Overview of the Office Of Research Assurances ORA






































- Slides: 39

Overview of the Office Of Research Assurances (ORA) Office of Research Assurances 205 Albrook, Pullman http: //www. ora. wsu. edu Updated January 2015

Recording date of this workshop is January 16, 2015 Some of the rules and procedures discussed in this workshop are subject to change. Please check university resources before relying exclusively on this recorded presentation.

ORA WSU Office of Research Assurances 3

Overview of ORA • Introduction • Institutional Animal Care and Use Committee (IACUC) • Institutional Biosafety Committee (IBC) • Institutional Review Board (IRB) • Radiation Safety Committee (RSC) • Conflict of Interest Committee (COIC) • Export Controls • Responsible Conduct of Research • Hazardous Materials Shipping • Contacts 4

Introduction • ORA, established in 2002, is part of the overall effort of WSU to ensure the responsible conduct of research. • We work collaboratively with researchers to stay in compliance with applicable internal and external policies and regulations. • By staying in compliance we avoid funding delays, funding denials, unpublished research, and in some cases fines and jail. Our philosophy is centered on customer service and helping PIs and departments navigate the labyrinth of laws and policies that affect research at WSU. 5

Institutional Animal Care and Use Committee (IACUC) 6

IACUC The IACUC will… • Monitor animal research • Address occupational health requirements • Address training requirements • Inventory and track animals used for research at all campuses In order for… Animals to be used safely and humanely in research at WSU 7

IACUC Who Oversees Animal Research? USDA U. S. Department of Agriculture NIH National Institutes of Health OLAW Office of Lab Animal Welfare OCV Office of the Campus Vet (WSU) IACUC 8

IACUC • Training requirements: • Animal Awareness Seminars and the Animal Contact Program • • • Review procedures that use animals. Address occupational health requirements Review of protocols to minimize ethical cost by following: Replacement Reduction Refinement Animals can be used safely and humanely in research 9

Institutional Biosafety Committee (IBC) 10

IBC • Reviews procedures for working with potentially hazardous biological materials including: – Genetically Modified Organisms (GMOs) – Infectious Agents and Pathogens • Human • Animal • Plants – Human Tissue and Cells (used in research) • Verifies containment practices and facilities – Biosafety levels (BSL-1, -2, -3) 11

IBC Who Oversees Research With Biological Materials? NIH National Institutes of Health USDA U. S. Department of Agriculture § APHIS Animal and Plant Health Inspection Service BSO Biological Safety Officer (Local – WSU’s ORA) WSU’s IBC 12

Institutional Review Board (IRB) 13

IRB US National Research Act 1974 • Established National Commission for Protection of Human Subjects § The Belmont Report, 1979 • Code of Federal Regulations, 1981 § Institutional Review Boards (IRBs) § Informed Consent • Common Rule (45 CFR Part 46), 1991 • HIPAA Privacy Rule (45 CFR Parts 160, 162, & 164), 2003 14

IRB The Institutional Review Board IRB is responsible for the review and approval of all research activities involving human subjects. The IRB protects the rights and welfare of human subjects to ensure that all are treated in such a way as to minimize risk and avoid harm. The guiding principles of the IRB are: Respect for Persons Beneficence Justice 15

IRB Who Oversees Human Subjects Research? HHS U. S. Department of Health and Human Services § OHRP Office for Human Research Protections FDA U. S. Food and Drug Administration WSU’s IRB 16

Radiation Safety Office/Radiation Safety Committee (RSO/RSC) 17

RSO/RSC The WSU Radiation Safety Office and Radiation Safety Committee ensures the safe use of radioactive materials and radiation machines on the Pullman campus and other WSU sites around the state. As Low As Reasonably Achievable 18

RSO/RSC Who Oversees Radiation Safety? NRC U. S. National Radioactivity Commission DOH Washington Department of Health WSU’s RSC and RSO 19

RSO/RSC • Reviews procedures involving work with radioactive materials. • Addresses training requirements • Monitors radiation exposure of personnel • Inventories and tracks radioactive materials • Manages radioactive waste disposal • Audits labs for procedures, documentation, and properly working equipment. 20

Conflict of Interest Committee (COIC) 21

COIC The COI Committee will… • Increase accountability • Add transparency • Enhance regulatory compliance and effective administrative management of PIs’ financial conflicts of interest. We want to manage conflicts and potential conflicts in order to support entrepreneurship while protecting the reputations of PIs and WSU. 22

COIC Who Oversees Conflict of Interest? PHS U. S. Public Health Service NSF National Science Foundation FDA U. S. Food and Drug Administration Other funding agencies Professional organizations WSU’s COIC 23

COIC The COI Committee reviews all pertinent documentation, including COI management plans, relating to potential or actual financial COI applications. The COIC has the responsibility and authority to • assess whether a potential conflict exists • assess the extent of the conflict and • manage, reduce or eliminate the conflict before approving the research or technology transfer activity. 24

Export Controls 25

Export Controls • It is ultimately each person’s obligation to comply with Export Regulations in respect to their WSU associated activities. • Most research activities at WSU are exempted through the Fundamental Research Exemption. • Operation of export controlled equipment is allowed foreign nationals who would otherwise be restricted because of a very specific definition of “use”: • • • operating installing maintaining repairing overhauling and refurbishing 26

Export Controls Who Oversees Export Controls? U. S. Department of Commerce (Bureau of Industry and Security) controls dualuse items Export Administration Regulations (EAR) – Commerce Control List U. S. Department of State (Office of Defense Trade Controls) controls defense articles, defense services, and related technical data International Traffic in Arms Regulations (ITAR) – US Munitions List U. S. Department of the Treasury oversees U. S. trade embargoes (Office of Foreign Assets Control) List of specifically designated nations, nationals, and individuals 27

Export Controls Responsibilities of PIs: • Review in advance research for potential EAR/ITAR issues. • Apply for a license BEFORE project begins--process can take 2 -6 months or longer. *Embargoed Countries Delivery of services to embargoed countries is not allowed without a license or exception. Contact the ORA before entering into an agreement or providing a service to individuals or institutions from Cuba, Iran, North Korea, Sudan, or Syria. 28

Responsible Conduct of Research (RCR) 29

RCR Who Oversees RCR? • • • State of Washington NIH/NSF Professional Organizations Vice President for Research Office Research Assurances Principal Investigators 30

RCR trainings on My. Research for both Faculty/Staff and students. Retraining is required every 5 years. Anyone who is credited on a grant must be current on training. • • • Data Acquisition, Management, Sharing and Ownership Conflict of Interest and Commitment Use of Humans Use of Animals Research Misconduct Publishing Practices and Authorship Mentor/Trainee Relationships and Responsibilities Peer Review Collaborations 31

Hazardous Materials Shipping (HMS) 32

Hazardous Materials Shipping If a person knowingly, willingly, or recklessly violates a hazardous material regulation, special permit, or approval issued by the DOT, this is considered to be a criminal violation. ORA Hazmat Shipping 509 -432 -3869 Hazardous materials shipments from WSU are handled by the ORA Shipping Coordinators. We will come to your lab or main office, pick up your package with a completed/signed request for shipment, and generate a label and verify that contents are packaged according to the shipping regulations. 33

Hazardous Materials Shipping Is my shipment (potentially) hazardous? • • • Dry Ice Chemicals (including preservatives) Pathogens Human specimens Animal specimens Radioactive materials* If your material is hazardous or you are not sure, call: ORA Hazmat Shipping 509 -432 -3869 *Radiation Safety 335 -8916 34

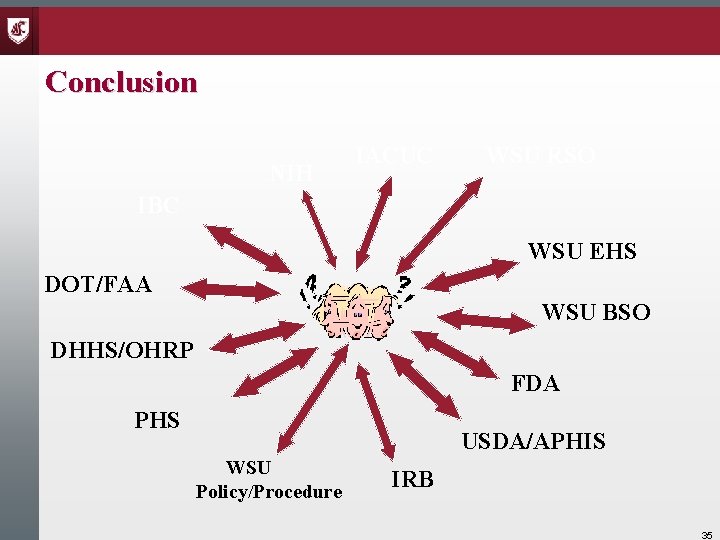
Conclusion NIH IACUC WSU RSO IBC WSU EHS DOT/FAA WSU BSO DHHS/OHRP FDA PHS USDA/APHIS WSU Policy/Procedure IRB 35

ORA/RSO Contacts Director (Interim) & Biological Safety Officer (BSO) Biosafety and Hazmat Shipping (HMS) Industrial Hygienist Mike Kluzik 335 -9553 Ryan Schwager 335 -4462 mkluzik@wsu. edu rschwager@wsu. edu rani_m@wsu. edu COI, Export Controls, RCR, & HMS Sheri Glaesman 335 -0039 sglaesman@wsu. edu IRB Coordinator IACUC Coordinator Rani Muthukrishnan 335 -7951 Malathi Jandhyala 335 -3668 mjandhyala@wsu. edu IRB & IBC Program Coordinator Patrick Conner 335 -7183 patrick_conner@wsu. edu Ayesha Ahmed 368 -6667 ayesha. ahmed@wsu. edu

ORA/RSO Contacts Radiation Safety Officer Jean Cloran 335 -7057 jcloran@wsu. edu Assistant RSO Rey Mc. Gehee 335 -6763 rmcgehee@wsu. edu Health Physicist Kreta Johnson 335 -7058 kretaj@wsu. edu Radiation Safety Technician III Scott Finch 335 -4221 sfinch@wsu. edu Radiation Safety Technician III Sonja Fenimore 335 -8175 katmere@wsu. edu

Thank You! www. ora. wsu. edu

This has been a WSU Training Videoconference If you attended this live training session and wish to have your attendance documented in your training history, please notify Human Resource Services within 24 hours of today's date: hrstraining@wsu. edu
 Swara pambiwara iku kudu kung tegese
Swara pambiwara iku kudu kung tegese Confronto crin d'oro crespo e chiome d'argento fine
Confronto crin d'oro crespo e chiome d'argento fine Francesco petrarca laura
Francesco petrarca laura Zephyr assurances
Zephyr assurances Sueldos banco credicoop
Sueldos banco credicoop Sots meaning in research
Sots meaning in research Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Chụp tư thế worms-breton
Chụp tư thế worms-breton Alleluia hat len nguoi oi
Alleluia hat len nguoi oi Môn thể thao bắt đầu bằng chữ đua
Môn thể thao bắt đầu bằng chữ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 101012 bằng
101012 bằng Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế
Cái miệng nó xinh thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thế nào là giọng cùng tên? *
Thế nào là giọng cùng tên? * Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Tia chieu sa te
Tia chieu sa te Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dot
Dot Số nguyên tố là gì
Số nguyên tố là gì Tư thế ngồi viết
Tư thế ngồi viết































































