Proposed Nutrition Facts Update Diana Monaco RDN CDN



































- Slides: 39

Proposed Nutrition Facts Update Diana Monaco, RDN, CDN FDA – Office of Regulatory Affairs Division of Communications, Public Affairs and Editorial Services Branch 716 -846 -6204 Diana. monaco@fda. hhs. gov 1

NLEA • The Nutrition Labeling and Education Act (NLEA) of 1990 provided FDA with the authority to require nutrition labeling. • The objectives of NLEA were to reduce consumer confusion about labels, help consumers make better food choices and encourage product innovation by giving manufacturers an incentive to improve the nutritional value of food. 2

Why Change the Nutrition facts label? • The current label is more than 20 years old and it’s time to make changes based on • new nutrition and public health research, • the most recent dietary recommendations from expert groups, • and input from the public. 3

Does the label really change eating behavior? If it did, we wouldn’t have an obesity epidemic, would we? • Many factors contribute to the obesity epidemic, such as exercise and eating behaviors, which are not addressed by the Nutrition Facts label. • The label is ONE tool to help consumers make informed food choices and maintain healthy dietary practices. • The label can encourage manufacturers to reformulate existing products and produce new ones so that they have a more healthy nutrition profile. 4

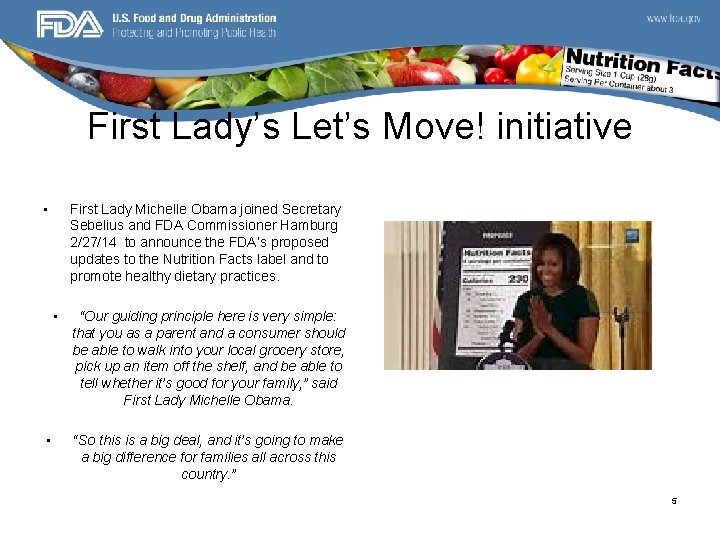
First Lady’s Let’s Move! initiative • First Lady Michelle Obama joined Secretary Sebelius and FDA Commissioner Hamburg 2/27/14 to announce the FDA’s proposed updates to the Nutrition Facts label and to promote healthy dietary practices. • • “Our guiding principle here is very simple: that you as a parent and a consumer should be able to walk into your local grocery store, pick up an item off the shelf, and be able to tell whether it’s good for your family, ” said First Lady Michelle Obama. “So this is a big deal, and it’s going to make a big difference for families all across this country. ” 5

MAJOR CHANGES BEING PROPOSED • Latest Nutrition Science • Refreshed Design • Updated Serving Sizes to reflect what people actually consume • New Labeling requirements for various package sizes 6

Who is affected by Label change? • This represents about 60, 000 manufacturers and over 700, 000 UPC’s. • In terms of sales they represent about $236. 78 billion in grocery sales, drug stores and mass merchandising stores. 7

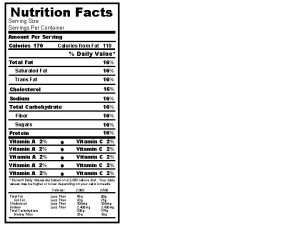
Old vs. New 8

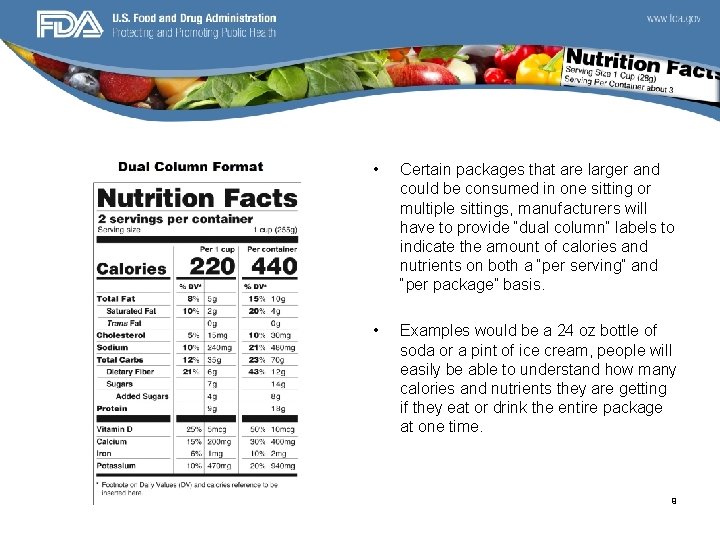
• Certain packages that are larger and could be consumed in one sitting or multiple sittings, manufacturers will have to provide “dual column” labels to indicate the amount of calories and nutrients on both a “per serving” and “per package” basis. • Examples would be a 24 oz bottle of soda or a pint of ice cream, people will easily be able to understand how many calories and nutrients they are getting if they eat or drink the entire package at one time. 9

10

Refreshed Design • “Calories” and “Servings Per Container” would be more prominent to emphasize parts of the label that are important in addressing obesity and cardiovascular disease • 11

Calories from Fats • “Calories from fat” no longer would be required because the type of fat is now known to be more important than the total amount of fat. 12

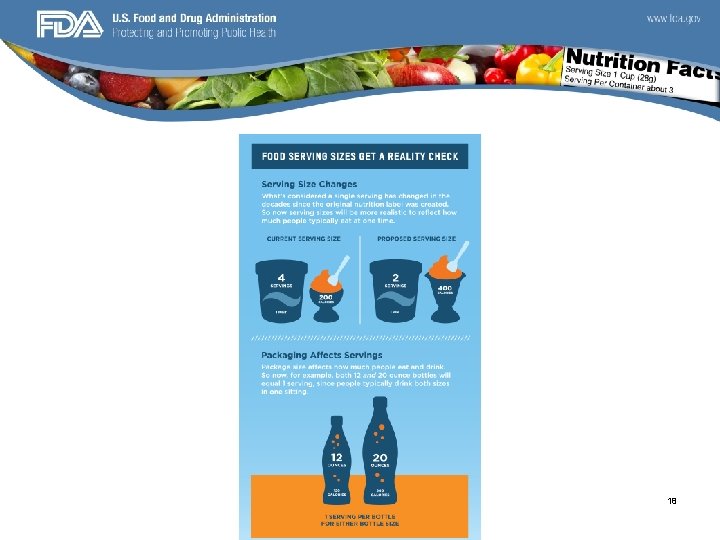
PORTION SIZES • Over the last 20 years portion sizes have increased by 60% for salty snacks and 52% for soft drinks. • Because larger package portion size have been shown to be correlated with increased food consumption the increased “supersizing” trend may represent of the drivers of the obesity epidemic. 13

Serving Size Changes • Right justifying the actual amounts of the serving size information • Reversing the order of “Serving Size” and “Servings Per Container” • Changing “Amount Per Serving” to “Amount per ______”, with the blank filled in with the serving size in a common household measure, e. g. amount to 2/3 rd cup. • Some serving sizes will increase and others will decrease because by law, the serving sizes must be based on the amounts of food and drink that people typically consume, not on how much they should consume. • Recent food consumption data show that some serving sizes need to be updated. 14

Serving Sizes con’t • Jillonne Kevala, Ph. D. , supervisory chemist at the Food and Drug Administration (FDA) says, "The fact is, for many foods, we're eating larger portions than we used to. ” • And the proposed changes to the Nutrition Facts label would reflect that. “Ice cream and soft drinks are just two food products that would be affected by changes in serving size requirements that are included in the proposed updates to the Nutrition Facts label. The goal: to bring serving sizes closer to what people actually eat so that when they look at calories and nutrients on the label, these numbers more closely match what they are consuming. • In some cases, the reference amounts used to set serving sizes would be smaller. Today's single serving yogurts more often come in 6 -ounce containers, versus the previous 8 -ounce ones. FDA is proposing a 6 -ounce reference amount for 15 yogurt.

• Ice cream was previously a ½ cup now it is 2/3 cup. • Soda is changing serving size from 8 oz. to 12 oz. 16

New Labeling requirements for various package sizes • We know that package size affects what people eat. • With the new requirements, more food products will be required to be labeled as a single serving, and the calorie and nutrient information would be based on the entire package. • Examples: • A 20 oz. Bottle of soda • A 15 oz can of soup 17

18

Fats • • Total Fat, Saturated Fat and Trans Fats will be required on the label. Trans Fat will be reduced, not eliminated, so FDA will continue to require it on the label. • The FDA published a final determination that partially hydrogenated oils (PHO’s), the source of artificial trans fat, may not be generally regarded as safe (GRAS), but this determination would not affect naturally occurring trans fat, which would still exist in the food supply. • Trans fat is present naturally in food from some animals, mainly ruminants such as cows and goats. • Also industry can currently use some oils that are approved as food additives and can still petition FDA for certain uses of PHO’s. 19

Sodium • Small reduction in the daily values for sodium, from the current 2400 mg/day to 2300 mg/day to be consistent with the 2005 by the Institute of Medicine (IOM) Dietary Reference Intake Electrolytes report and the 2013 IOM Sodium Intake in Populations report. 20

TOTAL CARBOHYDRATES • Replacing “Total Carbohydrate” with “Total Carbs” and indenting “Added Sugars” directly beneath the listing for “Total Sugars” • FDA no longer permitting the voluntary declaration of Other carbohydrates on the label • Total Carbs – Total Sugars • Includes ______grams Added Sugars 21

Dietary Fiber • We are establishing a definition for fiber that can be declared on the label to include naturally occurring fibers and only fibers added to foods that show a physiological health benefit, • such as lower blood glucose and cholesterol levels, • a greater feeling of fullness (satiety) resulting in reduced calorie intake, • and improved bowel function. 22

Added Sugars • The proposed rule would require declaration of “added sugars” as well, indented under “sugars, ” to help consumers understand how much sugar is NATURALLY OCCURRING and how much is ADDED to the product. • %DV is also required • “empty calories” • “although added sugars are not chemically different from naturally occurring sugars, many foods and beverages that are major sources of added sugars have lower micronutrient densities compared to foods and beverages that are major sources of naturally occurring sugars”. 23

Why must “added sugars” be added? • -Scientific data shows that it is difficult to meet nutrient needs while staying within calorie requirements if you consume more than 10% of your total daily calories from added sugar • Empty calories • The 2015 -2020 Dietary Guidelines for Americans recommend reducing caloric intake from added sugars; as does the American Heart Association, The American Academy of Pediatrics, the Institute of Medicine, and the World Health Organization • On average, Americans get about 13% of their total calories from added sugars – Major sources: soft drinks, fruit drinks, coffee and tea, sports and energy drinks, and alcoholic beverages 24

How is FDA defining added sugars? • Sugars added during the processing of foods, or are packaged as such, • and include sugars (free, mono-, and disaccharides), • sugars from syrups and honey, • and sugars from concentrated fruit or vegetable juice of the same type. 25

Excludes… • This definition excludes fruit or vegetable juice concentrated from 100% fruit juice that is sold to consumers (e. g. frozen 100% fruit juice concentrate) as well as some sugars found in fruit and vegetable juices, jellies, jams, preserves, and fruit spreads. • These are excluded because FDA has standards of identity which are requirements for what a food product must contain to be marketed under a certain name. 26

% Daily Value or %DV • Shifting to the left of the label the %Daily Value or %DV. This is to help consumers place nutrient information in the context of a total daily diet. • 5/20 Rule • Revise the DV for a variety of nutrients including sodium, dietary fiber and Vitamin D. These are used to calculate the %DV on the label, which help consumers to understand the nutrition information in the context of a total daily diet 27

%DV for Vitamins and Minerals • Declaring the actual amount, in addition to the %DV, of mandatory vitamins and minerals and voluntary vitamins and minerals. 28

Potassium and Vitamin D • Potassium and Vitamin D would have to be declared because they are new “nutrients of public health significance”. Vitamin D is important in bone health and potassium helps to lower blood pressure. • Vitamin A& C would no longer be required on the label, though manufacturers could include them voluntarily. 29

Potassium and Vitamin D • Vitamin D is important for its role in bone development and general health. • Potassium is beneficial in lowering blood pressure and preventing hypertension. • “…evidence that people are not consuming enough of these nutrients to protect against chronic diseases…” 30

Bottom Section • It now will read: “The % Daily value tells you how much a nutrient in a serving of food contributes to a daily diet. 2, 000 calories a day is used for general nutrition advice. ” 31

COST • The one-time cost to industry of labeling, reformulation, and initial recordkeeping is $2. 3 billion, with an small annual cost associated with recurring recordkeeping. 32

Side notes • FSIS/USDA, which regulates labeling for meat, poultry and processed eggs, is planning to propose similar changes for products they regulate (meat, poultry and some processed egg products) 33

Imported Foods • Foods imported to the United States will need to meet the final requirements. 34

Dietary Supplements • Except for a few minor changes to be consistent with the Nutrition facts label, the Supplement Facts label appearing on dietary supplement products is not changing. • Requires declaration of added sugars, if added. • Changes the units of measure. Vitamin E & D now to be listed in micrograms or milligrams and not IU. • Serving sizes are not changing on dietary supplements. 35

FOP labeling • The Nutrition Facts is distinct from front-of-pack (FOP) labeling, which is not required by the FDA at this time. • FDA seeking a science-based solution to the proliferation of FOP labeling and is evaluating the IOM’s FOP Report released in 2011. 36

PUBLIC COMMENT WAS SOUGHT How to Comment on the Proposed Nutrition Label FDA issued two proposed rules on the nutrition facts label. The rules are published in the Federal Register (FR) so that members of the public can review them and send their comments to us. The public is given a period of time to submit their comments. The comment period was extended to and closed on August 1, 2014. You may view submitted comments in the docket folder of each rule. 37

Compliance Dates • Manufacturers will need to use the new label by 2 years and 2 months after publication date. Small businesses (defined for the purposes of this rule to have less than $10 million in annual food sales) will have an additional year to comply. 38

FDA. GOV • Updates for consumers and industry on FDA webpage. Spanish available as well. • http: //www. fda. gov/For. Consumers/Consumer. Up dates/ucm 387114. htm 39
 Monaco drink nutrition label
Monaco drink nutrition label Hijos de sergio y estibaliz
Hijos de sergio y estibaliz ........ is an alternative of log based recovery.
........ is an alternative of log based recovery. Zozhnik indian
Zozhnik indian Cdn architectures
Cdn architectures Https //fontawesome.bootstrapcheatsheets.com cdn
Https //fontawesome.bootstrapcheatsheets.com cdn Iptv cdn
Iptv cdn What is cedexis radar
What is cedexis radar 12112336 pix cdn org m p 0 83 83878 hevtgvi2 html
12112336 pix cdn org m p 0 83 83878 hevtgvi2 html Azure cdn zones
Azure cdn zones Cdn free
Cdn free Apache traffic server
Apache traffic server Carbohydrates
Carbohydrates 7 servings per container
7 servings per container Monaco cartina fisica
Monaco cartina fisica Monaco harta europei
Monaco harta europei Philippe denain monaco
Philippe denain monaco Ics guido monaco
Ics guido monaco Illumination monaco
Illumination monaco Scnrs
Scnrs Femmes leaders mondiales monaco
Femmes leaders mondiales monaco Xxcmd
Xxcmd Isabel monaco
Isabel monaco Andrea giuliani monaco
Andrea giuliani monaco Kristina vukaj
Kristina vukaj Ebis monaco
Ebis monaco Chirurgie bariatrique monaco
Chirurgie bariatrique monaco Kristen monaco
Kristen monaco Caisses sociales de monaco téléservices
Caisses sociales de monaco téléservices Calcul retraite monaco
Calcul retraite monaco Purge scene
Purge scene Monegasque
Monegasque Eutimio monaco
Eutimio monaco Monaco sulla spiaggia friedrich
Monaco sulla spiaggia friedrich Patrick fietje
Patrick fietje Ime monaco
Ime monaco Michele rubinelli monaco
Michele rubinelli monaco 64:8+9:9-63:7
64:8+9:9-63:7 The term ecosystem was proposed by *
The term ecosystem was proposed by * Environmental scanning techniques
Environmental scanning techniques