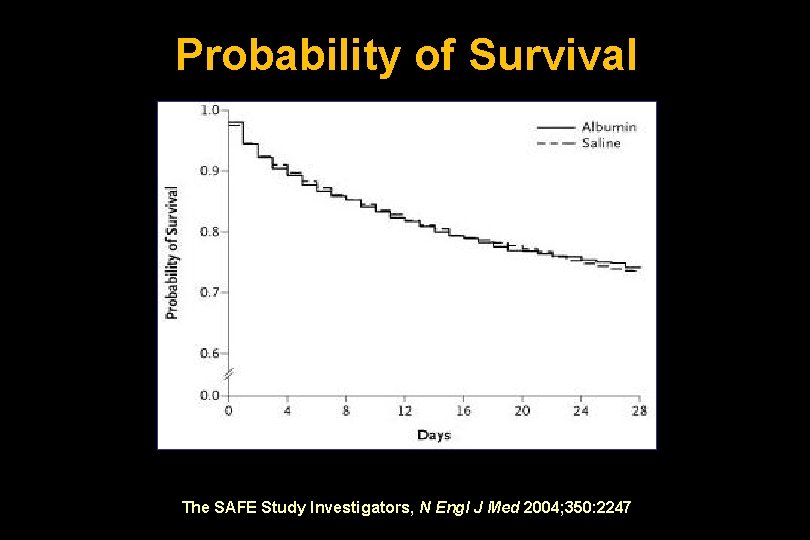
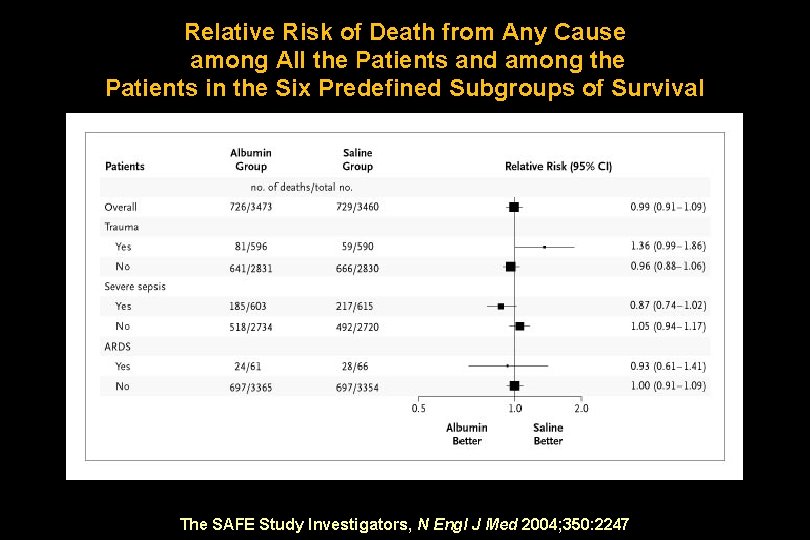
Probability of Survival The SAFE Study Investigators, N Engl J Med 2004; 350: 2247

Relative Risk of Death from Any Cause among All the Patients and among the Patients in the Six Predefined Subgroups of Survival The SAFE Study Investigators, N Engl J Med 2004; 350: 2247

Sepsis Induced Hypotension • Fluid challenge – Minimum of 20 ml/kg crystalloid or colloid equivalent

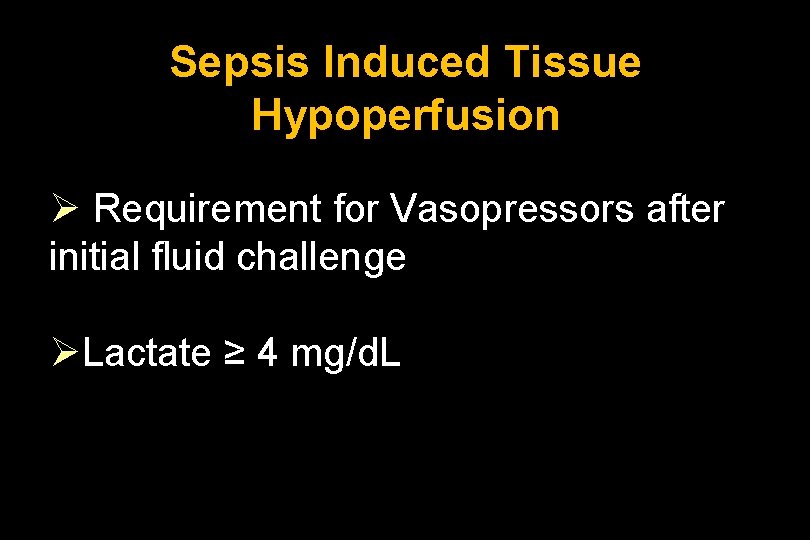
Sepsis Induced Tissue Hypoperfusion Ø Requirement for Vasopressors after initial fluid challenge ØLactate ≥ 4 mg/d. L

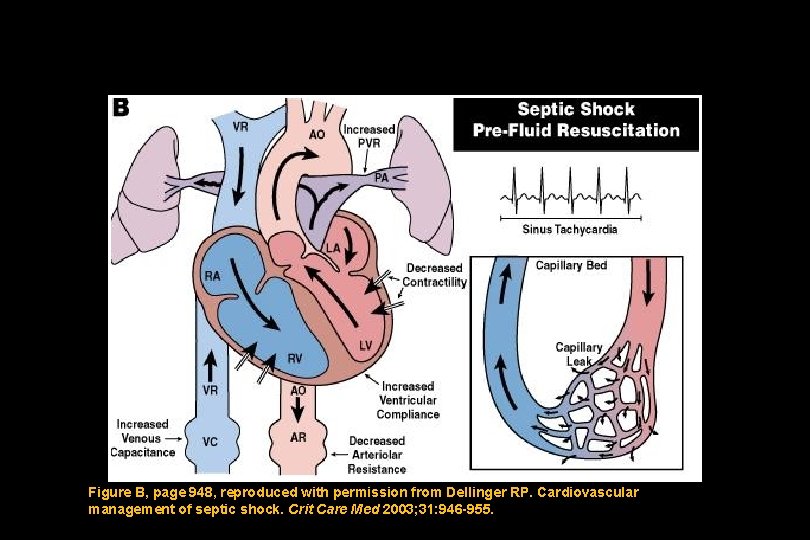
Figure B, page 948, reproduced with permission from Dellinger RP. Cardiovascular management of septic shock. Crit Care Med 2003; 31: 946 -955.

Which two adrenergic agents are most appropriate to maintain acceptable blood pressure in a patient with septic shock? A. B. C. D. Dopamine or epinephrine Epinephrine or vasopressin Vasopressin or norepinephrine Norepinephrine or dopamine

Which two adrenergic agents are most appropriate to maintain acceptable blood pressure in a patient with septic shock? A. B. C. D. Dopamine or epinephrine Epinephrine or vasopressin Vasopressin or norepinephrine Norepinephrine or dopamine

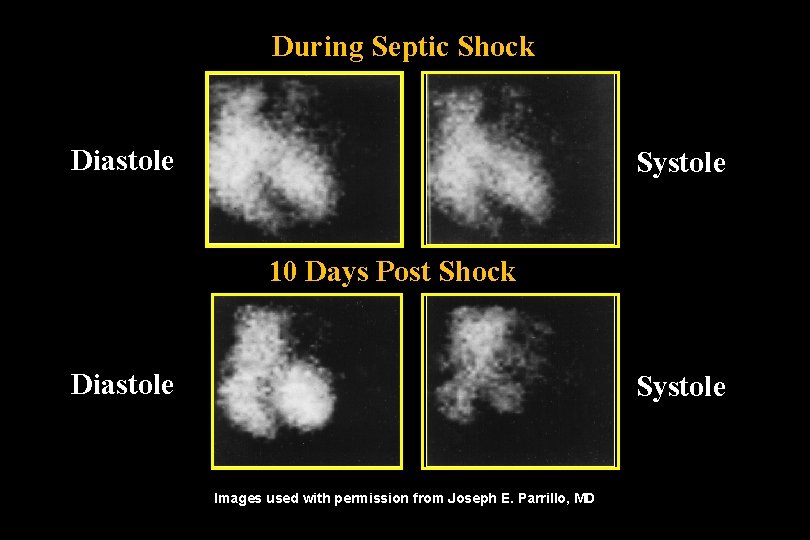
During Septic Shock Diastole Systole 10 Days Post Shock Diastole Systole Images used with permission from Joseph E. Parrillo, MD

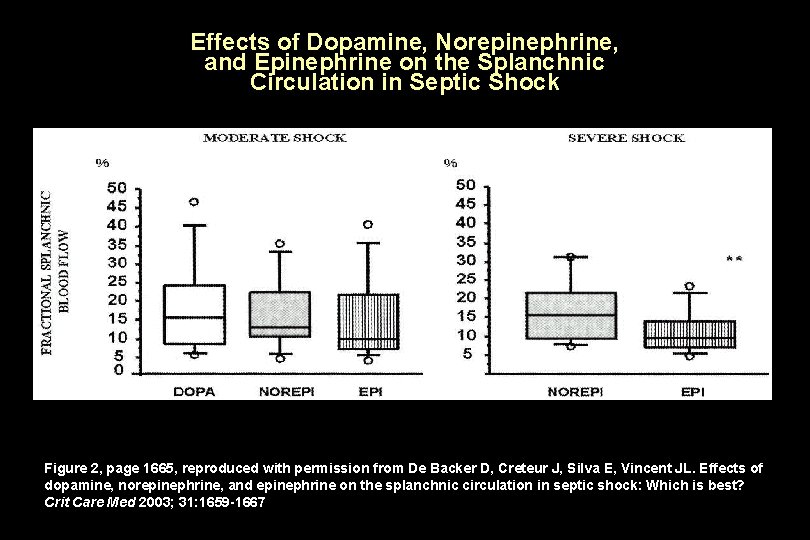
Effects of Dopamine, Norepinephrine, and Epinephrine on the Splanchnic Circulation in Septic Shock Figure 2, page 1665, reproduced with permission from De Backer D, Creteur J, Silva E, Vincent JL. Effects of dopamine, norepinephrine, and epinephrine on the splanchnic circulation in septic shock: Which is best? Crit Care Med 2003; 31: 1659 -1667

Norepinephrine vs. Dopamine

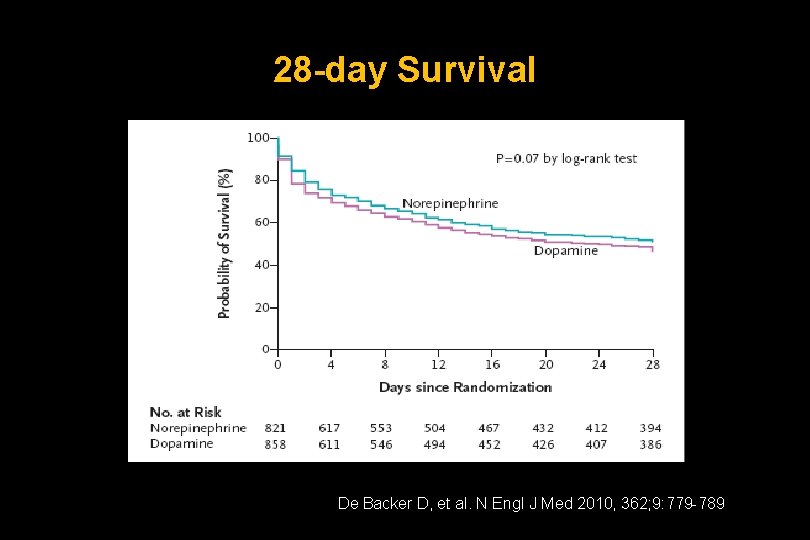
28 -day Survival De Backer D, et al. N Engl J Med 2010, 362; 9: 779 -789

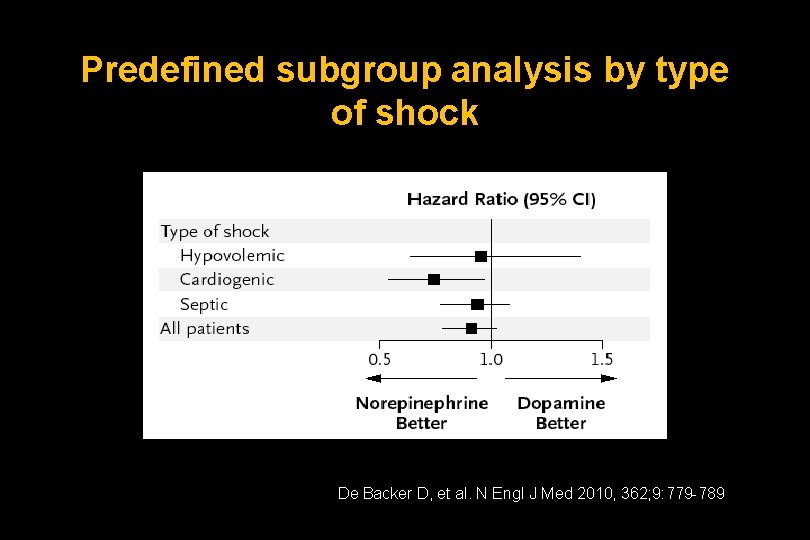
Predefined subgroup analysis by type of shock De Backer D, et al. N Engl J Med 2010, 362; 9: 779 -789

Phenylephrine • Pure vasoconstrictor in general should be avoided – Decreases cardiac output • Rare exceptions – Cardiac output measured and high and difficulty with maintaining MAP with other vasopressor agents – Profound tachycardia or severe ventricular arrhythmias with norepinephrine

Initial Resuscitation of Persistent Hypotension or Lactate >4 Recommend Insertion central venous catheter Recommended goals : • Central venous pressure: 8– 12 mm Hg • Higher with altered ventricular compliance or increased intrathoracic pressure • Scv. O 2 saturation (SVC) 70% Grade 1 C

The Early Bird Gets the Worm

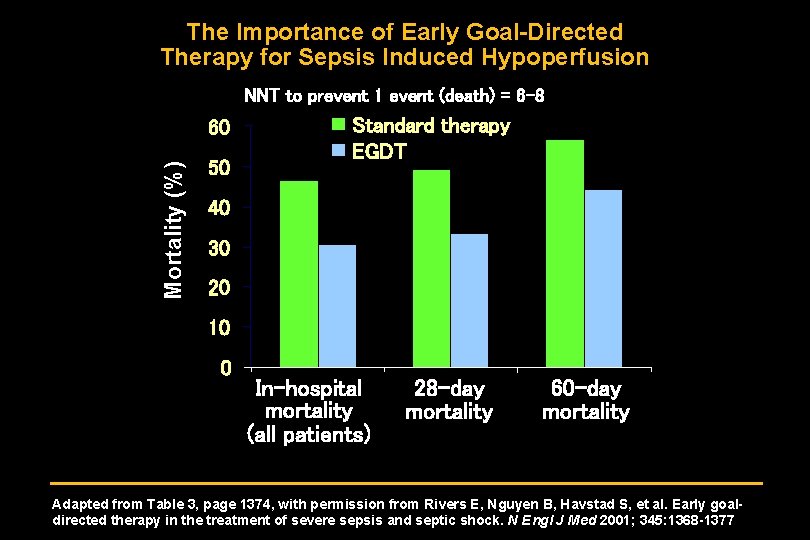
The Importance of Early Goal-Directed Therapy for Sepsis Induced Hypoperfusion NNT to prevent 1 event (death) = 6 -8 Mortality (%) 60 50 Standard therapy EGDT 40 30 20 10 0 In-hospital mortality (all patients) 28 -day mortality 60 -day mortality Adapted from Table 3, page 1374, with permission from Rivers E, Nguyen B, Havstad S, et al. Early goaldirected therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345: 1368 -1377

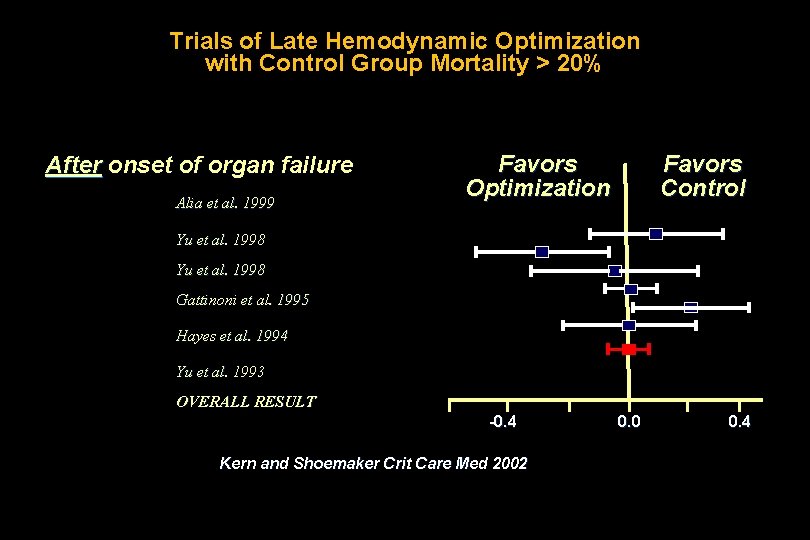
Trials of Late Hemodynamic Optimization with Control Group Mortality > 20% After onset of organ failure Alia et al. 1999 Favors Optimization Favors Control Yu et al. 1998 Gattinoni et al. 1995 Hayes et al. 1994 Yu et al. 1993 OVERALL RESULT -0. 4 Kern and Shoemaker Crit Care Med 2002 0. 0 0. 4

Role of Collaboration ED ICU

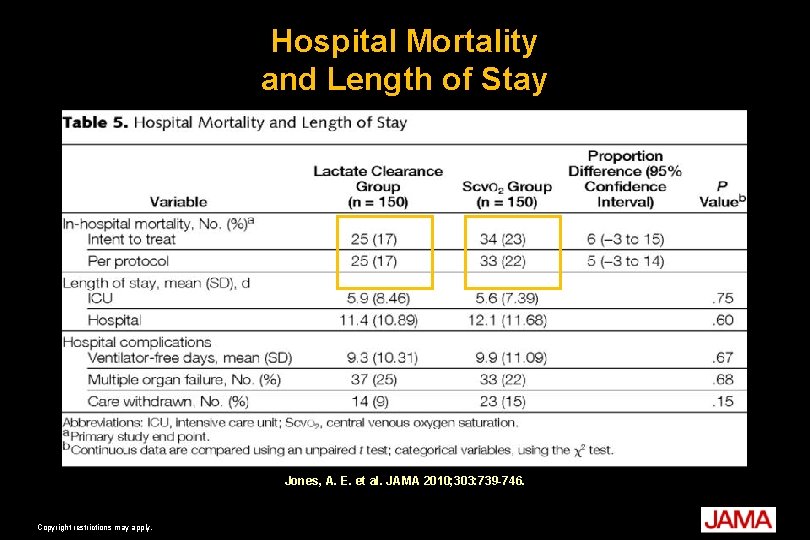
Jones AE, Shapiro NI, Trzeciak S, et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010; 303(8): 739 -46.

Hospital Mortality and Length of Stay Jones, A. E. et al. JAMA 2010; 303: 739 -746. Copyright restrictions may apply.

Vasopressin • Continue to recommend against using high doses of vasopressin (unless salvage therapy) • Vasopressin. 03 units per min plus NE equivalent to norepinephrine alone – VASST trial


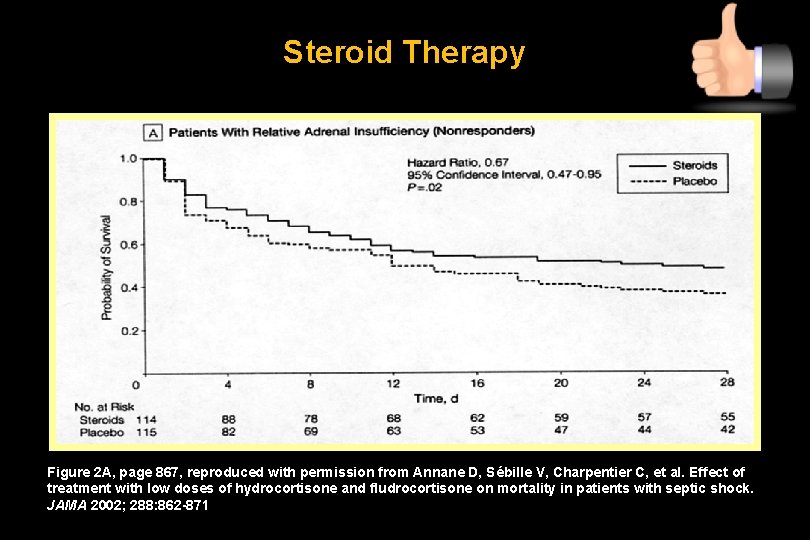
Steroid Therapy Figure 2 A, page 867, reproduced with permission from Annane D, Sébille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002; 288: 862 -871

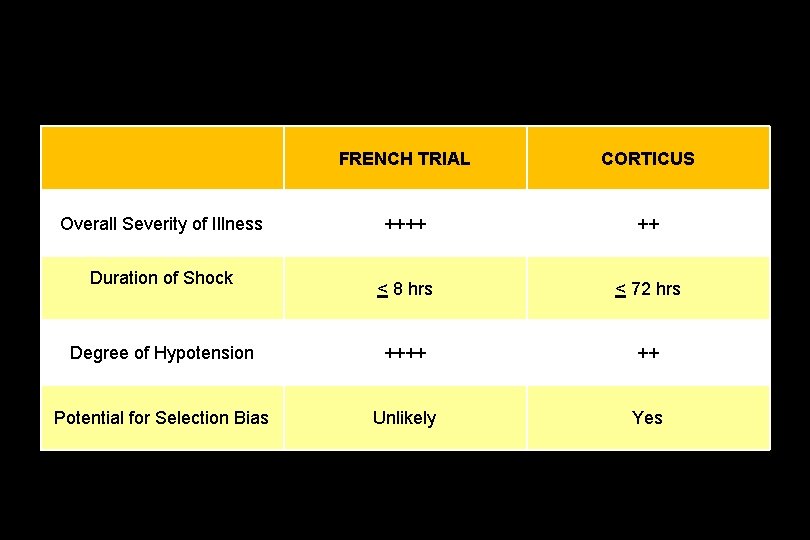
CORTICUS: Results • No benefit in intent to treat – Mortality – Shock reversal • Earlier reversal of shock with steroids in those that had shock reversal

FRENCH TRIAL CORTICUS ++++ ++ < 8 hrs < 72 hrs Degree of Hypotension ++++ ++ Potential for Selection Bias Unlikely Yes Overall Severity of Illness Duration of Shock

Steroids Suggest intravenous hydrocortisone be given only to adult septic shock patients after blood pressure is identified to be poorly responsive to fluid resuscitation and vasopressor therapy Grade 2 C

Recombinant Human Activated Protein C (rh. APC) • Suggest use in patients with clinical assessment of high risk of death due to sepsis induced organ dysfunction typically with APACHE II 25 or greater or multiple organ failure • No absolute contraindications • Weigh relative contraindications Grade 2 B

PROWESS SHOCK

As to Guidelines Publications • “Knowledge is Good”

“Knowledge is Good”


Guidelines Are Not Enough • Protocols • Performance Improvement Programs

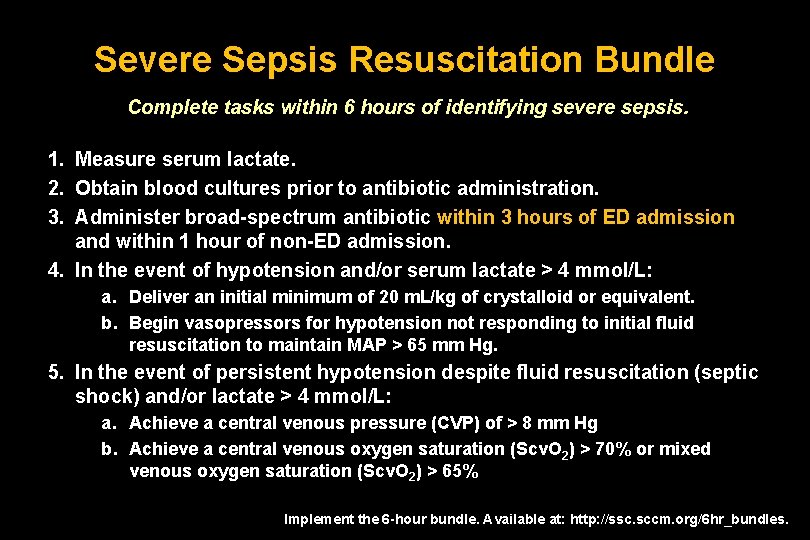
Severe Sepsis Resuscitation Bundle Complete tasks within 6 hours of identifying severe sepsis. 1. Measure serum lactate. 2. Obtain blood cultures prior to antibiotic administration. 3. Administer broad-spectrum antibiotic within 3 hours of ED admission and within 1 hour of non-ED admission. 4. In the event of hypotension and/or serum lactate > 4 mmol/L: a. Deliver an initial minimum of 20 m. L/kg of crystalloid or equivalent. b. Begin vasopressors for hypotension not responding to initial fluid resuscitation to maintain MAP > 65 mm Hg. 5. In the event of persistent hypotension despite fluid resuscitation (septic shock) and/or lactate > 4 mmol/L: a. Achieve a central venous pressure (CVP) of > 8 mm Hg b. Achieve a central venous oxygen saturation (Scv. O 2) > 70% or mixed venous oxygen saturation (Scv. O 2) > 65% Implement the 6 -hour bundle. Available at: http: //ssc. sccm. org/6 hr_bundles.


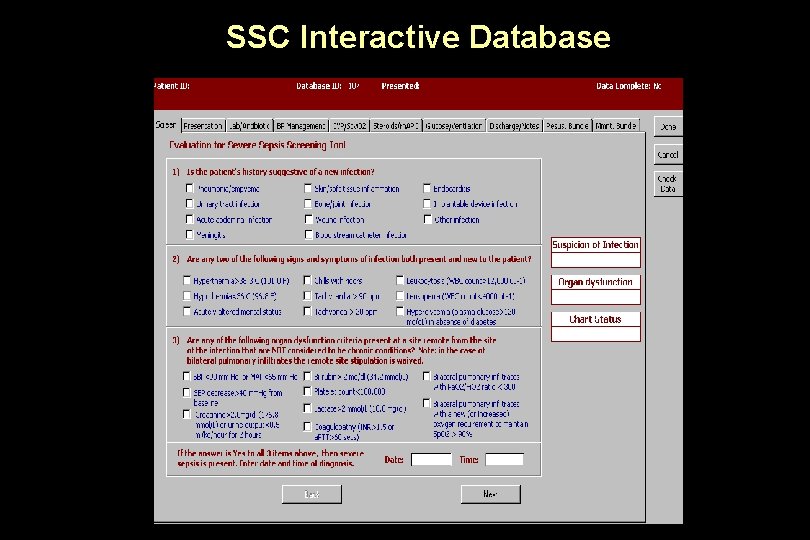
SSC Interactive Database

Surviving Sepsis Campaign: Phase III • A global, multi-center, 2 -year trial – Multiple hospital networks – 166 sites – 15, 022 patients • Primary outcome – The impact of a model for changing bedside management of sepsis • Secondary outcome – Mortality


Findings • Primary outcome of SSC: behavior change – 20% increase in bundle compliance over 24 months • Secondary outcome - Mortality -- 7. 0% ARR, 19% RRR -- 5. 4% ARR after severity adjustment

Septic Shock Research at Cooper

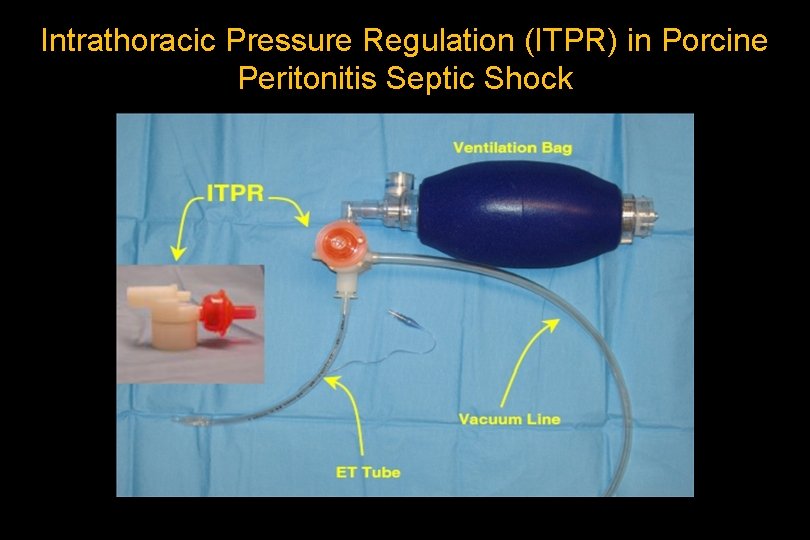
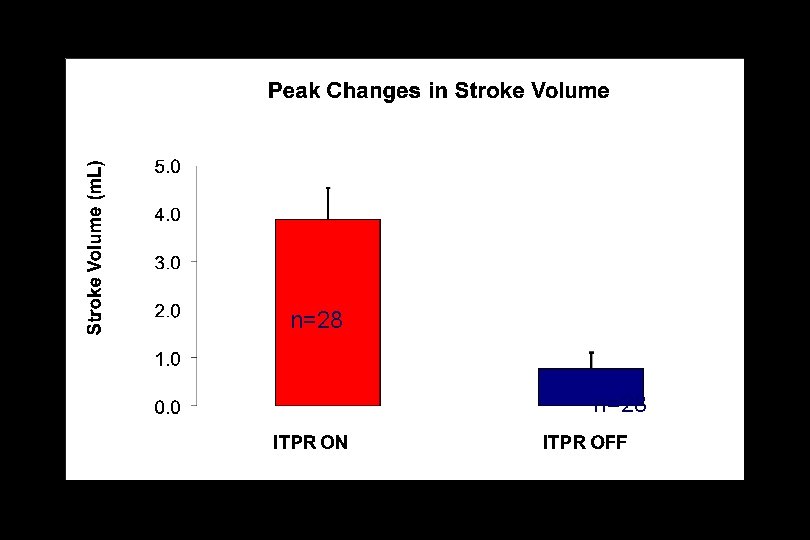
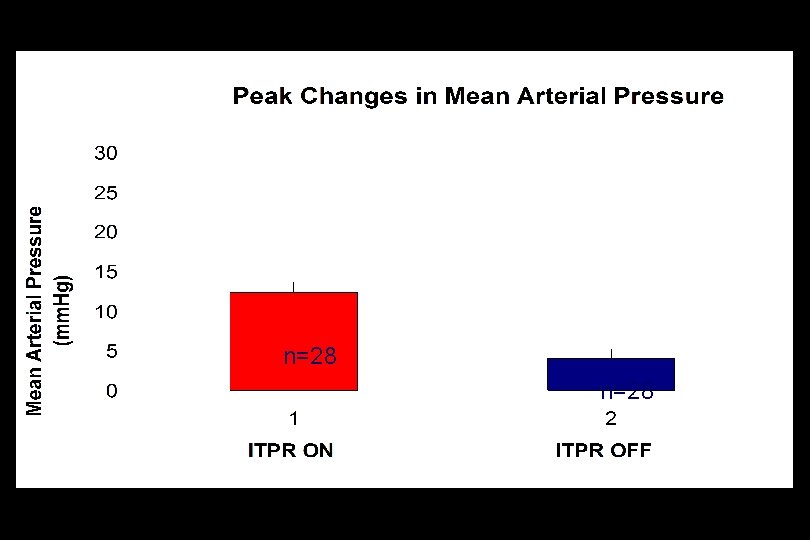
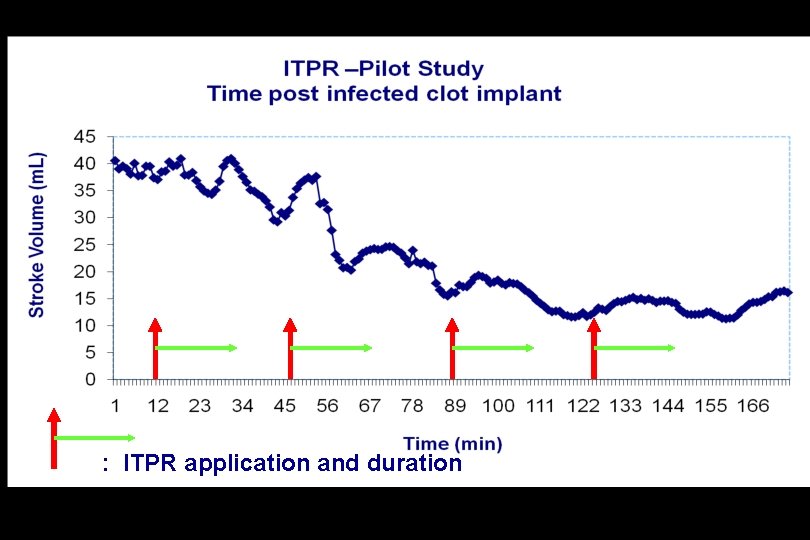
Intrathoracic Pressure Regulation (ITPR) in Porcine Peritonitis Septic Shock

p<0. 001 n=28

p<0. 001 n=28

: ITPR application and duration

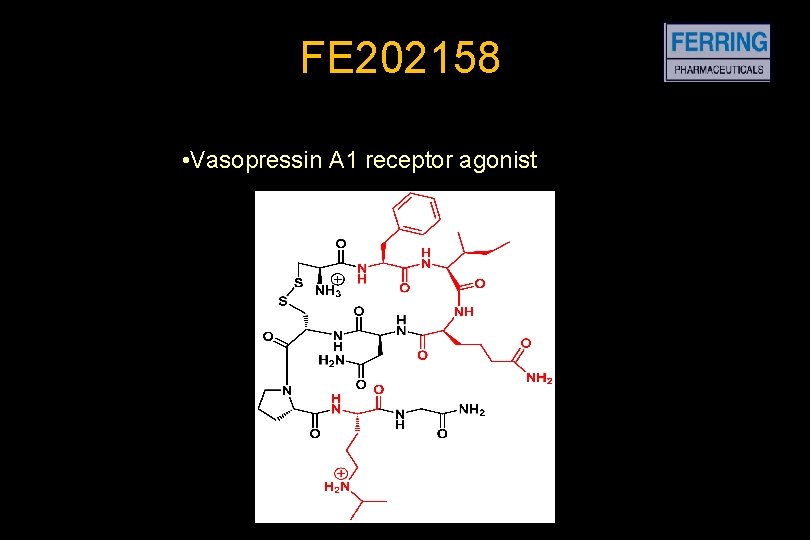
FE 202158 • Vasopressin A 1 receptor agonist

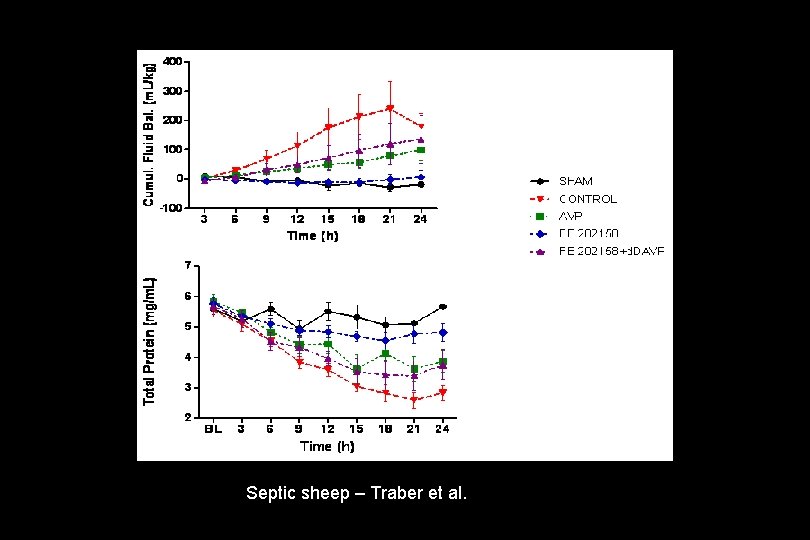
Septic sheep – Traber et al.

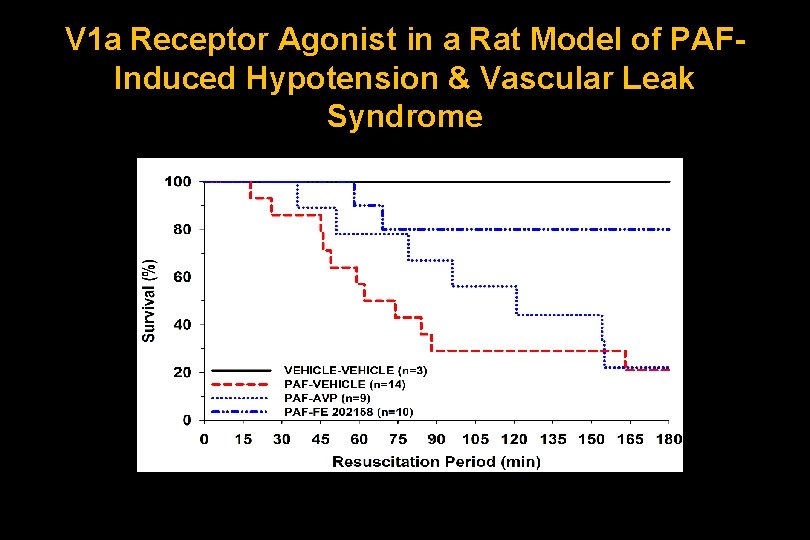
V 1 a Receptor Agonist in a Rat Model of PAFInduced Hypotension & Vascular Leak Syndrome

The EUPHRATES Trial Evaluating the Use of Polymyxin B Hemoperfusion in a Randomized controlled trial of Adults Treated for Endotoxemia and Septic shock

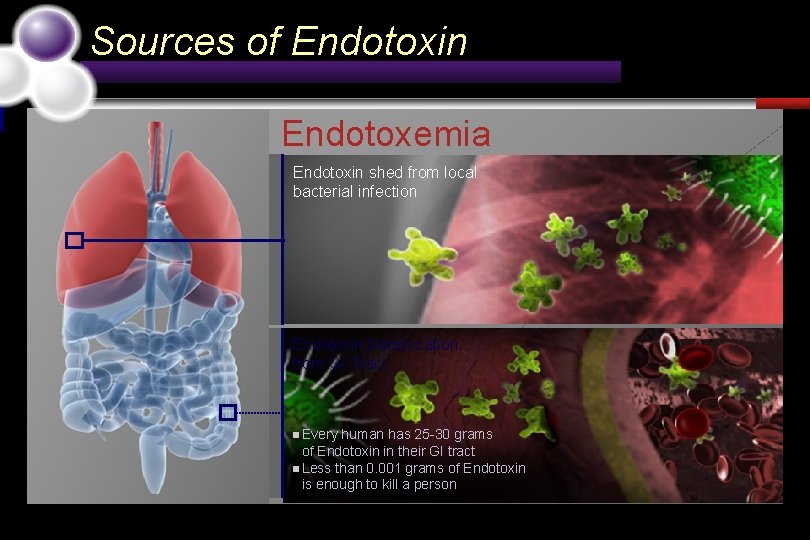
Sources of Endotoxin Endotoxemia Endotoxin shed from local bacterial infection Endotoxin translocation from GI Tract n Every human has 25 -30 grams of Endotoxin in their GI tract n Less than 0. 001 grams of Endotoxin is enough to kill a person

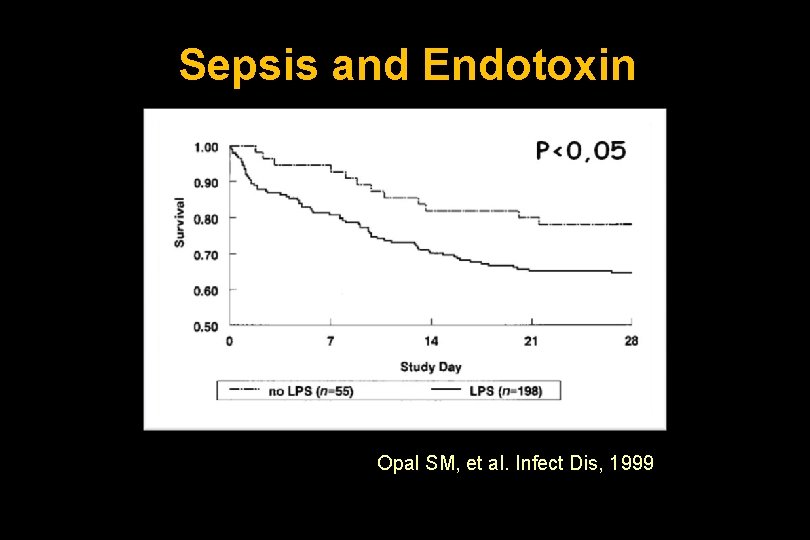
Sepsis and Endotoxin Opal SM, et al. Infect Dis, 1999

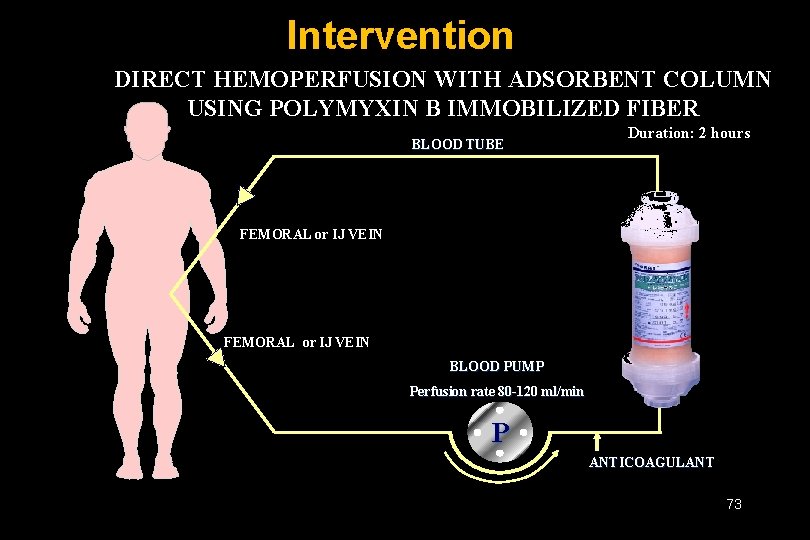
Intervention DIRECT HEMOPERFUSION WITH ADSORBENT COLUMN USING POLYMYXIN B IMMOBILIZED FIBER BLOOD TUBE Duration: 2 hours FEMORAL or IJ VEIN BLOOD PUMP Perfusion rate 80 -120 ml/min P ANTICOAGULANT 73


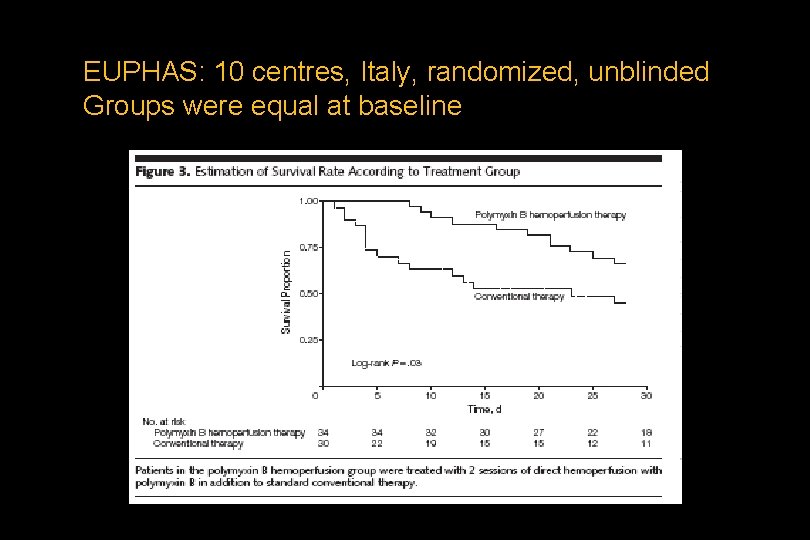
EUPHAS: 10 centres, Italy, randomized, unblinded Groups were equal at baseline

Endotoxin Activity Assay EAA™ The only FDA cleared test for detection of Endotoxin Spectral Diagnostics Inc. 76

THANK YOU
 Alister stevens
Alister stevens Vnode unix
Vnode unix Boeing forever new frontiers
Boeing forever new frontiers Cauda equina vs conus medullaris
Cauda equina vs conus medullaris Normovolemico
Normovolemico Spinal shock vs neurogenic shock
Spinal shock vs neurogenic shock Spinal shock vs neurogenic shock
Spinal shock vs neurogenic shock Spinal shock vs neurogenic shock
Spinal shock vs neurogenic shock Line current and phase current
Line current and phase current Power formula three phase
Power formula three phase Semiconductor
Semiconductor Ac systems lesson 4
Ac systems lesson 4 Drift current density unit
Drift current density unit Ceramic composition resistors
Ceramic composition resistors Balanced wye delta connection
Balanced wye delta connection Slideplayer
Slideplayer Drift current density unit
Drift current density unit Chapter 9 frontiers of biotechnology
Chapter 9 frontiers of biotechnology Richard walker frontiers
Richard walker frontiers Frontiers of biotechnology chapter 9
Frontiers of biotechnology chapter 9 Chapter 9 frontiers of biotechnology
Chapter 9 frontiers of biotechnology Frontiers in chemical engineering
Frontiers in chemical engineering Hochedlinger
Hochedlinger Frontiers in molecular bioscience impact factor
Frontiers in molecular bioscience impact factor Weakening frontiers meaning
Weakening frontiers meaning Frontiers of information technology
Frontiers of information technology Frontiers in bioinformatics
Frontiers in bioinformatics Frontiers
Frontiers Hfsp letter of intent
Hfsp letter of intent Jea septic tank phase out
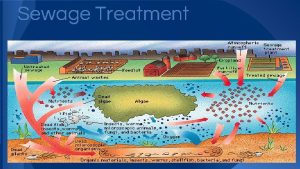
Jea septic tank phase out Septic tank contamination groundwater
Septic tank contamination groundwater Boutonniere nodes
Boutonniere nodes Indianapolis septic tank elimination program
Indianapolis septic tank elimination program Septic squeeze test tonsillitis
Septic squeeze test tonsillitis Advantex septic system reviews
Advantex septic system reviews Barnstable septic loan
Barnstable septic loan Septic implantation syndrome
Septic implantation syndrome Septic arthritis complications
Septic arthritis complications Panhandle health septic
Panhandle health septic Septic arthritis antibiotics
Septic arthritis antibiotics Septic arthritis antibiotics
Septic arthritis antibiotics Qqq10
Qqq10 Multiflow septic system
Multiflow septic system Puraflo peat septic system
Puraflo peat septic system Septic arthritis complications
Septic arthritis complications Doc's septic tank cleaning
Doc's septic tank cleaning Bakgil
Bakgil Haemophilus influenzae septic arthritis
Haemophilus influenzae septic arthritis Haemophilus influenzae septic arthritis
Haemophilus influenzae septic arthritis Haemophilus influenzae septic arthritis
Haemophilus influenzae septic arthritis Chemical oxygen demand principle
Chemical oxygen demand principle Thymoma location
Thymoma location Septic workup
Septic workup Septic
Septic Summit county septic permit
Summit county septic permit Admixtures
Admixtures Carmody septic
Carmody septic Gonococcal vs nongonococcal arthritis
Gonococcal vs nongonococcal arthritis At cutoff the jfet channel is
At cutoff the jfet channel is Dcep welding
Dcep welding Touch current vs leakage current
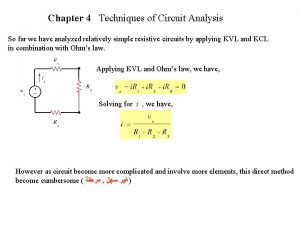
Touch current vs leakage current Kcl mesh analysis
Kcl mesh analysis Distributive shock
Distributive shock Therapeutic community
Therapeutic community Therapeutic class and pharmacologic class
Therapeutic class and pharmacologic class Association for applied and therapeutic humor
Association for applied and therapeutic humor Management of current assets
Management of current assets Symptoms of shock
Symptoms of shock Jones and bartlett learning
Jones and bartlett learning Shock and vibe symposium
Shock and vibe symposium Biphasic and monophasic shock
Biphasic and monophasic shock Navmat p-9492
Navmat p-9492 Therapeutic orientations
Therapeutic orientations Therapeutic feeding center adalah
Therapeutic feeding center adalah Therapeutic exercise chapter 1 mcqs
Therapeutic exercise chapter 1 mcqs Microwave diathermy block diagram
Microwave diathermy block diagram Therapeutic drift
Therapeutic drift Introduction of therapeutic communication
Introduction of therapeutic communication