Total Parenteral Nutrition TPN 1 What is Total

















- Slides: 41

Total Parenteral Nutrition (TPN) 1

What is Total Parenteral Nutrition (TPN) Hyperalimentation? Definitions Composition Candidats Calculation Methods of administration Monitoring 2

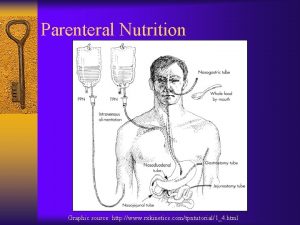
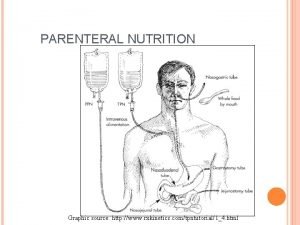
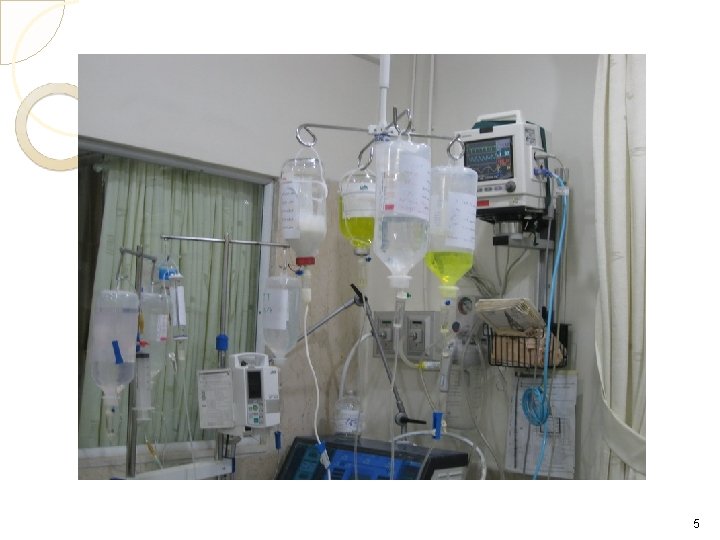
What is Total Parenteral Nutrition (TPN) Hyperalimentation? TPN : is the administration of concentrated glucose & amino acid solutions via a central or large diameter peripheral vein. TPN therapy is necessary when the GI tract cannot be used or is not used to meet the Patient nutritional needs. TPN solutions may contain 20%-60% glucose and 3. 5% to 10% protein (in the form of amino acids) in addition to various amounts of electrolytes, vitamins, minerals, & trace elements. These solutions can be modified, depending on the presence of organ system impairment and/or the specific nutritional needs of the Patient. To provide necessary amounts of fat and the fat soluble vitamins (A, D, E, and K), intralipids are often administered 2 -3 x a week along with TPN (monitor triglyceride levels) TPN is often used in hospital, long term care, and subacute care, but is also frequently used in the home-care setting. 3

What is Total Parenteral Nutrition (TPN) Hyperalimentation? TPN or Hyperalimentation is the IV infusion of a nutritionally, complete formula, including ◦ amino acids (protein/nitrogen) ◦ dextrose (carbohydrate/glucose) ◦ fat emulsions (fatty acids) ◦ vitamins ◦ electrolytes ◦ minerals ◦ trace elements 4

5

(Purpose of TPN) Why TPN? • Promote wound healing • Avoid malnutrition • Examples: severe burns, sepsis, cardiac conditions, trauma, liver failure, GI conditions impairing absorption, anorexia nervosa • Nutrition through the GI tract is best & should be used when the Patient GIT is functional before initiating parental nutrition 6

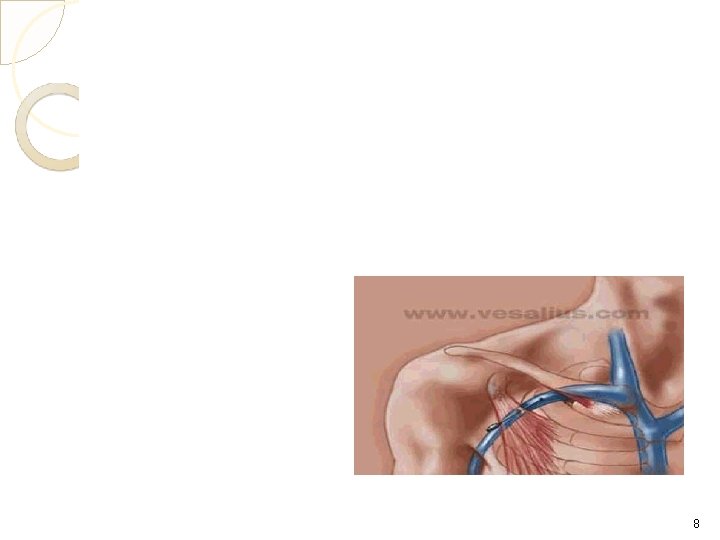
Candidates for TPN Patient unable to take nutrition orally or enterally Who are at risk of malnutrition because of actual or anticipated prolonged inability to ingest, digest, or absorb nutrients Patient who are severely injured 7

8

Expected Outcomes • Expected Outcomes 1. Patient will achieve/maintain ideal body weight 2. Patient will achieve/maintain fluid & electrolyte balance 3. Patient will maintain serum glucose levels at less than 200 mg 4. Patient will remain free of local & systemic infections TPN Expected Outcomes 1. Patient will achieve/maintain ideal body weight Wt gain is usually between 1 & 2 pounds per week Wt is an indicator of how well the Patient is doing nutritionally & determines fluid volume A wt gain greater than 1 pound per day indicates fluid retention 9

Expected Outcomes 2. Patient will achieve/maintain fluid & electrolyte balance • Patient who have ◦ ◦ ◦ electrolyte disturbances, elevated blood glucose level, renal dysfunction, or hepatic dysfunction may require their TPN therapy to be adapted by composition or volume (requires MD order) 3. Patient will maintain serum glucose levels at less than 200 mg – Serum glucose level less than 200 mg will reflect a metabolic tolerance to the concentrated glucose solution in TPN 4. Patient will remain free of local & systemic infections – Ensures that TPN is infusing into the vein rather than into surrounding tissues & there are no signs of an access device infection 10

unexpected Outcomes Unexpected Outcomes & Related to Interventions Exit site infection indicated by : There is redness, swelling, & tenderness around the venous access site. Action: Notify MD. Apply warm compress, Antibiotic therapy may begin systemic infection indicated by : Patient develops fever, malaise, chills, signs of exit site infection may also be present. Action: Notify MD & consult about the need to obtain cultures of exit site or blood. Antibiotic therapy may begin. stops flowing or flows at a rate slower than ordered: Venous access device may have become occluded with fibrin or particulate matter. Action: Report occlusion to MD. If the device is a surgically placed device, a thrombolytic agent may be ordered 11

Unexpected Outcomes & Related Interventions Patient has weight gain bout 1 pound per day. Taut skin turgor & dependent edema may be present. Crackles over lung fields. Symptoms indicate fluid retention rather than restoration of body proteins. Serum glucose is greater than 200 mg. Indicates Patient intolerance to glucose load in TPN solution. May document need for addition of insulin to the TPN, modification of TPN solution, or sliding scale insulin coverage Serum electrolytes are out of normal range. The electrolyte levels in the solution may need to be adjusted 12

TPN Evaluation We must be monitor the following: 1. daily weights 2. I &O & evaluate for fluid overload or dehydration 3. finger stick blood sugar q 6 h or as ordered 4. sign and symptoms of infection, either at infusion site (redness, swelling, tenderness, or drainage) or systemic signs of infection (fever, elevated WBC & malaise) Complications of Central Parenteral Nutrition • Air Embolism • Infection – Localized infection or system infection • Hyperglycemia or Hypoglycemia • Pneumothorax • Arterial Laceration 13

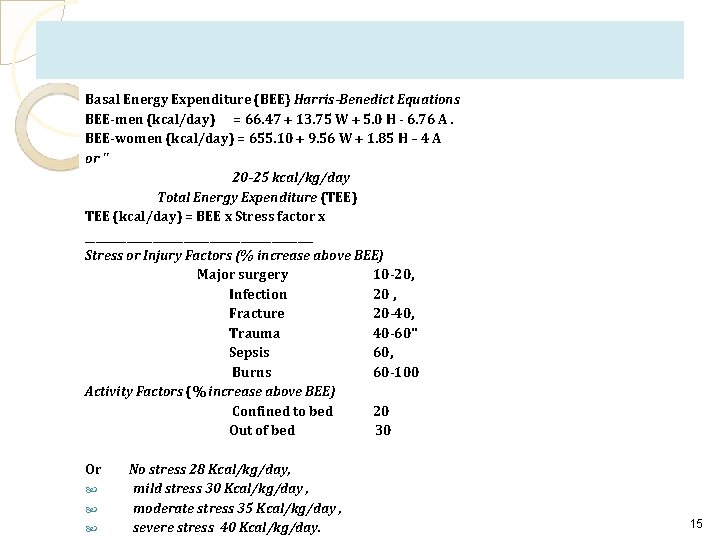
TPN Evaluation Estimation of Energy Expenditure • The traditional method of assessing energy expenditure is to first calculate basal energy expenditure (BEE), which is the amount of energy (kilocalories (kcal) needed to support basic metabolic functions in a state of complete rest. • BEE is most commonly calculated using the Harris-Benedict equations. 2) Alternatively, BEE can be estimated at 20 to 25 kcal/kg per day. • Resting energy expenditure (REE) is the energy expended in the postabsorptive state is approximately 10% greater than BEE. • Determination of BEE or REE does not include additional energy needed for stress or activity. The Harris-Benedict equation can be modified to include stress and physical activity factors, or these variables can be estimated at ◦ ◦ 30 to 35 kcal/kg per day for moderate stress and up to 45 kcal/kg per day for severe stress as in the following table. 14

Basal Energy Expenditure (BEE} Harris-Benedict Equations BEE-men (kcal/day) = 66. 47 + 13. 75 W + 5. 0 H - 6. 76 A. BEE-women (kcal/day) = 655. 10 + 9. 56 W + 1. 85 H – 4 A or " 20 -25 kcal/kg/day Total Energy Expenditure (TEE) TEE (kcal/day) = BEE x Stress factor x ______________________ Stress or Injury Factors (% increase above BEE) Major surgery 10 -20, Infection 20 , Fracture 20 -40, Trauma 40 -60" Sepsis 60, Burns 60 -100 Activity Factors (% increase above BEE) Confined to bed 20 Out of bed 30 Or No stress 28 Kcal/kg/day, mild stress 30 Kcal/kg/day , moderate stress 35 Kcal/kg/day , severe stress 40 Kcal/kg/day. 15

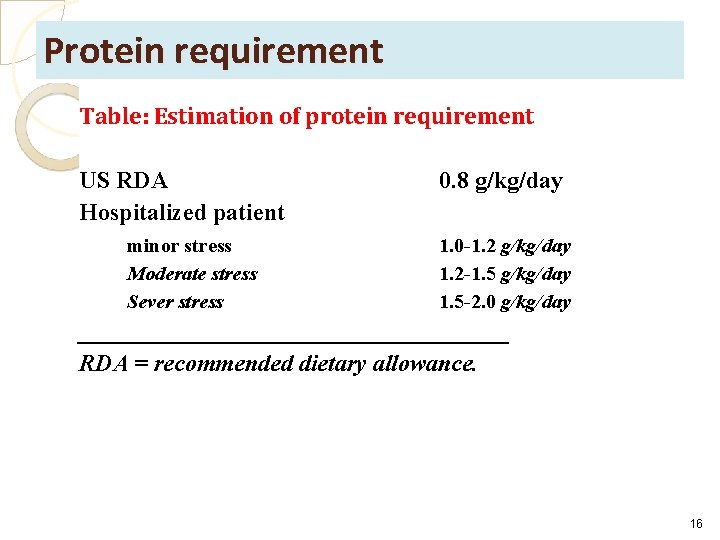
Protein requirement Table: Estimation of protein requirement US RDA Hospitalized patient minor stress Moderate stress Sever stress 0. 8 g/kg/day 1. 0 -1. 2 g/kg/day 1. 2 -1. 5 g/kg/day 1. 5 -2. 0 g/kg/day __________________ RDA = recommended dietary allowance. 16

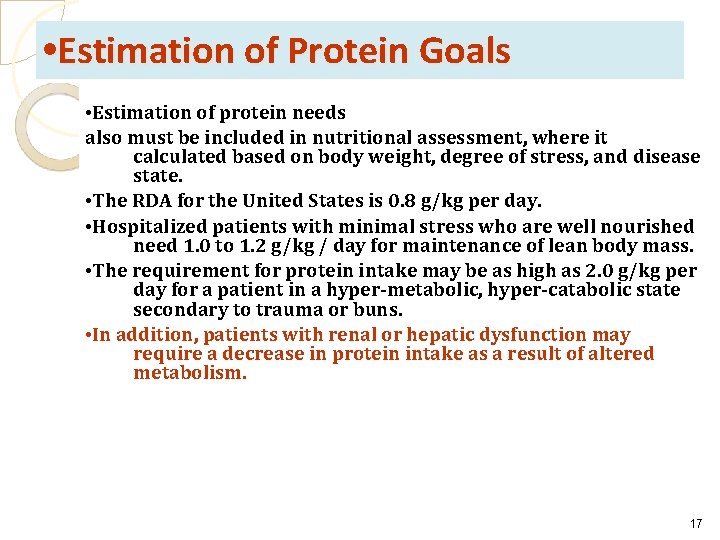
• Estimation of Protein Goals • Estimation of protein needs also must be included in nutritional assessment, where it calculated based on body weight, degree of stress, and disease state. • The RDA for the United States is 0. 8 g/kg per day. • Hospitalized patients with minimal stress who are well nourished need 1. 0 to 1. 2 g/kg / day for maintenance of lean body mass. • The requirement for protein intake may be as high as 2. 0 g/kg per day for a patient in a hyper-metabolic, hyper-catabolic state secondary to trauma or buns. • In addition, patients with renal or hepatic dysfunction may require a decrease in protein intake as a result of altered metabolism. 17

Components of Parenteral Nutrient Formulations Parenteral nutrient formulations are mixtures containing: carbohydrate, protein, lipids, water, electrolytes, vitamins, and trace minerals. These admixtures must be prepared under aseptic conditions. Although parenteral feeding is an important adjuvant therapy for many disease states, errors have occurred in managing this complex therapy resulting in patient harm and death. In 1994 the FDA issued a Safety Alert after two deaths related to errors in compounding parenteral nutrient formulations occurred. guidelines have been developed for parenteral nutrition therapy. 18

• Carbohydrate • Dextrose in water is the most common carbohydrate for IV use. It is available commercially in concentrations ranging from 2. 5 to 70%. These dextrose solutions are mixed with other components of the parenteral nutrient formulation and diluted to various final concentrations. • IV dextrose is monohydrated and provides 3. 4 kcal/g. • Glycerol also is available (as a 3% mixture with 3% amino acids) for administration as a peripheral parenteral nutrient formulation. Glycerol has a caloric density of 4. 3 kcal/g. • Other carbohydrates such as fructose, sorbitol, and invert sugar have been used: ◦ ◦ ◦ used investigationally in parenteral nutrient formulations are associated with adverse effects and are not available commercially. 19

5 g/kg/day or 3. 5 mg/kg/minute (maximum rate: 4 -7 mg/kg/minute) Minimum recommended amount: 400 calories/day or 100 g/day 20%, 50% ( from CV-line) 60 -70% of calorie requirements should be provided with dextrose 30 -40 m. L/kg fluid per day 20

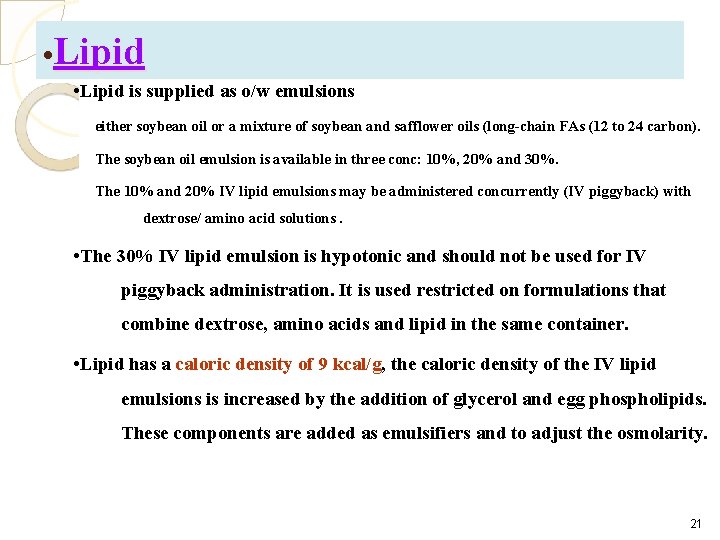
• Lipid is supplied as o/w emulsions either soybean oil or a mixture of soybean and safflower oils (long-chain FAs (12 to 24 carbon). The soybean oil emulsion is available in three conc: 10%, 20% and 30%. The 10% and 20% IV lipid emulsions may be administered concurrently (IV piggyback) with dextrose/ amino acid solutions. • The 30% IV lipid emulsion is hypotonic and should not be used for IV piggyback administration. It is used restricted on formulations that combine dextrose, amino acids and lipid in the same container. • Lipid has a caloric density of 9 kcal/g, the caloric density of the IV lipid emulsions is increased by the addition of glycerol and egg phospholipids. These components are added as emulsifiers and to adjust the osmolarity. 21

Initial: 20% to 40 % of total calories (maximum: 60% of total calories or 2. 5 g/kg/day) ◦ Note: Monitor triglycerides while receiving intralipids. Safe for use in pregnancy I. V. lipids are safe in adults with pancreatitis if triglyceride levels <400 mg/d. L 22

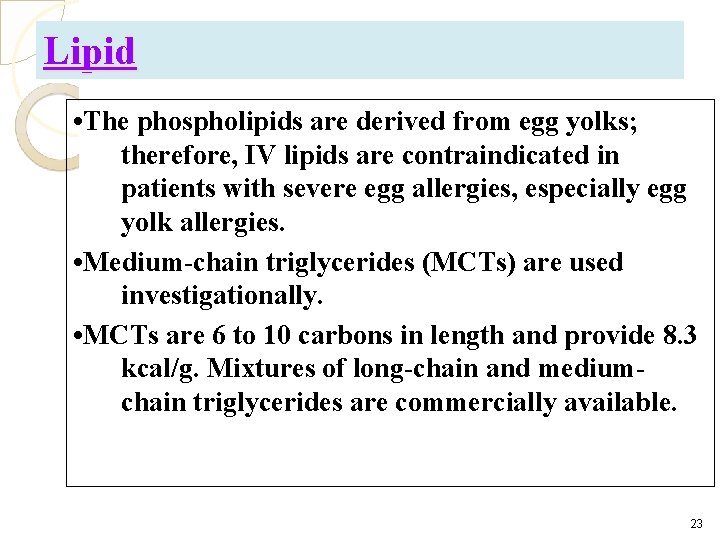
Lipid • The phospholipids are derived from egg yolks; therefore, IV lipids are contraindicated in patients with severe egg allergies, especially egg yolk allergies. • Medium-chain triglycerides (MCTs) are used investigationally. • MCTs are 6 to 10 carbons in length and provide 8. 3 kcal/g. Mixtures of long-chain and mediumchain triglycerides are commercially available. 23

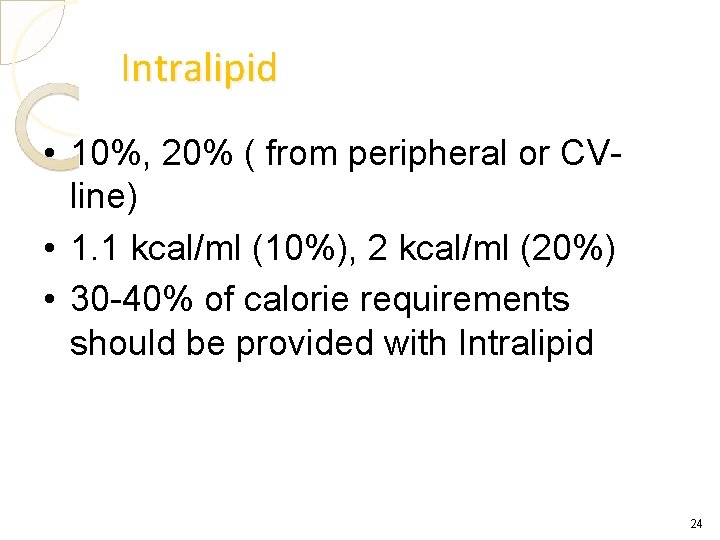
Intralipid • 10%, 20% ( from peripheral or CVline) • 1. 1 kcal/ml (10%), 2 kcal/ml (20%) • 30 -40% of calorie requirements should be provided with Intralipid 24

25

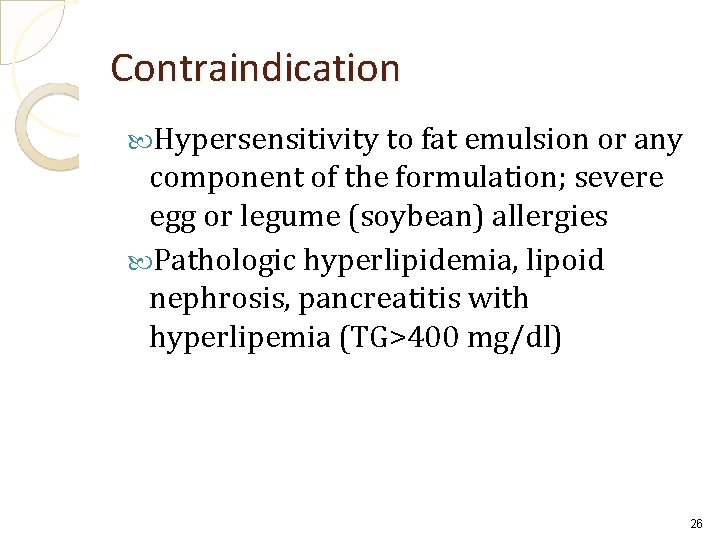
Contraindication Hypersensitivity to fat emulsion or any component of the formulation; severe egg or legume (soybean) allergies Pathologic hyperlipidemia, lipoid nephrosis, pancreatitis with hyperlipemia (TG>400 mg/dl) 26

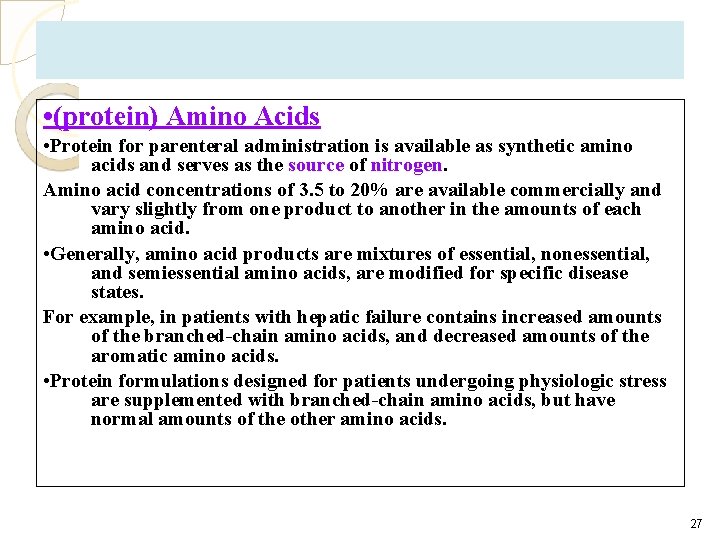
• (protein) Amino Acids • Protein for parenteral administration is available as synthetic amino acids and serves as the source of nitrogen. Amino acid concentrations of 3. 5 to 20% are available commercially and vary slightly from one product to another in the amounts of each amino acid. • Generally, amino acid products are mixtures of essential, nonessential, and semiessential amino acids, are modified for specific disease states. For example, in patients with hepatic failure contains increased amounts of the branched-chain amino acids, and decreased amounts of the aromatic amino acids. • Protein formulations designed for patients undergoing physiologic stress are supplemented with branched-chain amino acids, but have normal amounts of the other amino acids. 27

• Amino Acids • Amino acid products for patients with renal failure either have increased amounts of the essential amino acids or provide only essential amino acids. • Amino acid products designed to meet the needs of neonates are also available. Protein or amino acids have a caloric density of 4 kcal/g. Traditionally, protein calories were not always included in the calculation of energy needs for patients receiving parenteral nutrient formulations. Ideally, protein is used for tissue repair and not oxidized for energy. Today, the conventional wisdom is to include the protein calories in these calculations. 28

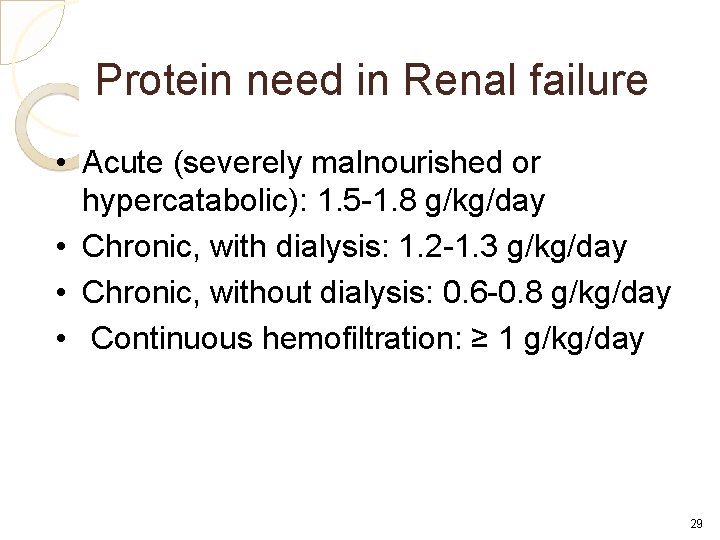
Protein need in Renal failure • Acute (severely malnourished or hypercatabolic): 1. 5 -1. 8 g/kg/day • Chronic, with dialysis: 1. 2 -1. 3 g/kg/day • Chronic, without dialysis: 0. 6 -0. 8 g/kg/day • Continuous hemofiltration: ≥ 1 g/kg/day 29

Protein need in Hepatic failure • Acute management when other treatments have failed: – With encephalopathy: 0. 6 -1 g/kg/day – Without encephalopathy: 1 -1. 5 g/kg/day • Chronic encephalopathy – Use branch chain amino acid enriched diets only if unresponsive to pharmacotherapy • Pregnant women in second or third trimester – Add an additional 10 -14 g/day 30

Aminofusion • 5%, 10% ( from CV-line) • 1 -1. 5 g/kg/day • Should not be used as a calorie source 31

• Micronutrients are the electrolytes, vitamins, and trace minerals needed for metabolism. These nutrients are available from various manufacturers as either single entities or in combinations. For example, the trace element zinc is available commercially as a single trace element product or as a combination product with the other trace elements, copper, chromium, manganese, and selenium. • The following table summarizes available nutrients and their caloric density. 32

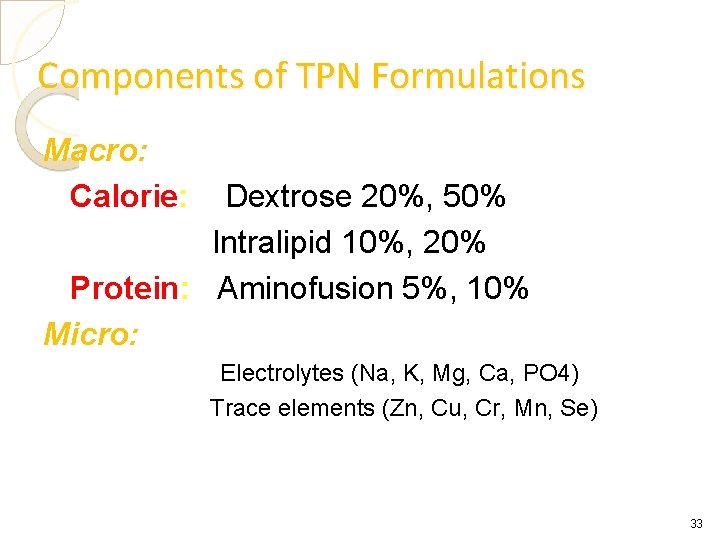
Components of TPN Formulations Macro: Calorie: Dextrose 20%, 50% Intralipid 10%, 20% Protein: Aminofusion 5%, 10% Micro: Electrolytes (Na, K, Mg, Ca, PO 4) Trace elements (Zn, Cu, Cr, Mn, Se) 33

Case D. C a 38 y. o man with a 12 -year history of crohn’s disease is admitted to surgery ward of Imam hospital in Sari for a compliant of increasing abdominal pain, nausea & vomiting for 7 days and no stool output for 5 days. Because of N & V, he has been drinking only liquids during the past weeks. His crohn disease had several exacerbations during the past 2 years and 10 cm of his ileum has been resected 6 month ago. Drugs: Mesalamine 1000 mg qid + prednisolone 10 mg/d. Abdominal x-ray is consisting with bowel obstruction. Exploratory laparotomy was performed and 25 cm of his ileum resected. Bowel sounds are absent. He has a right subclavian CV-line. Considering that his Ht=180 cm, Wt=60 kg (6 month ago: 70 kg) and Age=38 y. o, what is your recommended TPN formula for him? 34

BEE= 66. 47+13. 75× 60+5× 180 -6. 76× 38=1535 kcal/d TEE= 1535× 1. 2 = 2200 kcal/d Intralipid 10%= ? 2200 × 30%= 660 kcal 1 ml ≡ 1. 1 kcal 660 : 1. 1 = 600 ml ( 500 ml) Dext 50%= ? 2200 – 550= 1650 kcal 1 g dextrose ≡ 3. 4 kcal 1650 : 3. 4= 485 g Dext 50 g ≡ 100 ml 485 g ≡ 970 ml (1000 ml) Aminofusion 10 %= ? 1. 5 g/kg/d × 60 kg= 90 g/day 10 g ≡ 100 ml 90 g ≡ 900 ml (1000 ml) 35

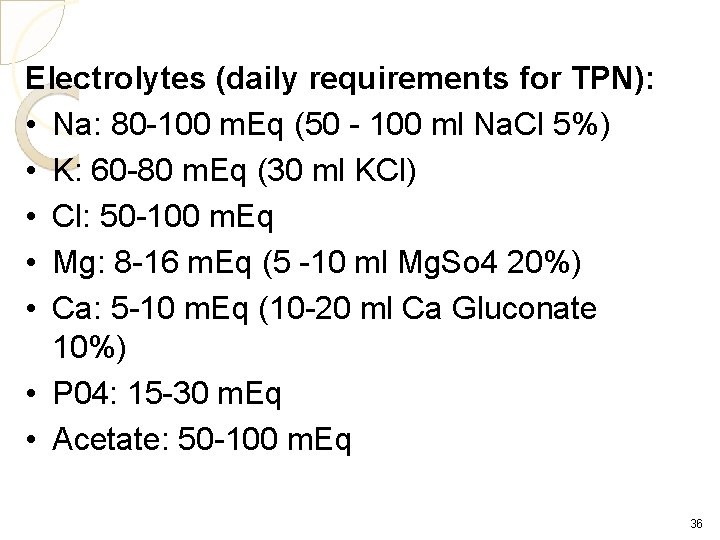
Electrolytes (daily requirements for TPN): • Na: 80 -100 m. Eq (50 - 100 ml Na. Cl 5%) • K: 60 -80 m. Eq (30 ml KCl) • Cl: 50 -100 m. Eq • Mg: 8 -16 m. Eq (5 -10 ml Mg. So 4 20%) • Ca: 5 -10 m. Eq (10 -20 ml Ca Gluconate 10%) • P 04: 15 -30 m. Eq • Acetate: 50 -100 m. Eq 36

37

38

• Vitamins: A, D, E, Water soluble vitamins • Trace Elements: • Zn, Se, Cu, Cr, Mn – ↓ Zn • Delayed ulcer healing, Dermatitis, Alopcia (5α reductase), Diarrhea – ↓ Se: Low activity of SOD & Deiodinase • Amp B Complex + Amp Vit C MV Therapeutic ( Zn, Cu, Mn) 39

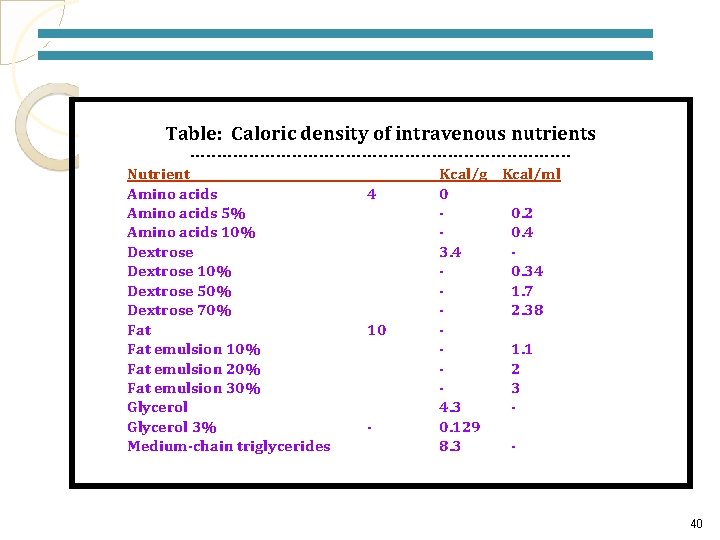
Table: Caloric density of intravenous nutrients -----------------------------------Nutrient Kcal/g Kcal/ml Amino acids 4 0 Amino acids 5% 0. 2 Amino acids 10% 0. 4 Dextrose 3. 4 Dextrose 10% 0. 34 Dextrose 50% 1. 7 Dextrose 70% 2. 38 Fat 10 Fat emulsion 10% 1. 1 Fat emulsion 20% 2 Fat emulsion 30% 3 Glycerol 4. 3 Glycerol 3% 0. 129 Medium-chain triglycerides 8. 3 - 40

Special Considerations • Max infusion rate of dextrose: 0. 5 g/kg/h (to avoid hyperglycemia, glycosuria, fatty liver, hyperosmolar coma) • K should be added to dextrose solutions • Slow starting & slow tapering of Dext 50% • If BS>200, Insulin should be added • some brands of lipids can be mixed with Dext+Aminifusion in the same IV container 41
 Cost of tpn
Cost of tpn Calculating tpn calories
Calculating tpn calories Tpn nutrition
Tpn nutrition Complication of parenteral nutrition
Complication of parenteral nutrition Small bowel obstruction medical nutrition therapy
Small bowel obstruction medical nutrition therapy Ppn vs tpn
Ppn vs tpn Enteral
Enteral Tpn tapering guidelines
Tpn tapering guidelines Tpn complication
Tpn complication Santral parenteral beslenme
Santral parenteral beslenme Enteral beslenme komplikasyonları
Enteral beslenme komplikasyonları Ppn vs tpn
Ppn vs tpn Kanadsk
Kanadsk Piccline spola
Piccline spola Tpn pharmacy
Tpn pharmacy Ppn vs tpn
Ppn vs tpn Ppn vs tpn
Ppn vs tpn Ruptura perinei
Ruptura perinei R 8 314
R 8 314 Ppn vs tpn
Ppn vs tpn Tpn chapter 47
Tpn chapter 47 Tpn avec instillation
Tpn avec instillation Aseptic
Aseptic How to do tpn calculations
How to do tpn calculations Ppn vs tpn
Ppn vs tpn Regiony evropy
Regiony evropy Blood transfusion
Blood transfusion Tpn içeriği
Tpn içeriği Escan tpn
Escan tpn Tpn komplikasyonları
Tpn komplikasyonları Starter tpn
Starter tpn Isaac asimov introduction
Isaac asimov introduction Rxkinetics osmolarity
Rxkinetics osmolarity Rossi tpn
Rossi tpn Nina novkov
Nina novkov Rxkinetics
Rxkinetics Vanilla tpn
Vanilla tpn Tpn component
Tpn component Tpn epilogue
Tpn epilogue Sog en medicina
Sog en medicina Tipos de nutrición parenteral
Tipos de nutrición parenteral Parenteral administration routes
Parenteral administration routes