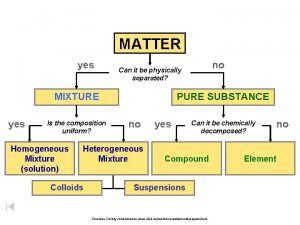
Nutrition and Composition of Food What is Food






















































































- Slides: 140

Nutrition and Composition of Food

What is Food? � � Any substance taken into the assimilated by a plant or animal to keep it alive and enable it to grow and repair tissue; nourishment Anything that nourishes or stimulates; whatever helps something to keep active, grow, etc ◦ Webster’s New World College Dictionary – 4 th edition. 1999. � Complex mixture of chemicals that an organism takes in and assimilates to: ◦ Promote growth ◦ Expend energy ◦ Replace worn or injured tissue ◦ Prevent some diseases

Nutrition… � Encompasses definitions many processes= many � The series of processes by which an organism takes in and assimilates food for promoting growth and replacing worn or injured tissues ◦ Webster’s New World College Dictionary – 4 th edition. 1999.

Most Foods are Actually… Extremely complex mixtures of thousands of chemicals � 97% of food’s mass is made up of: � �Proteins �Carbohydrates �Lipids � The remainder of food consists of thousands of compounds that exist in small amounts (measuring parts per million) and are often important in: � Taste � Odor � Color � Vitamin and minerals also exist in minute amounts and are very important in body function.

Obesity Rates

Defining Obesity � � For adults, overweight and obesity ranges are determined by using weight and height to calculate a number called the “body mass index” (BMI). BMI is used because, for most people, it correlates with their amount of body fat. An adult who has a BMI between 25 and 29. 9 is considered overweight. An adult who has a BMI of 30 or higher is considered obese. BMI is an estimate of body fat so sometimes it can show that a person is overweight when they are not actually �Like athletes

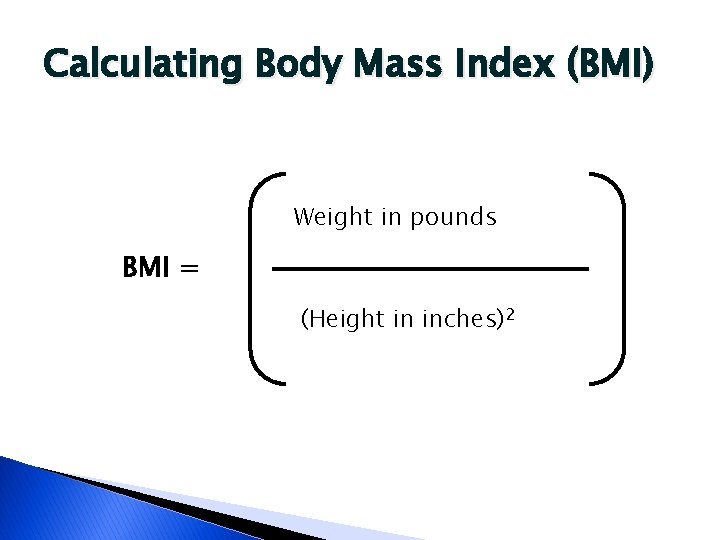
Calculating Body Mass Index (BMI) Weight in pounds BMI = (Height in inches)2

Calculate Your BMI = ( )2

Obesity is associated with increased risk for � Diabetes � Hypertension � Cancer � Stroke ◦ Breast ◦ Colon � Heart � Liver disease � Respiratory � Sleep issues disturbances

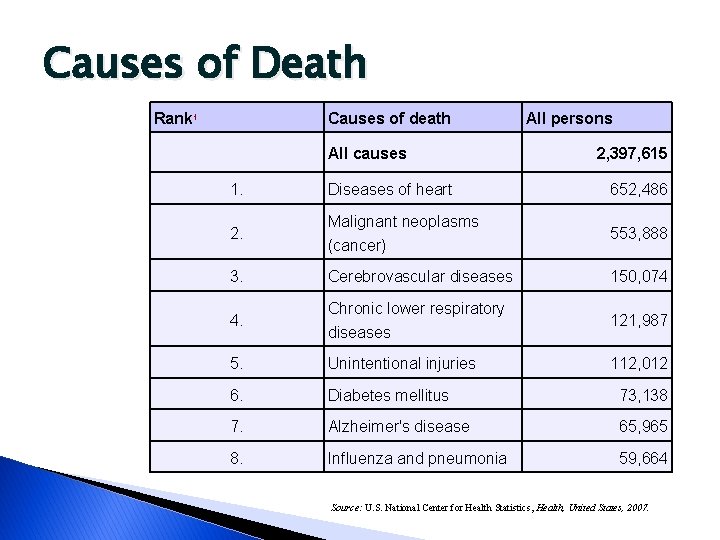
Causes of Death Rank 1 Causes of death All causes All persons 2, 397, 615 1. Diseases of heart 652, 486 2. Malignant neoplasms (cancer) 553, 888 3. Cerebrovascular diseases 150, 074 4. Chronic lower respiratory diseases 121, 987 5. Unintentional injuries 112, 012 6. Diabetes mellitus 73, 138 7. Alzheimer's disease 65, 965 8. Influenza and pneumonia 59, 664 Source: U. S. National Center for Health Statistics, Health, United States, 2007.

“New” Food Pyramid

Calculating % Fat in Food 1. Multiply grams of fat times 9 calories per gram to get calories from fat 2. Divide calories from fat by total calories and multiply by 100 to get % of calories from fat 3. Multiply grams of saturated fat by 9 calories per gram to get calories from saturated fat 4. Divide calories from saturated fat by total calories and multiply by 100 to get percent calories from saturated fat

Try It for Yourself � Quarter Pounder from Mc. Donalds ◦ Calories – 410 ◦ Total fat – 19 grams ◦ Saturated fat – 7 grams � � Calculate the % fat and % saturated fat in the quarter pounder. Dietary recommendations are to consume less than 10% of calories from saturated fats and keep total fat intake between 20 -35% of total calories…does a cheeseburger do this?

Food vs. Nutrients � Food ◦ Anything that nourishes or stimulates; whatever helps something to keep active, grow, etc �Webster’s New World College Dictionary – 4 th edition. 1999. � Nutrient ◦ Nutritious ingredient or substance in a food �Webster’s New World College Dictionary – 4 th edition. 1999.

Classes of Nutrients �Carbohydrates �Vitamins �Proteins �Minerals �Lipids �Water

PROTEINS

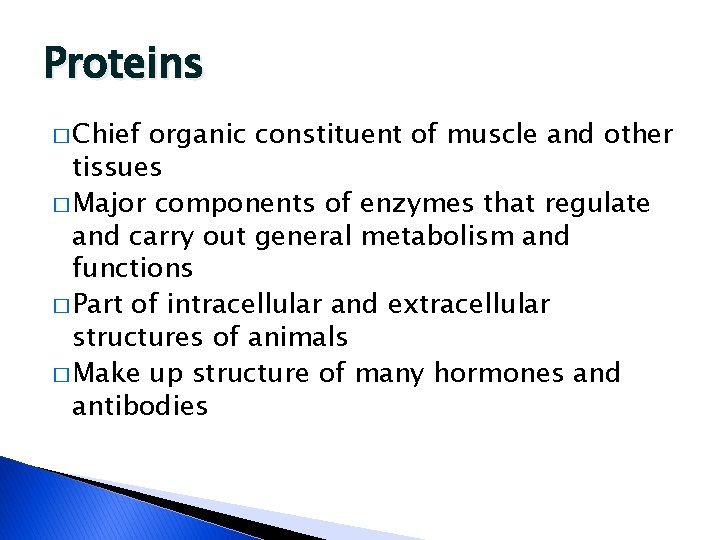
Proteins � Chief organic constituent of muscle and other tissues � Major components of enzymes that regulate and carry out general metabolism and functions � Part of intracellular and extracellular structures of animals � Make up structure of many hormones and antibodies

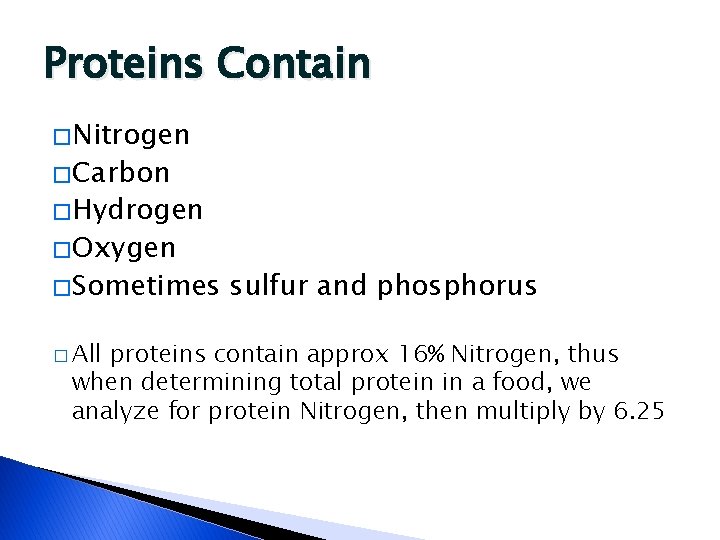
Proteins Contain � Nitrogen � Carbon � Hydrogen � Oxygen � Sometimes � All sulfur and phosphorus proteins contain approx 16% Nitrogen, thus when determining total protein in a food, we analyze for protein Nitrogen, then multiply by 6. 25

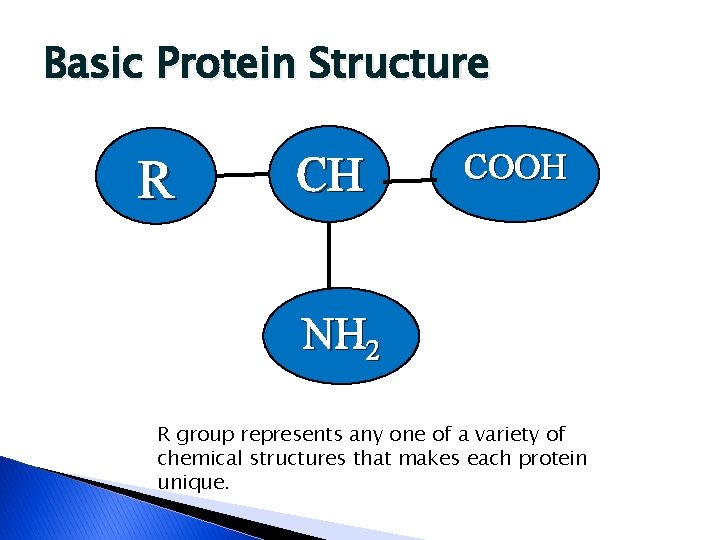
Basic Protein Structure R CH COOH NH 2 R group represents any one of a variety of chemical structures that makes each protein unique.

Quality of a Protein � Not all protein is made up of suitable materials to properly supply the body with what it needs � Proteins quality ◦ Meat ◦ Milk ◦ Eggs � High from animal products are considered high quality proteins contain ◦ All essential amino acids in amounts needed to support protein tissue formation by body

Quality…cont… � Most plant proteins are NOT considered complete ◦ Soybeans are close �Some consider them to be complete or high quality proteins

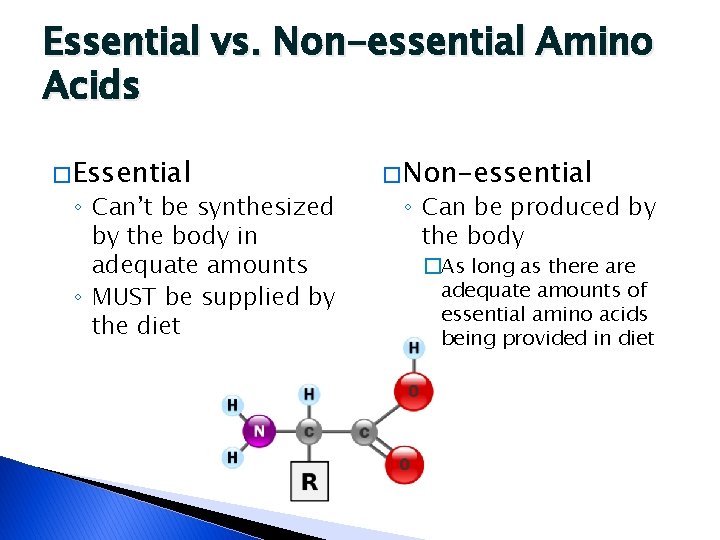
Essential vs. Non-essential Amino Acids � Essential ◦ Can’t be synthesized by the body in adequate amounts ◦ MUST be supplied by the diet � Non-essential ◦ Can be produced by the body �As long as there adequate amounts of essential amino acids being provided in diet

Why are Essentials Needed for Non -Essentials? � If ANY essential amino acids are missing from the diet, NO proteins formed. � WHY? ? �Because if the body did not stop all protein production, cells would end up with an imbalance of proteins �Seriously affect cell function

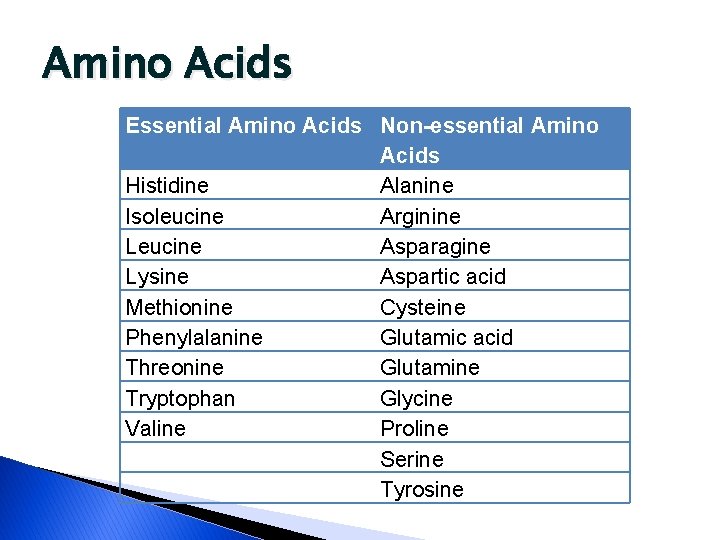
Amino Acids Essential Amino Acids Non-essential Amino Acids Histidine Alanine Isoleucine Arginine Leucine Asparagine Lysine Aspartic acid Methionine Cysteine Phenylalanine Glutamic acid Threonine Glutamine Tryptophan Glycine Valine Proline Serine Tyrosine

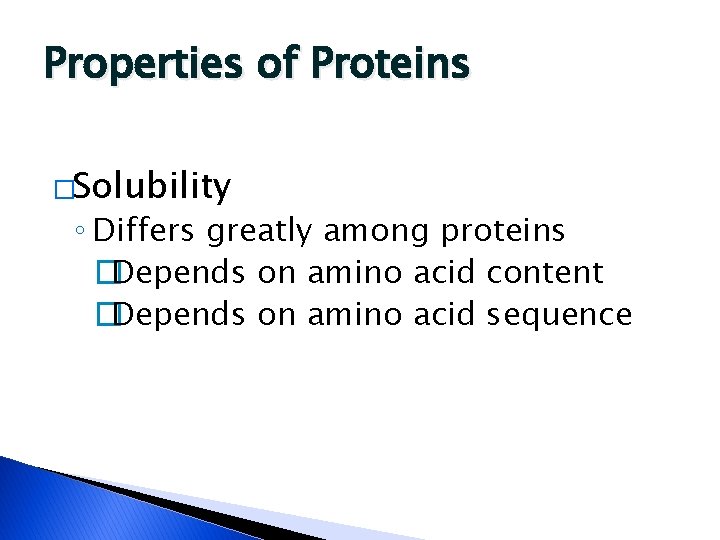
Properties of Proteins �Solubility ◦ Differs greatly among proteins �Depends on amino acid content �Depends on amino acid sequence

Solubility of Proteins Protein Type Function Solubility Histones Protein replication Soluble in pure water Albumins Soluble in pure water Keratin Collagen Hair and fingernails Myosin Muscle tissue Insoluble in water; Soluble in weak salt solution Lactoglobulins Milk Insoluble in water; Soluble in weak salt solution Wheat, glutenin, orzenin (rice 0 ovalbumin - egg white lactalbumin – milk blood albumins bones cartilage connective tissue epidermis Insoluble in water Not soluble in water; soluble in acids or alkali

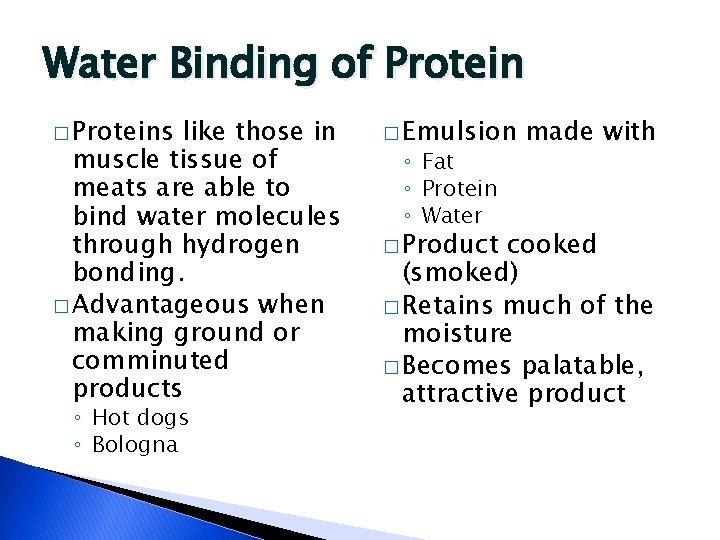
Water Binding of Protein � Proteins like those in muscle tissue of meats are able to bind water molecules through hydrogen bonding. � Advantageous when making ground or comminuted products ◦ Hot dogs ◦ Bologna � Emulsion ◦ Fat ◦ Protein ◦ Water � Product made with cooked (smoked) � Retains much of the moisture � Becomes palatable, attractive product

Structure of Proteins � Primary structure ◦ made up of ◦ Molecular weight ◦ Amino acid composition and ◦ Sequence along the polypeptide chain � Secondary structure ◦ Shape of a coiled helix � Tertiary structure ◦ Result of the folding of the chain over itself ◦ Three-dimensional state ◦ Very important to level of protein activity

Structure of Proteins… � Quaternary structure ◦ Possible of two or more polypeptide chains join together ◦ Huge tangled, complicated chain of amino acids ◦ Fragile molecules �Good to be aware of these reactions when exposing product to �Heat �Acid �Salt �Other conditions that could disturb their stability


Denaturation of Proteins � The change in molecular structure without breaking covalent bonds or altering amino acid sequence � Protein conformation (secondary, tertiary, and quaternary) structures can be very fragile and thus can be altered by a number of factors that can be used in food processing


Denaturation of Proteins cont… � Denaturation usually results in the loss of biological activity and significant changes in physical and functional properties (such as solubility) � Occurs only in proteins and protein-like substances

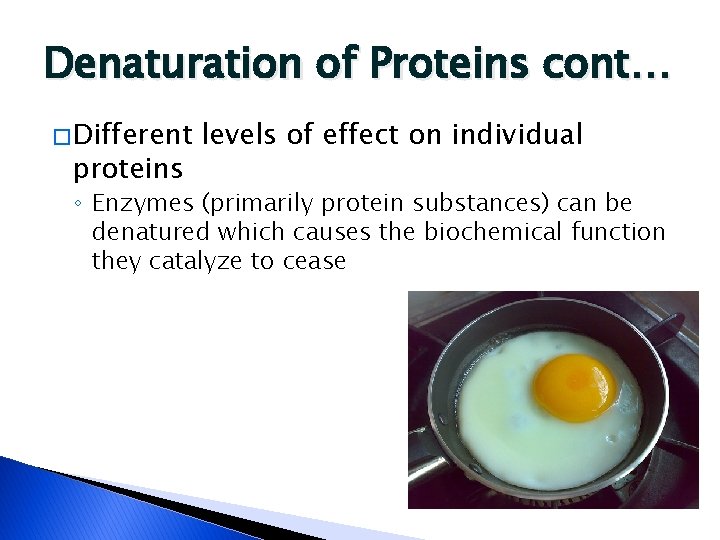
Denaturation of Proteins cont… � Different proteins levels of effect on individual ◦ Enzymes (primarily protein substances) can be denatured which causes the biochemical function they catalyze to cease

Denaturation of Proteins cont… � Caused factors by many ◦ Heat ◦ Acids ◦ Solvents �Ethyl alcohol ◦ Concentrated solutions of some salts ◦ Surface forces � Loss of biological activity and solubility � Irreversible gels may be formed ◦ More susceptible to enzymatic hydrolysis �Makes them more digestible

Denaturation of Proteins cont… � Practically irreversible � Control of denaturation is essential to food technology � Inactivation of enzymes = storage problems � “Blanching” step in freezing and canning of fruits and veggies done to denature enzymes � Contributes to flavor and texture of food

Denaturation of Proteins cont… � Cheeses and yogurt are direct results of denaturation of milk proteins � Cooking affects denaturation of proteins and impacts flavor of protein-rich foods like eggs and milk ◦ Hardening of egg whites in frying pan = denaturation ◦ Whipping of egg whites to form foam results from exposure to surface forces ◦ Many meat proteins are cooked to 134°F to 167°F = profound changes in �Texture �Water holding capacity �Shrinkage

CARBOHYDRATES

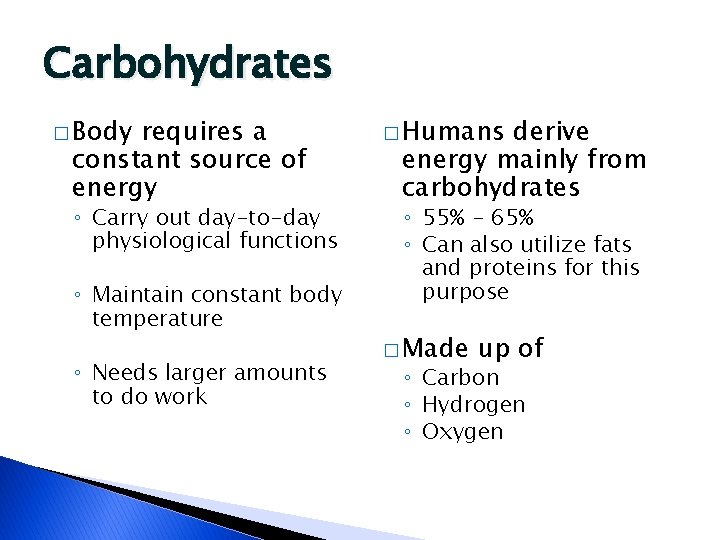
Carbohydrates � Body requires a constant source of energy ◦ Carry out day-to-day physiological functions ◦ Maintain constant body temperature ◦ Needs larger amounts to do work � Humans derive energy mainly from carbohydrates ◦ 55% - 65% ◦ Can also utilize fats and proteins for this purpose � Made up of ◦ Carbon ◦ Hydrogen ◦ Oxygen

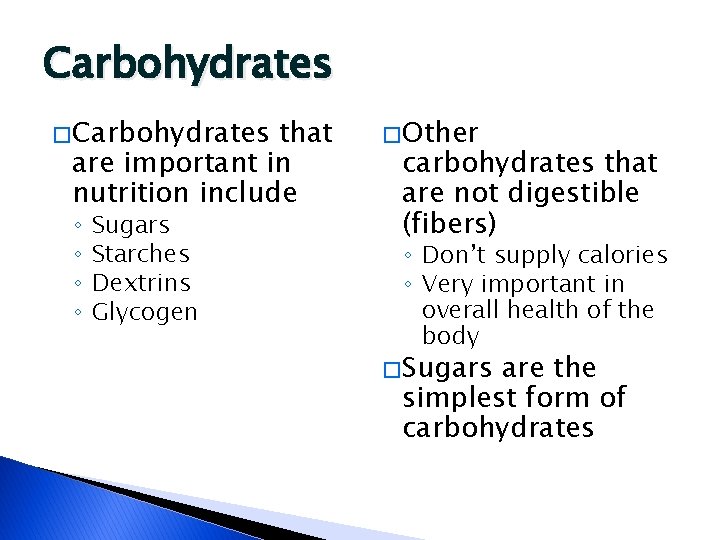
Carbohydrates � Carbohydrates that are important in nutrition include ◦ ◦ Sugars Starches Dextrins Glycogen � Other carbohydrates that are not digestible (fibers) ◦ Don’t supply calories ◦ Very important in overall health of the body � Sugars are the simplest form of carbohydrates

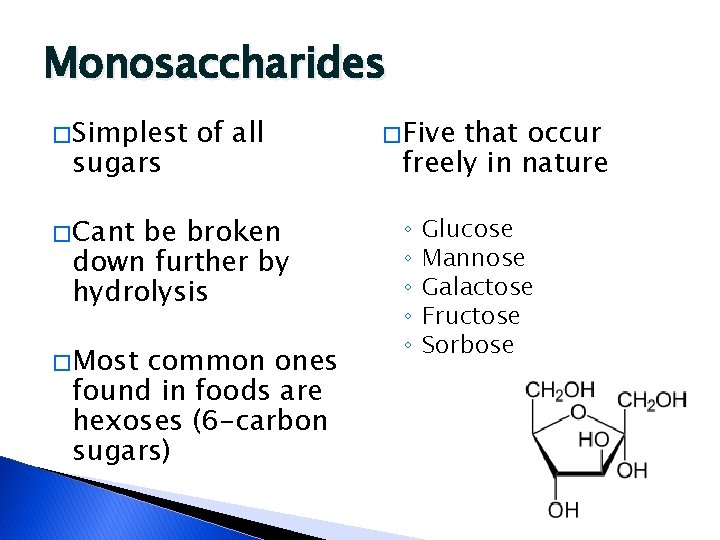
Monosaccharides � Simplest sugars of all � Cant be broken down further by hydrolysis � Most common ones found in foods are hexoses (6 -carbon sugars) � Five that occur freely in nature ◦ ◦ ◦ Glucose Mannose Galactose Fructose Sorbose

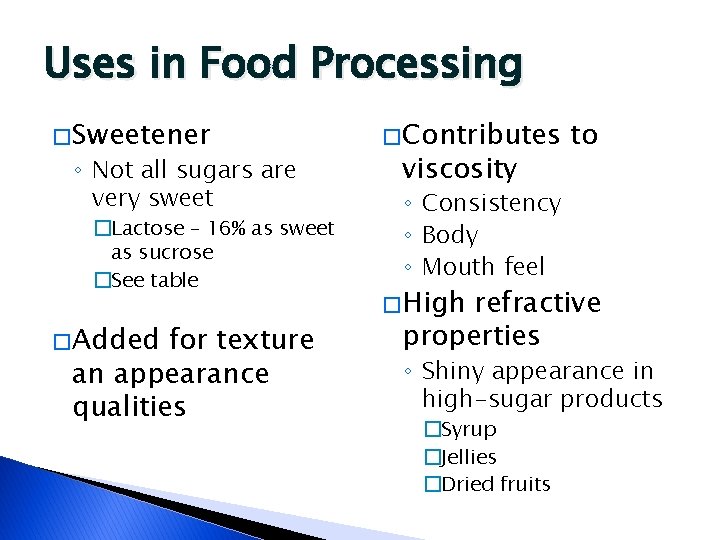
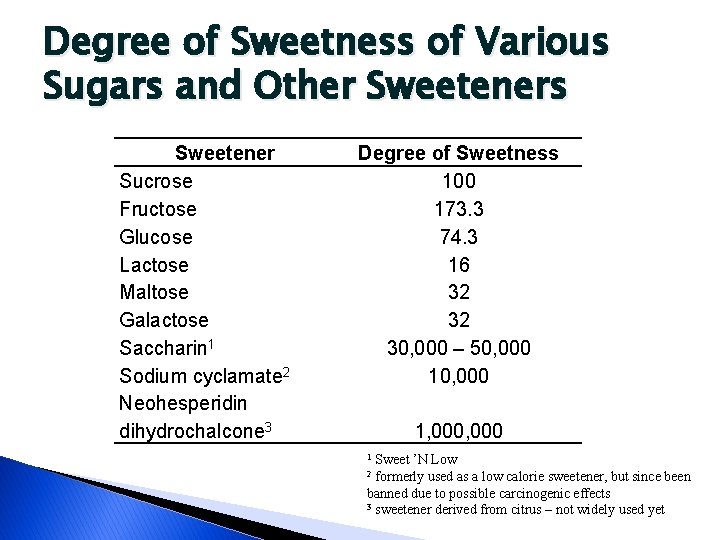
Uses in Food Processing � Sweetener ◦ Not all sugars are very sweet �Lactose – 16% as sweet as sucrose �See table � Added for texture an appearance qualities � Contributes viscosity to ◦ Consistency ◦ Body ◦ Mouth feel � High refractive properties ◦ Shiny appearance in high-sugar products �Syrup �Jellies �Dried fruits

Uses in Food Processing � Water can be available for microbial use ◦ Can be bound by sugar and unavailable for microbes ◦ Still offer moist product ◦ Water available for microbial use is measured �Water activity (Aw) ◦ Sugars have affinity for water �Suppress water activity �Jams, jellies, etc �Preservative effect

Degree of Sweetness of Various Sugars and Other Sweeteners Sweetener Sucrose Fructose Glucose Lactose Maltose Galactose Saccharin 1 Sodium cyclamate 2 Neohesperidin dihydrochalcone 3 Degree of Sweetness 100 173. 3 74. 3 16 32 32 30, 000 – 50, 000 1, 000 Sweet ’N Low 2 formerly used as a low calorie sweetener, but since been banned due to possible carcinogenic effects 3 sweetener derived from citrus – not widely used yet 1

Polysaccharides � Polymers ◦ Compounds of many smaller molecules � Simple monosaccharides joined together by glycosidic bonds � May contain the same monosaccharides or several different monosaccharides joined together ◦ When more than 10 units are joined together = polysaccharide �Most contain hundreds of thousands of monosaccharides

Polysaccharides cont… � NOT sweet � Responsible for Texture – including: ◦ ◦ ◦ Viscosity Mouth feel Consistency Gelatin Smoothness Toughness � Nutritionally, starch is the most important polysaccharide ◦ Main source of calories in human diet

Starch � Made up of two polymers (large molecule made up of repeating chemical structures) ◦ Amylose – linear compound ◦ Amylopectin – branched compound

Major Enzymes that Catalyze Starch Hydrolysis � Alpha-amylase ◦ Widely distributed in nature ◦ Saliva ◦ Pancreatic excretions in mammals ◦ Plants ◦ Microorganisms � Beta-amylase ◦ Found almost exclusively in higher plants � Pullulanase � Amyloglucosidase ◦ Found mainly in molds � Maltase

Starch � Generally not readily soluble in cold water ◦ When heated, uptake water, swell, and gelatinize �Viscosity increases; forming a paste �Gel is formed when cooled �Often used to thicken foods �Can be modified when combined with sugar or acid and used in puddings

Gel Strength � Very important ◦ Starches used in products like �Canned soup/stews ◦ Important because of exposure to heat for long periods of time during canning etc. �Maintain a smooth but thick texture ◦ Generally – amylase rich structures will form a stronger gel �Pile of branches = amylopectin �Pile of cut logs = amylase

Starch… � Can be treated with acids or enzymes ◦ Hydrolysis of some bonds ◦ Results in low viscosity �For some sauces, toppings, and gravy � Can be treated with oxidizing agents such as sodium hypochlorate ◦ Results in reduced viscosity and paste clarity ◦ Used as emulsion thickeners and stabilizers in dressings and spreads

Glycogen � Animal starch � Produced in liver from glucose � Stored in liver and muscles ◦ Available for immediate use as energy ◦ Can only store limited amount � Excess carbohydrate intake = excess glycogen production = excess carbohydrate converted to fat and stored in the body as such

Glycogen… � Body maintains an equilibrium between glucose (energy-producing sugar) and glycogen (can quickly be converted to glucose) � Nutritional minimal � Animal value of glycogen in foods is foods not considered a good source of carbohydrate

Polydextrose � Used as food additive for texture � Not readily digested � Contains 1 calorie per gram � Used as “bulking agent” in low-calorie products to ◦ Replace sugar ◦ Bind water � Add to textural attributes without greatly increasing calories

Fibers � Includes the nondigestible carbohydrates � Water soluble ◦ Cereal brans ◦ Pectin ◦ Lower serum cholesterol levels �Binding with bile acids and causing removal of cholesterol in feces � Water insoluble ◦ Wheat products ◦ Wheat bran ◦ Thought to reduce colon cancer �Increases bulk and dilutes effect of secondary bile acids

Fibers… � Cellulose ◦ Makes up most of the structural material in plants ◦ Main component of many industrially important substances �Wood �Paper �Fibers (cotton) � In nature ◦ Fibers that are very high mechanical strength ◦ Insoluble in water � Pectin ◦ Water soluble fiber ◦ Intercellular spaces of plant tissue

Fibers… � Gums ◦ Have ability to give highly viscous solutions at relatively low concentrations ◦ Used for �Gelling �Stabilizing �Suspending ◦ Used in �Candies �Fruit sauces �Syrups �Toppings �Spreads �Baked goods �Salad dressings �Beverages

Lipids

Lipids… �Definition ◦ The heterogeneous group of substances, associated with living systems, which have the common property of insolubility in water but solubility in nonpolar solvents such as hydrocarbons or alcohols.

Lipids… � Types ◦ ◦ ◦ of lipids Fats Oils Waxes Phospholipids Sphingolipids Sterols � Fats and oils ◦ Contain 9 calories per gram ◦ Proteins and carbohydrates contain 4 calories per gram

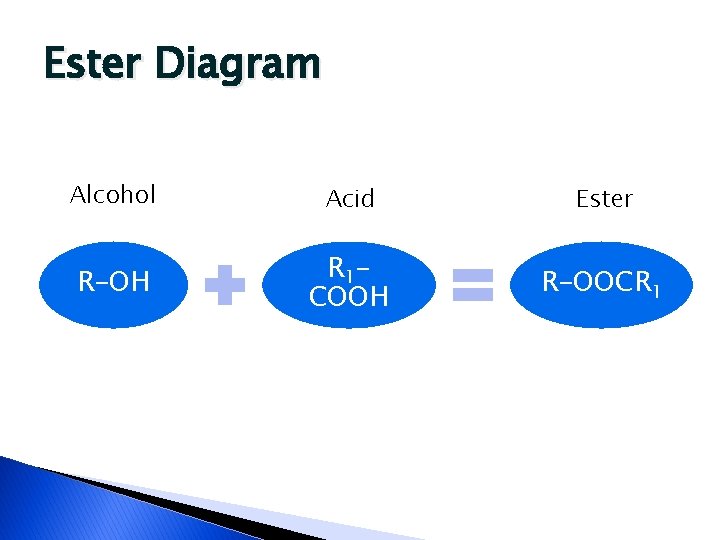
Fats and Oils � Glycerol esters of fatty acids ◦ Ester = alcohol and acid join and produce a water molecule. The result is an ester � Contain ◦ Carbon ◦ Hydrogen ◦ Oxygen �Proportion of oxygen is much less than in carbohydrates

Ester Diagram Alcohol Acid Ester R-OH R 1 COOH R-OOCR 1

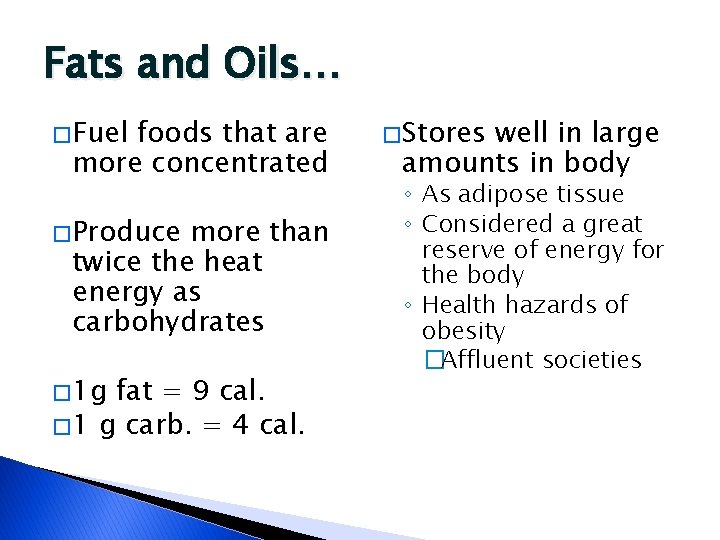
Fats and Oils… � Fuel foods that are more concentrated � Produce more than twice the heat energy as carbohydrates � 1 g fat = 9 cal. � 1 g carb. = 4 cal. � Stores well in large amounts in body ◦ As adipose tissue ◦ Considered a great reserve of energy for the body ◦ Health hazards of obesity �Affluent societies

Fats and Oils � Occur in foods as ◦ Lipid materials �Solid at room temperature ◦ Oils �Liquid at room temperature � Variation of lipids vs. oils depends on fatty acid components

Fatty Acids � Open chain carboxylic acids � Natural fatty acids most commonly found in fats and oils almost always contains an even number of carbon atoms ◦ Ranging from 4 – 28 � Fatty acid chain may be ◦ Saturated �Have the maximum number of hydrogen atoms attached ◦ Unsaturated �Lacking hydrogen atoms at certain points

Saturated vs. Unsaturated Fatty Acids

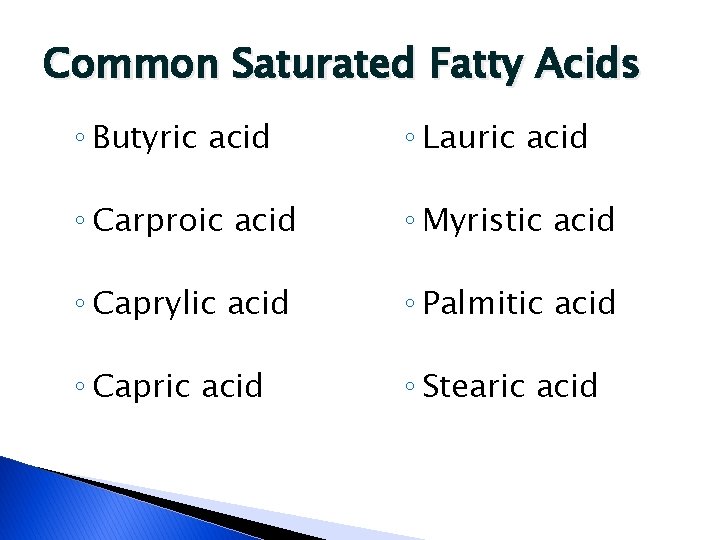
Common Saturated Fatty Acids ◦ Butyric acid ◦ Lauric acid ◦ Carproic acid ◦ Myristic acid ◦ Caprylic acid ◦ Palmitic acid ◦ Capric acid ◦ Stearic acid

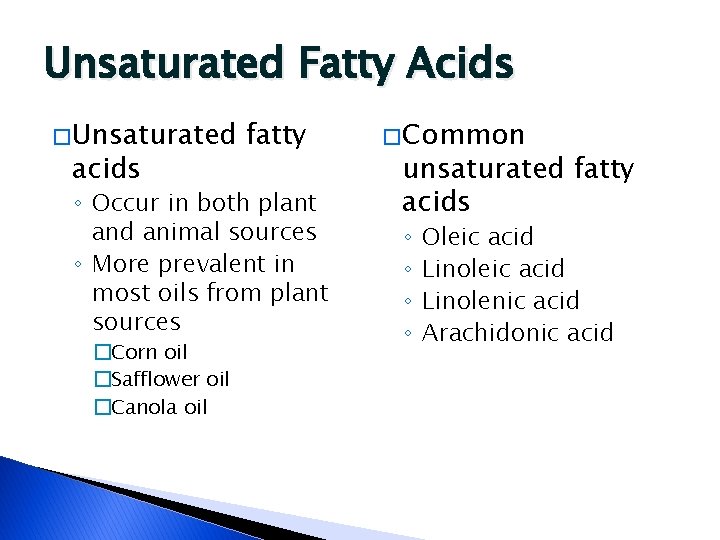
Unsaturated Fatty Acids � Unsaturated acids fatty ◦ Occur in both plant and animal sources ◦ More prevalent in most oils from plant sources �Corn oil �Safflower oil �Canola oil � Common unsaturated fatty acids ◦ ◦ Oleic acid Linolenic acid Arachidonic acid

Fatty Acids… � Monounsaturated �One double bond (one H missing) � Polyunsaturated �Two or more double bonds � Essential fatty acids ◦ Must be supplied by the diet �Linoleic �Alpha-linolenic

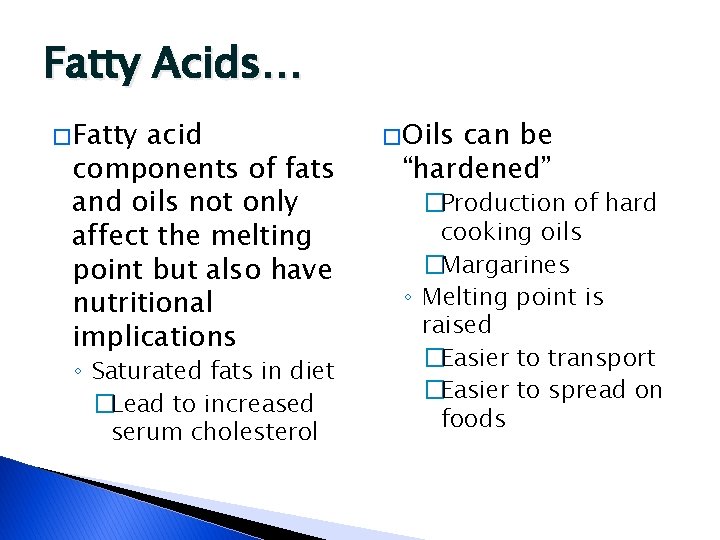
Fatty Acids… � Fatty acid components of fats and oils not only affect the melting point but also have nutritional implications ◦ Saturated fats in diet �Lead to increased serum cholesterol � Oils can be “hardened” �Production of hard cooking oils �Margarines ◦ Melting point is raised �Easier to transport �Easier to spread on foods

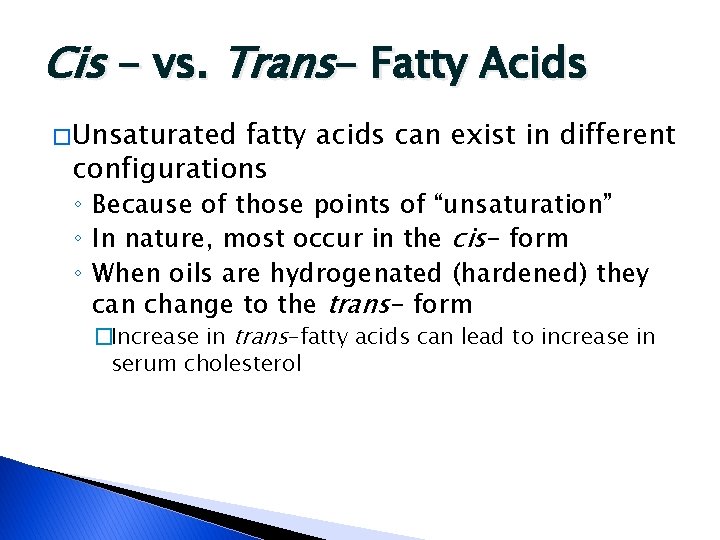
Cis - vs. Trans- Fatty Acids � Unsaturated fatty acids can exist in different configurations ◦ Because of those points of “unsaturation” ◦ In nature, most occur in the cis- form ◦ When oils are hydrogenated (hardened) they can change to the trans- form �Increase in trans-fatty acids can lead to increase in serum cholesterol

Two Paths of Fats Once Ingested � Hydrolyzed to glycerine and fatty acids ◦ By lipase enzymes ◦ In small intestine ◦ Eventually oxidized at the cellular level for energy, carbon dioxide, and water � Emulsified (particles dispersed within) ◦ Carried to adipose tissue ◦ Stored

Cis – and Trans- Fatty Acids

Phospholipids, Waxes, Sphingolipids, and Sterols

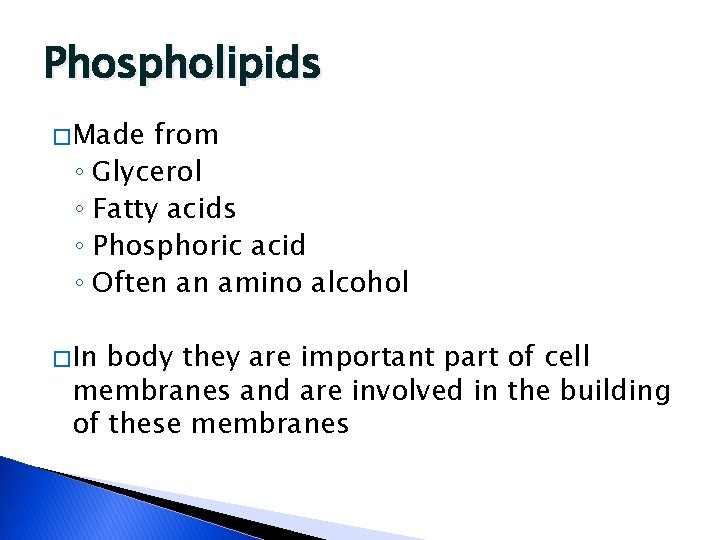
Phospholipids � Made from ◦ Glycerol ◦ Fatty acids ◦ Phosphoric acid ◦ Often an amino alcohol � In body they are important part of cell membranes and are involved in the building of these membranes

Phospholipids… � Very good emulsifiers in food industry ◦ Due to their polarity at one end and nonpolarity at the other ◦ Used in �Chocolate �Salad dressing �Mayonnaise �Component of egg yolks ◦ Help hold stuff together (polarity)

Waxes � Made from fatty acids and monohydric alcohols � In body ◦ Serve as protective, water repellent coatings on tissue surfaces ◦ Functions �Prevent over evaporation of moisture �Prevent invasion of water from the environment into tissues � In food industry ◦ Used in some packing ◦ As ingredients in some candies and confections �For texture or appearance ◦ Not digestible

SPHINGOLIPIDS and STEROLS � SPHINGOLIPIDS ◦ Sphingomyelin is important constituent of nerve and brain tissues � STEROLS ◦ Sterol cholesterols �Involved in �Composition of bile salts �Play role in emulsification of fats in the intestines �In other words, digestion of fats ◦ Ergosterol �May be converted to vitamin D in the body under the influence of sunlight or UV light

Vitamins

Vitamins � Many vitamins are required in small amounts by the body � 2 types ◦ Fat-soluble ◦ Water-soluble

Fat-Soluble Vitamins � Vitamin A � Vitamin D � Vitamin E � Vitamin K

Vitamin A � Found only in animals ◦ Retinol � Plants contain carotene ◦ Vitamin A can be produced in body ◦ Beta-carotene � Formed in body from yellow pigments (containing carotene) ◦ From many fruits and veggies ◦ Especially carrots ◦ Also found in fats and oils �Especially in liver oils of many saltwater fish

Vitamin A… � Required for vision and resistance to infection � Epithelial cells ◦ Cells in the lining of body cavities and in the skin and glands ◦ Require vitamin A � Deficiency may cause ◦ Impairment in bone formation ◦ Impairment of night vision ◦ Malfunction of epithelial tissues ◦ Defects n teeth enamel

Vitamin D � Necessary for normal tooth and bone formation � Fish � Deficiencies � Body ◦ Rickets �Deformities of bone �Bow-legs �Spine curvature �Tooth defects oils (especially fish liver oils) = great source can make vitamin D by converting sterols (cholesterol) with use of UV light

Vitamin E � Four different forms (tocopherols) ◦ Alpha- �Most common �Antioxidant �Unsaturated fatty acids ◦ Beta◦ Gamma◦ Delta� Enhance absorption of iron

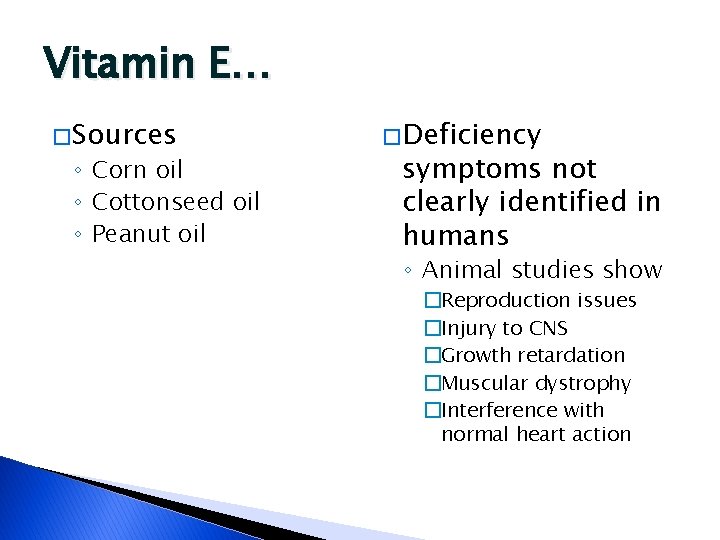
Vitamin E… � Sources ◦ Corn oil ◦ Cottonseed oil ◦ Peanut oil � Deficiency symptoms not clearly identified in humans ◦ Animal studies show �Reproduction issues �Injury to CNS �Growth retardation �Muscular dystrophy �Interference with normal heart action

Vitamin K � Essential in production of prothrombin ◦ Compound involved in the clotting of blood � Sources ◦ ◦ ◦ Cabbage Spinach Cauliflower Liver Can be synthesized by bacteria in human intestine �Antimicrobial therapies that destroy intestinal bacteria can lead to deficiencies in vitamin K

Vitamin K… � Symptoms of deficiency ◦ Loss of ability to clot blood ◦ Humans typically get adequate amounts of vitamin K in the diet

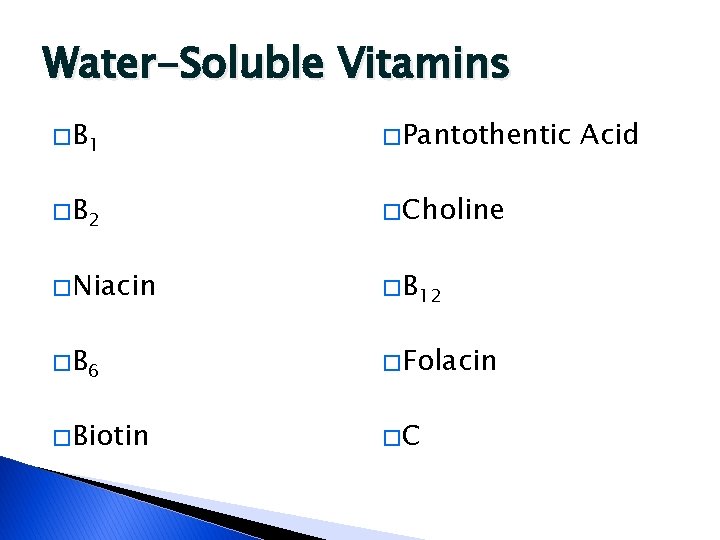
Water-Soluble Vitamins � B 1 � Pantothentic � B 2 � Choline � Niacin � B 12 � B 6 � Folacin � Biotin �C Acid

Vitamin B 1 � Also called thiamin � Involved in bodily oxidations that lead to formation of carbon dioxide � Necessary for: ◦ Nerve function ◦ Appetite ◦ Normal digestion �Growth �Fertility �Lactation

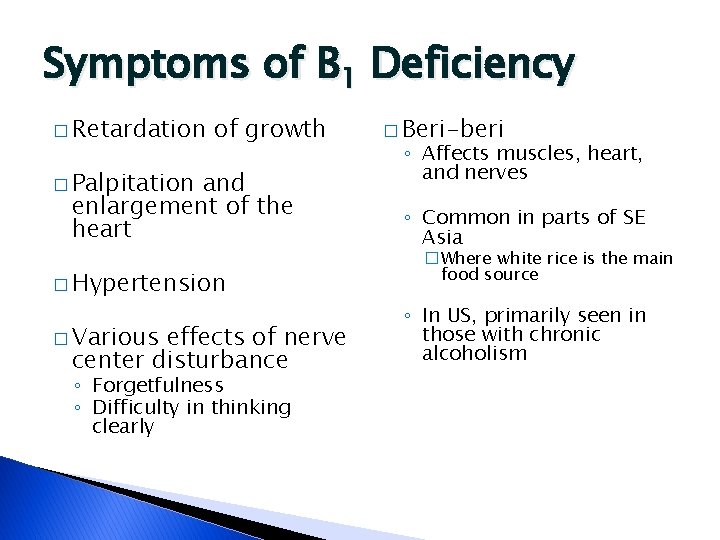
Symptoms of B 1 Deficiency � Retardation of growth � Palpitation and enlargement of the heart � Hypertension � Various effects of nerve center disturbance ◦ Forgetfulness ◦ Difficulty in thinking clearly � Beri-beri ◦ Affects muscles, heart, and nerves ◦ Common in parts of SE Asia �Where white rice is the main food source ◦ In US, primarily seen in those with chronic alcoholism

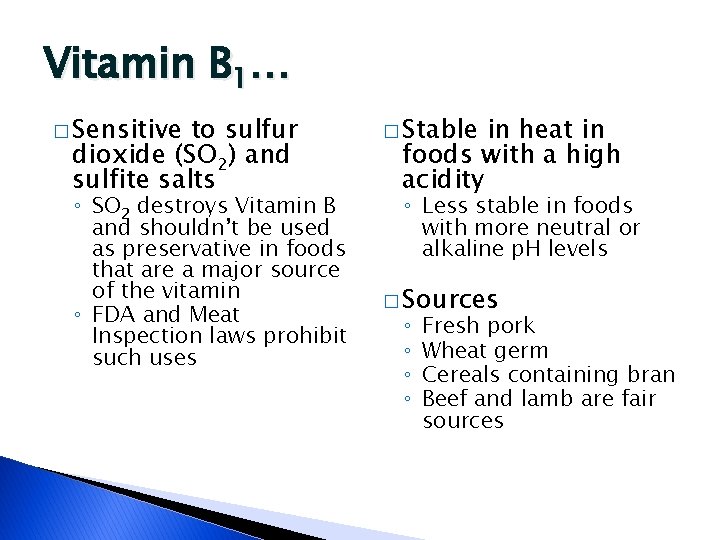
Vitamin B 1… � Sensitive to sulfur dioxide (SO 2) and sulfite salts ◦ SO 2 destroys Vitamin B and shouldn’t be used as preservative in foods that are a major source of the vitamin ◦ FDA and Meat Inspection laws prohibit such uses � Stable in heat in foods with a high acidity ◦ Less stable in foods with more neutral or alkaline p. H levels � Sources ◦ ◦ Fresh pork Wheat germ Cereals containing bran Beef and lamb are fair sources

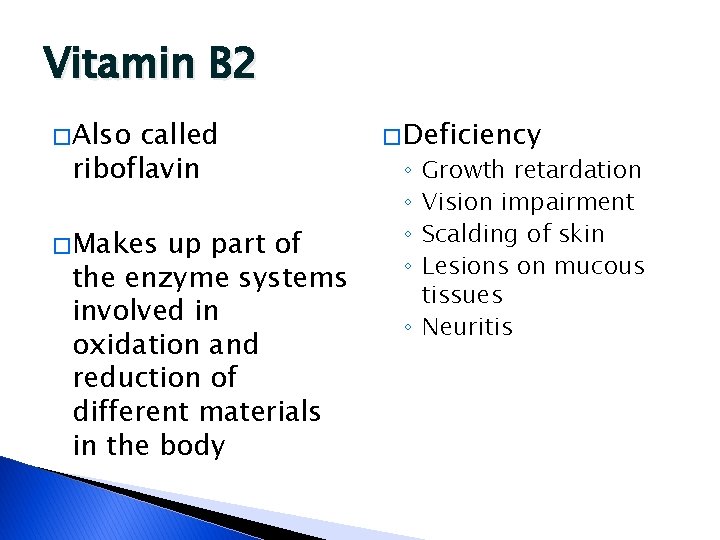
Vitamin B 2 � Also called riboflavin � Makes up part of the enzyme systems involved in oxidation and reduction of different materials in the body � Deficiency Growth retardation Vision impairment Scalding of skin Lesions on mucous tissues ◦ Neuritis ◦ ◦

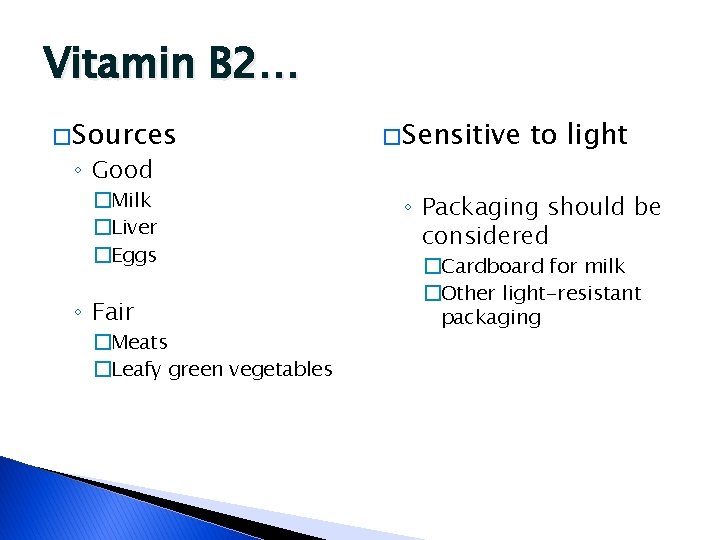
Vitamin B 2… � Sources ◦ Good �Milk �Liver �Eggs ◦ Fair �Meats �Leafy green vegetables � Sensitive to light ◦ Packaging should be considered �Cardboard for milk �Other light-resistant packaging

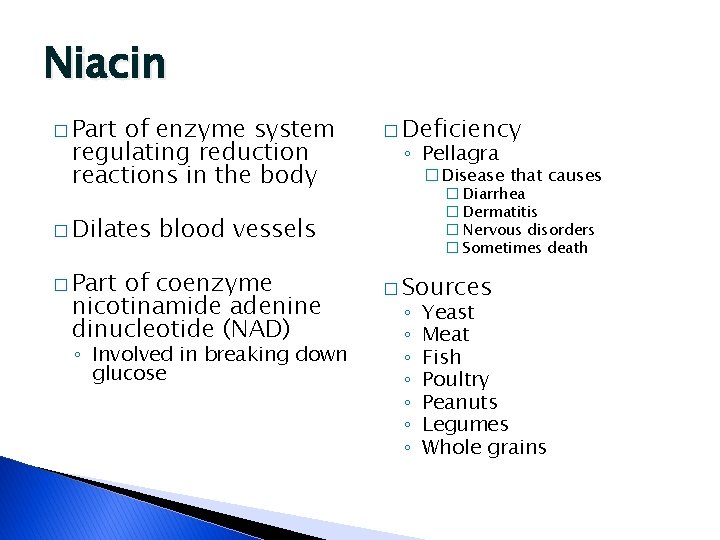
Niacin � Part of enzyme system regulating reduction reactions in the body � Dilates � Deficiency ◦ Pellagra �Disease that causes � Diarrhea � Dermatitis � Nervous disorders � Sometimes death blood vessels � Part of coenzyme nicotinamide adenine dinucleotide (NAD) ◦ Involved in breaking down glucose � Sources ◦ ◦ ◦ ◦ Yeast Meat Fish Poultry Peanuts Legumes Whole grains

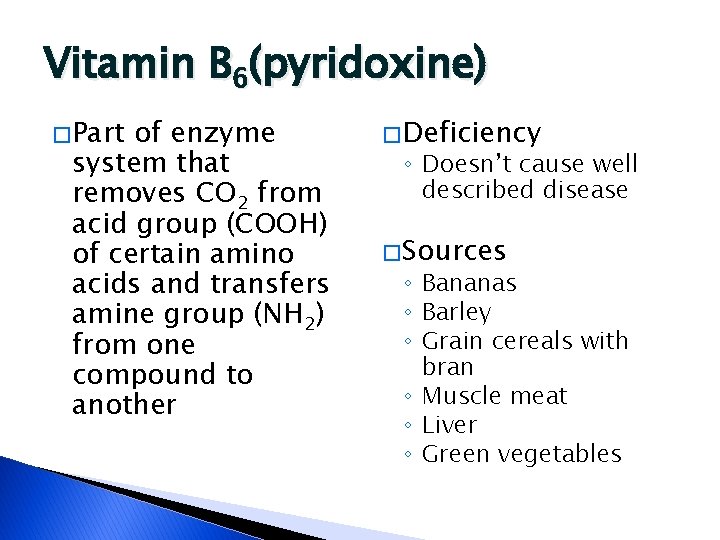
Vitamin B 6(pyridoxine) � Part of enzyme system that removes CO 2 from acid group (COOH) of certain amino acids and transfers amine group (NH 2) from one compound to another � Deficiency ◦ Doesn’t cause well described disease � Sources ◦ Bananas ◦ Barley ◦ Grain cereals with bran ◦ Muscle meat ◦ Liver ◦ Green vegetables

Vitamin B 6… � Has been taken by women taking steroid contraceptives � Has been used to treat PMS ◦ Not recommended without blood tests

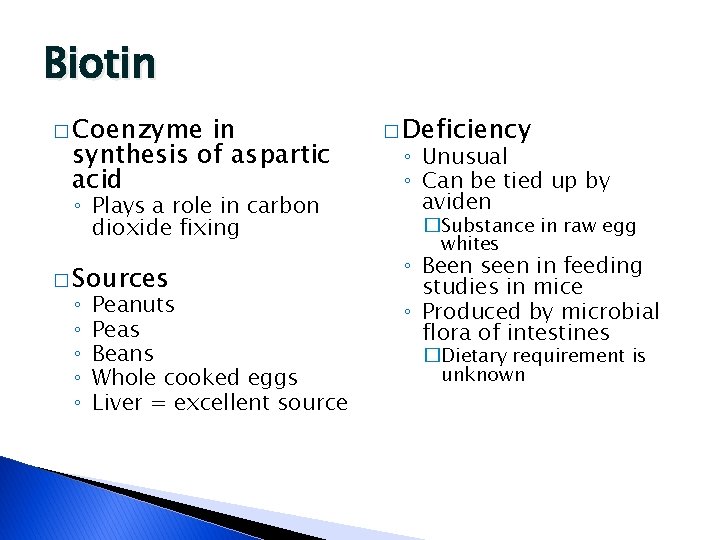
Biotin � Coenzyme in synthesis of aspartic acid ◦ Plays a role in carbon dioxide fixing � Sources ◦ ◦ ◦ Peanuts Peas Beans Whole cooked eggs Liver = excellent source � Deficiency ◦ Unusual ◦ Can be tied up by aviden �Substance in raw egg whites ◦ Been seen in feeding studies in mice ◦ Produced by microbial flora of intestines �Dietary requirement is unknown

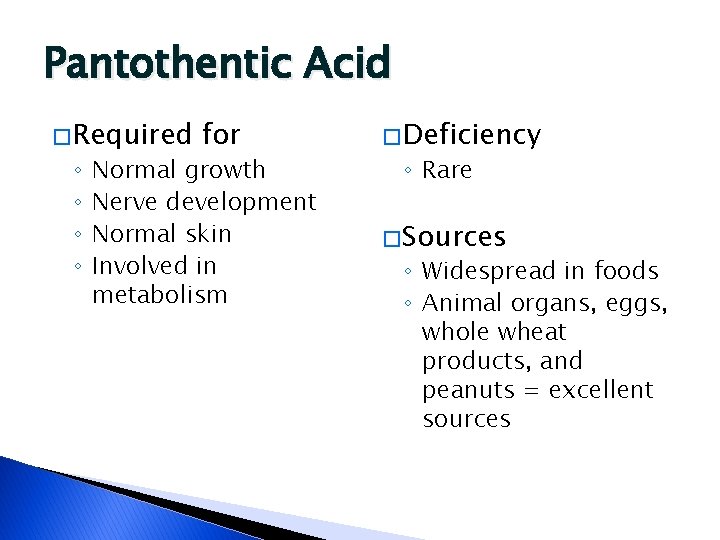
Pantothentic Acid � Required ◦ ◦ for Normal growth Nerve development Normal skin Involved in metabolism � Deficiency ◦ Rare � Sources ◦ Widespread in foods ◦ Animal organs, eggs, whole wheat products, and peanuts = excellent sources

Choline � Sometimes � Typically � Produced listed with B vitamins consumed in adequate amounts by intestinal flora � Component tissues of cell membranes and brain

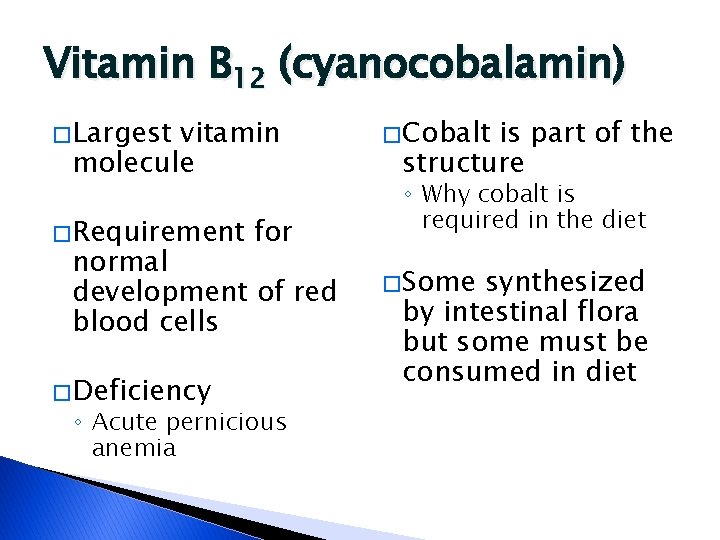
Vitamin B 12 (cyanocobalamin) � Largest vitamin molecule � Requirement for normal development of red blood cells � Deficiency ◦ Acute pernicious anemia � Cobalt is part of the structure ◦ Why cobalt is required in the diet � Some synthesized by intestinal flora but some must be consumed in diet

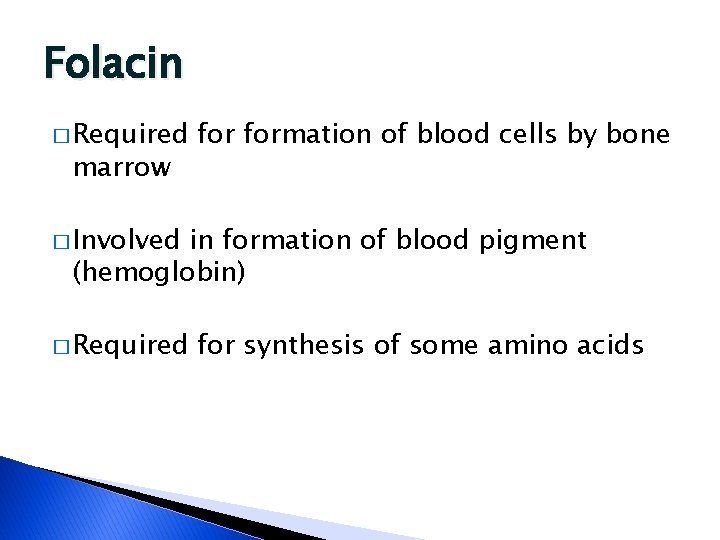
Folacin � Required marrow formation of blood cells by bone � Involved in formation of blood pigment (hemoglobin) � Required for synthesis of some amino acids

Folacin… � Deficiency ◦ Some types of anemia �Pernicious � Requirement is about 1. 25 times greater in pregnant women ◦ May act to prevent some birth defects � Sources ◦ ◦ ◦ Liver Leafy vegetables Legumes Cereal grains Nuts

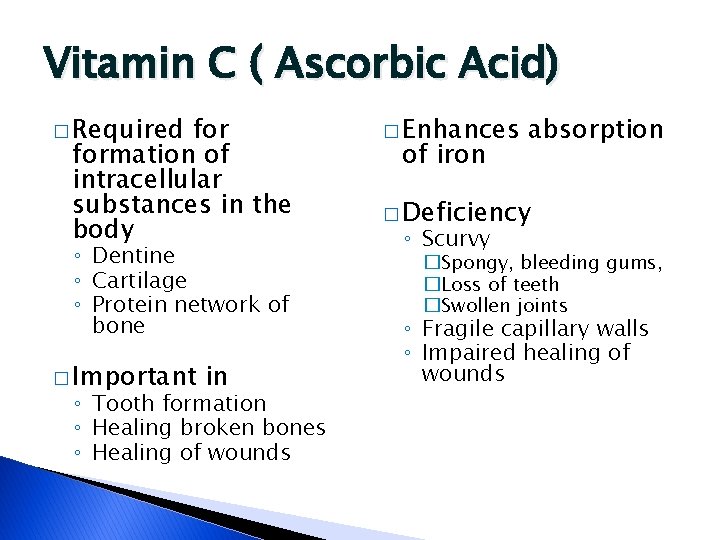
Vitamin C ( Ascorbic Acid) � Required formation of intracellular substances in the body ◦ Dentine ◦ Cartilage ◦ Protein network of bone � Important in ◦ Tooth formation ◦ Healing broken bones ◦ Healing of wounds � Enhances of iron absorption � Deficiency ◦ Scurvy �Spongy, bleeding gums, �Loss of teeth �Swollen joints ◦ Fragile capillary walls ◦ Impaired healing of wounds

Vitamin C… � Sources ◦ Excellent �Orange juice �Tomato juice �Green peppers �Broccoli �Cabbage �Brussels sprouts ◦ Fair �Potatoes �Fruits � Easily destroyed by oxidation and heat ◦ Can be lost in cooking water during processing ◦ Fortification may be necessary �Before and after processing

Minerals

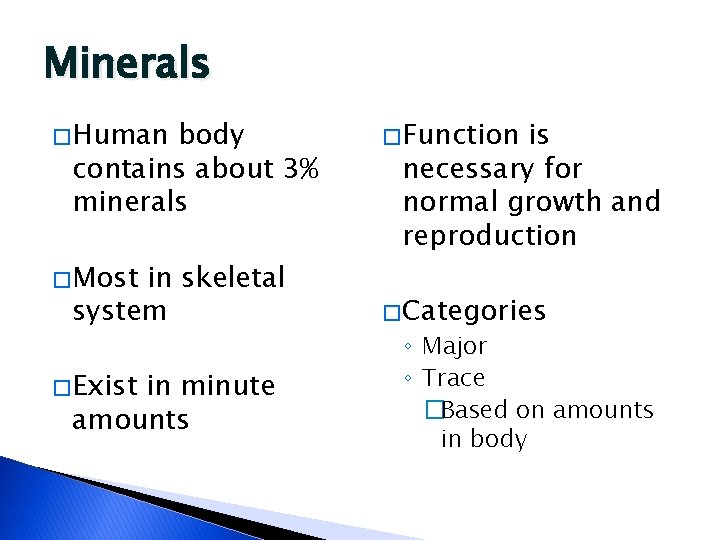
Minerals � Human body contains about 3% minerals � Most in skeletal system � Exist in minute amounts � Function is necessary for normal growth and reproduction � Categories ◦ Major ◦ Trace �Based on amounts in body

Major Minerals � Calcium � Phosphorous � Sodium � Magnesium � Chlorine � Sulfur � Potassium

Trace Minerals � Iron � Vanadium � Flourine � Tin � Iodine � Copper � Cobalt � Zinc � Manganese � Selinium � Silicon � Chromium � Aluminum � Boron � Cadmium

Minearals Major Minerals

Calcium � Required for ◦ Bone and tooth structure ◦ Function of nerves and muscles ◦ Blood clotting mechanism � Deficiencies ◦ Osteoporosis (especially in older women) ◦ Symptoms not apparent until later in life � Other ◦ Essential for calcium absorption ◦ Lactose may also help

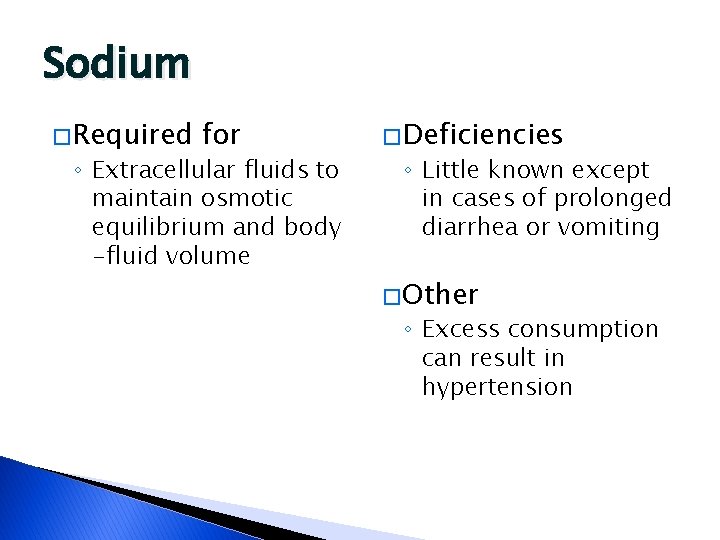
Sodium � Required for ◦ Extracellular fluids to maintain osmotic equilibrium and body -fluid volume � Deficiencies ◦ Little known except in cases of prolonged diarrhea or vomiting � Other ◦ Excess consumption can result in hypertension

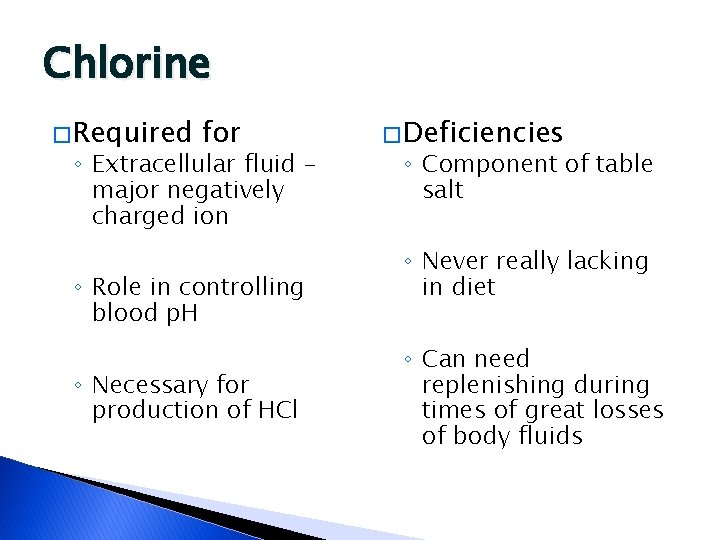
Chlorine � Required for ◦ Extracellular fluid – major negatively charged ion ◦ Role in controlling blood p. H ◦ Necessary for production of HCl � Deficiencies ◦ Component of table salt ◦ Never really lacking in diet ◦ Can need replenishing during times of great losses of body fluids

Potassium � Required for ◦ Present in body cells as chief intracellular cation ◦ Associated with function of muscles and nerves and with metabolism of CHO ◦ Maintaining fluid volume inside cells ◦ Maintaining acid-base balance

Potassium Sources… � Meats � Bananas � Eggs � Fresh � Oranges milk

Potassium… � Other ◦ Cell membranes = very permeable to K, but as leaks out, highly active pump returns it to the cell in exchange for sodium ◦ If as little as 6% of K contained in the cells escaped into the blood, heart would stop

Phosphorous is Required For: � 85% of P found in body is combined with Ca � Part of bodies major buffers (phosphoric acid and its salts) � Part of DNA and RNA � Some � Key lipids contain role in energy transfer

Phosphorous Sources: � Meat � Fish � Eggs � Nuts

Magnesium � Required for ◦ Minor component of bone ◦ Present in soft tissue cells where it is involved with protein synthesis � Deficiencies ◦ Unusual � Sources ◦ ◦ ◦ Veggies Cereal flours Beans Nuts

Sulfur � Required for ◦ Present in virtually all proteins � Deficiencies ◦ Associated with protein deficiencies

Minerals Trace Minerals

Iron – Required for: � May be due to poor absorption of sources found in plants � Animal sources and those found in fortified foods are more readily absorbed � Essential in hemoglobin and myoglobin

Iron Deficiencies � Most common deficient trace mineral in industrialized world � Cause anemia � Amount needed is related to growth rate and blood loss � Menstruating precautions women should take special

Iron… � Sources ◦ ◦ ◦ Liver Meat Eggs Oatmeal Wheat flour � Other ◦ Toxicity is rare but can happen with supplement tablets ◦ 6 -12 tablets can be fatal if ingested by small child ◦ Vitamins E and C aid in absorption

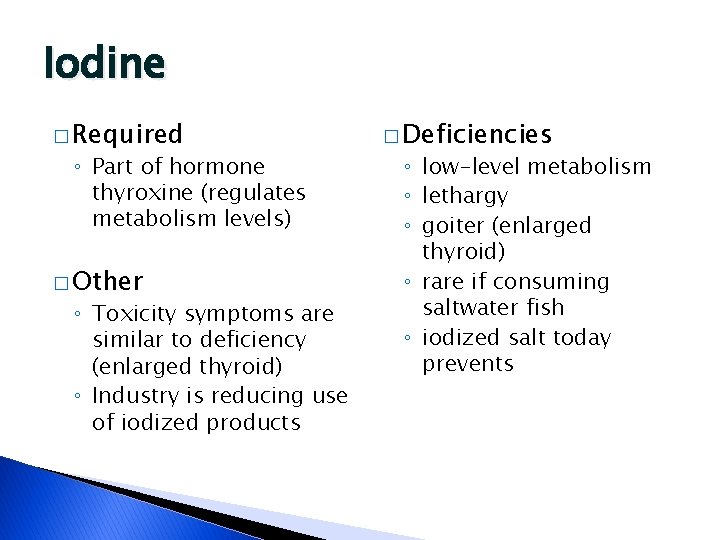
Iodine � Required ◦ Part of hormone thyroxine (regulates metabolism levels) � Other ◦ Toxicity symptoms are similar to deficiency (enlarged thyroid) ◦ Industry is reducing use of iodized products � Deficiencies ◦ low-level metabolism ◦ lethargy ◦ goiter (enlarged thyroid) ◦ rare if consuming saltwater fish ◦ iodized salt today prevents

Flourine � Required ◦ Helps prevent tooth decay � Sources ◦ Drinking water ◦ Fish � Other ◦ Too much fluorine via supplements can cause flourosis (mottling of tooth enamel)

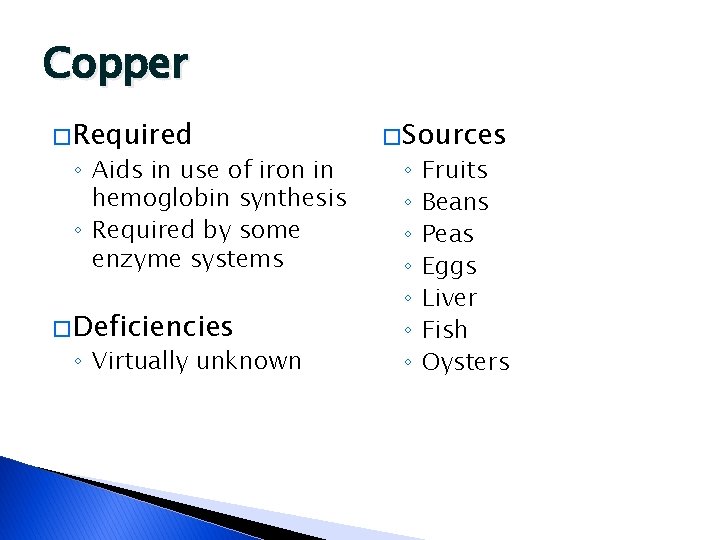
Copper � Required ◦ Aids in use of iron in hemoglobin synthesis ◦ Required by some enzyme systems � Deficiencies ◦ Virtually unknown � Sources ◦ ◦ ◦ ◦ Fruits Beans Peas Eggs Liver Fish Oysters

Copper…Other Info � Toxic in high concentrations � Happens when using copper utensils for storage or distribution of acidic foods (lining of tubs distributing lemonade)

Cobalt � Required ◦ Component of Vitamin B 12 ◦ Only place known in the body � Deficiencies ◦ Sufficient amounts present in foods ◦ Can be absorbed from some cooking utensils

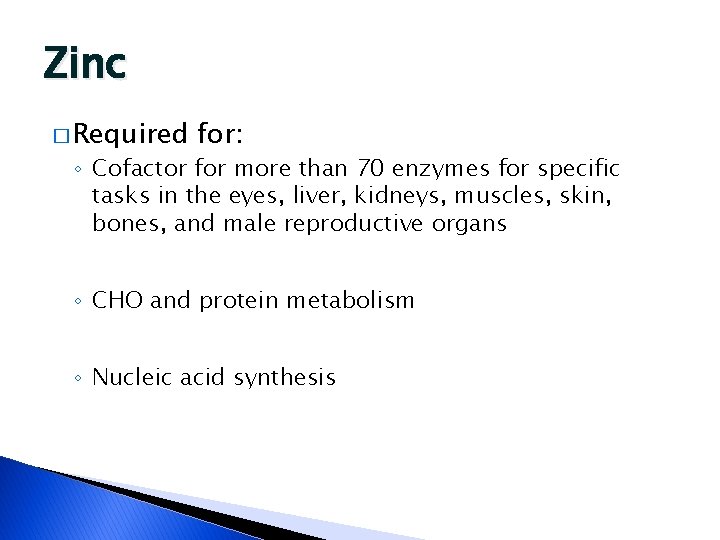
Zinc � Required for: ◦ Cofactor for more than 70 enzymes for specific tasks in the eyes, liver, kidneys, muscles, skin, bones, and male reproductive organs ◦ CHO and protein metabolism ◦ Nucleic acid synthesis

Zinc… � Deficiencies ◦ ◦ Rare Dwarfism Gonadal atrophy Possible damage to immune system � Sources ◦ Shellfish ◦ Meat ◦ Liver

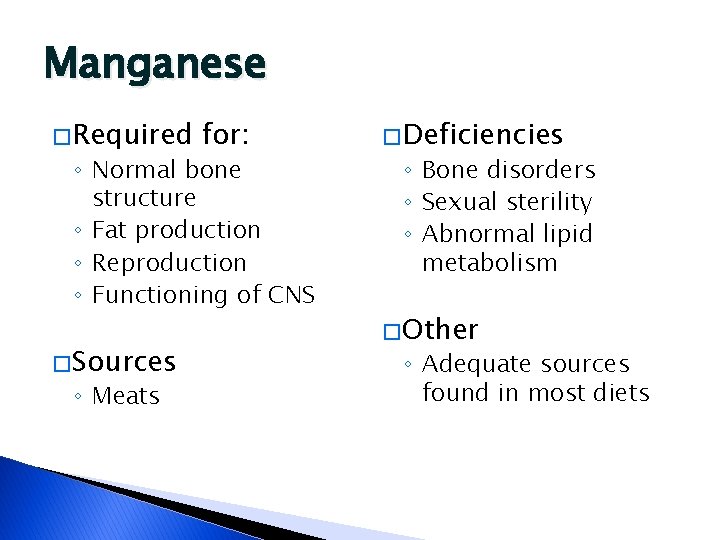
Manganese � Required for: ◦ Normal bone structure ◦ Fat production ◦ Reproduction ◦ Functioning of CNS � Sources ◦ Meats � Deficiencies ◦ Bone disorders ◦ Sexual sterility ◦ Abnormal lipid metabolism � Other ◦ Adequate sources found in most diets

Selenium � Required for: ◦ Antioxidant with Vitamin E � Sources ◦ Meat ◦ Seafood ◦ Grains � Deficiencies ◦ Anemia ◦ Muscle pain ◦ Sometimes heartfailure

Silicon � Sources ◦ Unpolished rice and grains � Deficiencies ◦ Diseases related to connective tissue

Tin � Required for: ◦ Growth rates ◦ Essential to structure of protein � Deficiencies ◦ None noted � Other ◦ Present in many foods

Chromium � Required for: ◦ Physiological role related to glucose metabolism � Sources ◦ Whole, unprocessed foods � Other ◦ Content decreases with age ◦ Linked to adult-onset diabetes

Aluminum, Boron, and Cadmium � Roles are unknown � Deficiencies unknown � Affected parts of brains of those with Alzheimer’s have found to have excess amounts of Aluminum � Still not good to store food long-term in aluminum containers

Natural Toxicants

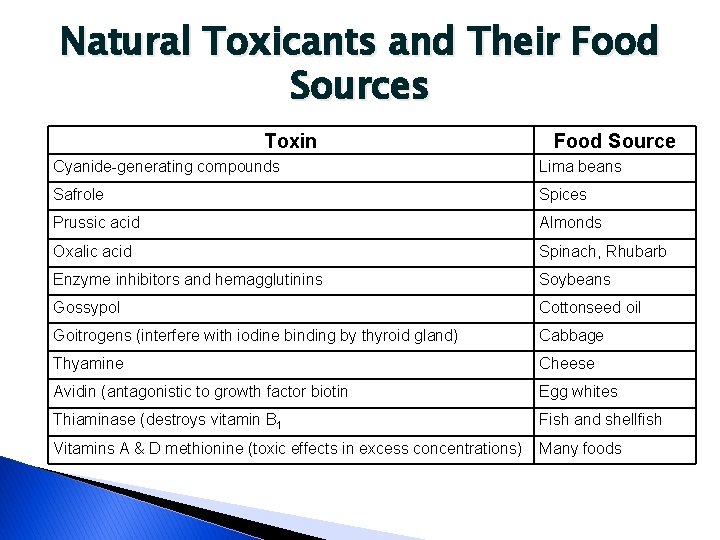
Natural Toxicants � Some plants can produce compounds that serve as protectors or help ensure reproduction ◦ May attract pollinating insects, repel animals or insects that may eat them ◦ Can be toxic to humans ◦ Some mushrooms produce specific nitrogencontainng bases or alkaloids that cause severe physiological issues ◦ Heavy metals such as lead, mercury and arseninc �Found in soils and water

Natural Toxicants and Their Food Sources Toxin Food Source Cyanide-generating compounds Lima beans Safrole Spices Prussic acid Almonds Oxalic acid Spinach, Rhubarb Enzyme inhibitors and hemagglutinins Soybeans Gossypol Cottonseed oil Goitrogens (interfere with iodine binding by thyroid gland) Cabbage Thyamine Cheese Avidin (antagonistic to growth factor biotin Egg whites Thiaminase (destroys vitamin B 1 Fish and shellfish Vitamins A & D methionine (toxic effects in excess concentrations) Many foods
 Illuminate aqa food
Illuminate aqa food Lesson 4 nutrition labels and food safety
Lesson 4 nutrition labels and food safety Food and nutrition unit 4
Food and nutrition unit 4 Section 38-1 food and nutrition answer key
Section 38-1 food and nutrition answer key Rda food label
Rda food label 38-1 food and nutrition
38-1 food and nutrition Chapter 10 nutrition for health lesson 1 answer key
Chapter 10 nutrition for health lesson 1 answer key Chapter 10 lesson 4 nutrition labels and food safety
Chapter 10 lesson 4 nutrition labels and food safety Usda food and nutrition service
Usda food and nutrition service Food and nutrition unit 5
Food and nutrition unit 5 Disadvantages of labour saving devices
Disadvantages of labour saving devices Define nutrition security
Define nutrition security Chapter 38 digestive and excretory systems
Chapter 38 digestive and excretory systems Nea 1 food preparation and nutrition example
Nea 1 food preparation and nutrition example Food and nutrition grade 11
Food and nutrition grade 11 Three sink method
Three sink method Chapter 8 food and nutrition
Chapter 8 food and nutrition Food and nutrition expo
Food and nutrition expo Extreme elite nutrition dog food
Extreme elite nutrition dog food Dri vs rda
Dri vs rda Nutrition crew
Nutrition crew Food nutrition
Food nutrition Baby food buying guide
Baby food buying guide Unit 2 food food food
Unit 2 food food food Eltonian pyramid
Eltonian pyramid Healthy food composition
Healthy food composition Food composition analysis
Food composition analysis Pros and cons of artificial nutrition and hydration
Pros and cons of artificial nutrition and hydration Discretionary calories
Discretionary calories Temperature regulation
Temperature regulation Nutrition for foodservice and culinary professionals
Nutrition for foodservice and culinary professionals Kelsey carbonetta
Kelsey carbonetta Objectives of nutrition and dietetics
Objectives of nutrition and dietetics Chapter 7 nutrition and your fitness
Chapter 7 nutrition and your fitness A saturated fatty acid holds all the hydrogen atoms it can.
A saturated fatty acid holds all the hydrogen atoms it can. Unit 3 nutrition lesson 6 anorexia nervosa and bulimia
Unit 3 nutrition lesson 6 anorexia nervosa and bulimia Chapter 4 nutrition and your personal fitness
Chapter 4 nutrition and your personal fitness Chapter 11 nutrition and diets
Chapter 11 nutrition and diets Chapter 11 nutrition and diets key terms
Chapter 11 nutrition and diets key terms Nutrition and hydration chapter 15
Nutrition and hydration chapter 15 Agriscience unit 26 self evaluation answers
Agriscience unit 26 self evaluation answers Journal on healthy food healthy mind
Journal on healthy food healthy mind Chapter 6 microbial nutrition and growth
Chapter 6 microbial nutrition and growth Project title for health and nutrition
Project title for health and nutrition Chapter 7 skin structure growth and nutrition
Chapter 7 skin structure growth and nutrition Chapter 6 microbial nutrition and growth
Chapter 6 microbial nutrition and growth Nutrition tools standards and guidelines
Nutrition tools standards and guidelines Chapter 11 nutrition and diet
Chapter 11 nutrition and diet Physical activity and nutrition coordinator
Physical activity and nutrition coordinator Fccla planning process worksheet
Fccla planning process worksheet Drive.google
Drive.google Seven nutrition and fitness
Seven nutrition and fitness Streak plate method
Streak plate method Chapter 55 nutrition and health
Chapter 55 nutrition and health Chapter 15 maternal and fetal nutrition
Chapter 15 maternal and fetal nutrition Nutrition and diet therapy nursing
Nutrition and diet therapy nursing Aims and objectives of nutrition
Aims and objectives of nutrition Energy input and output nutrition
Energy input and output nutrition Chapter 8 nutrition and hydration
Chapter 8 nutrition and hydration Deakin credit for prior learning
Deakin credit for prior learning Unit 26 animal anatomy physiology and nutrition
Unit 26 animal anatomy physiology and nutrition Nutrition for foodservice and culinary professionals
Nutrition for foodservice and culinary professionals Nutrition for foodservice and culinary professionals
Nutrition for foodservice and culinary professionals Nutrition and exercise concepts
Nutrition and exercise concepts Plant nutrition and soil science
Plant nutrition and soil science Lag phase
Lag phase Define optimal nutritional status
Define optimal nutritional status Chapter 14 nutrition and fluid balance
Chapter 14 nutrition and fluid balance Idaho academy of nutrition and dietetics
Idaho academy of nutrition and dietetics Chapter 15 digestion and nutrition
Chapter 15 digestion and nutrition Primary secondary and tertiary consumers
Primary secondary and tertiary consumers Producers food chain
Producers food chain How does the food chain go
How does the food chain go Food chain consumer levels
Food chain consumer levels Difference between food webs and food chains
Difference between food webs and food chains Food chains, food webs and ecological pyramids
Food chains, food webs and ecological pyramids Food webs and energy pyramids
Food webs and energy pyramids Yellowstone food web answer key
Yellowstone food web answer key Forest food chain
Forest food chain Role play on healthy food and junk food
Role play on healthy food and junk food Role play on healthy food and junk food
Role play on healthy food and junk food Deciduous forest energy pyramid
Deciduous forest energy pyramid Livelighter meal plan
Livelighter meal plan Fast food essay introduction
Fast food essay introduction Uml class
Uml class Difference aggregation and composition
Difference aggregation and composition Proportion and scale difference
Proportion and scale difference Uniform substance
Uniform substance What is resolution of force
What is resolution of force Cereal grain structure and composition
Cereal grain structure and composition Ideal properties of inlay wax
Ideal properties of inlay wax Lesson 6 - composition of linear functions
Lesson 6 - composition of linear functions Mouth temperature wax
Mouth temperature wax The study of composition structure and properties
The study of composition structure and properties Chapter 6 managing weight and body composition
Chapter 6 managing weight and body composition Unit 3 nutrition lesson 4 weight control
Unit 3 nutrition lesson 4 weight control Maintaining a healthy body composition and body image
Maintaining a healthy body composition and body image Ap english literature and composition flashcards
Ap english literature and composition flashcards Ap english language and composition exam format
Ap english language and composition exam format Academic writing and composition
Academic writing and composition Elements and principles of composition
Elements and principles of composition Chapter 6 managing weight and body composition
Chapter 6 managing weight and body composition Ap english language and composition midterm exam
Ap english language and composition midterm exam Inspection and acceptance committee
Inspection and acceptance committee Resolution and composition of vectors
Resolution and composition of vectors English comprehension and composition
English comprehension and composition Determiners of comprehension
Determiners of comprehension Draw and label the parts of an egg.
Draw and label the parts of an egg. Lesson 5-1 operations with functions
Lesson 5-1 operations with functions Still light tunic описание на русском
Still light tunic описание на русском Composition of substances and solutions
Composition of substances and solutions Maltese instruments
Maltese instruments Maintaining a healthy body composition and body image
Maintaining a healthy body composition and body image Composition with red, yellow, blue, and black
Composition with red, yellow, blue, and black Piet modriaan
Piet modriaan Cadbury silk
Cadbury silk Is the way you see your body.
Is the way you see your body. Basic camera shot
Basic camera shot Ideas about earth atmosphere
Ideas about earth atmosphere Which gland secretes bile juice
Which gland secretes bile juice What is the difference between essay and composition
What is the difference between essay and composition The area of polygons through composition and decomposition
The area of polygons through composition and decomposition Composition of human milk and cow milk
Composition of human milk and cow milk Properties of solid liquid and gas
Properties of solid liquid and gas Ap language and composition crash course
Ap language and composition crash course Poster composition and layout
Poster composition and layout Maintaining a healthy body composition and body image
Maintaining a healthy body composition and body image I can perform operations on functions
I can perform operations on functions Chapter 6 managing weight and body composition
Chapter 6 managing weight and body composition Nutrition care process
Nutrition care process Tanzania national nutrition survey 2020
Tanzania national nutrition survey 2020 Tanzania national nutrition survey 2020
Tanzania national nutrition survey 2020 Biobeyond unit 8 counting calories
Biobeyond unit 8 counting calories Unicef conceptual framework of nutrition
Unicef conceptual framework of nutrition Understanding nutrition 13th edition rental
Understanding nutrition 13th edition rental 1 tpn
1 tpn Swine nutrition management
Swine nutrition management Specialized nutritional support
Specialized nutritional support Nutrition 411
Nutrition 411 Monaco drink nutrition facts
Monaco drink nutrition facts Community nutrition assessment
Community nutrition assessment