Lecture Presentation Chapter 19 Radioactivity and Nuclear Chemistry







- Slides: 69

Lecture Presentation Chapter 19 Radioactivity and Nuclear Chemistry Catherine Mac. Gowan Armstrong Atlantic State University © 2013 Pearson Education, Inc.

A Quick Review of the Nucleus The nucleus of an atom: • is composed of nucleons – Protons (+) and neutrons (0) • has very small volume compared to volume of the atom • is essentially entire mass of atom • is very dense © 2013 Pearson Education, Inc.

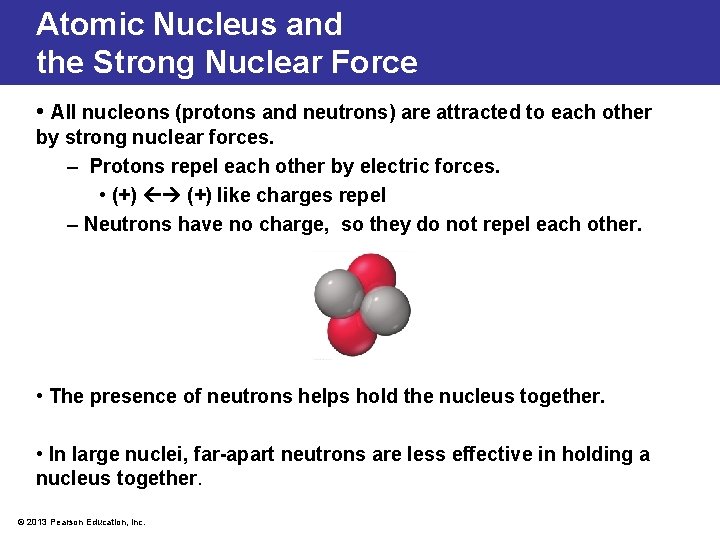
Atomic Nucleus and the Strong Nuclear Force • All nucleons (protons and neutrons) are attracted to each other by strong nuclear forces. – Protons repel each other by electric forces. • (+) like charges repel – Neutrons have no charge, so they do not repel each other. • The presence of neutrons helps hold the nucleus together. • In large nuclei, far-apart neutrons are less effective in holding a nucleus together. © 2013 Pearson Education, Inc.

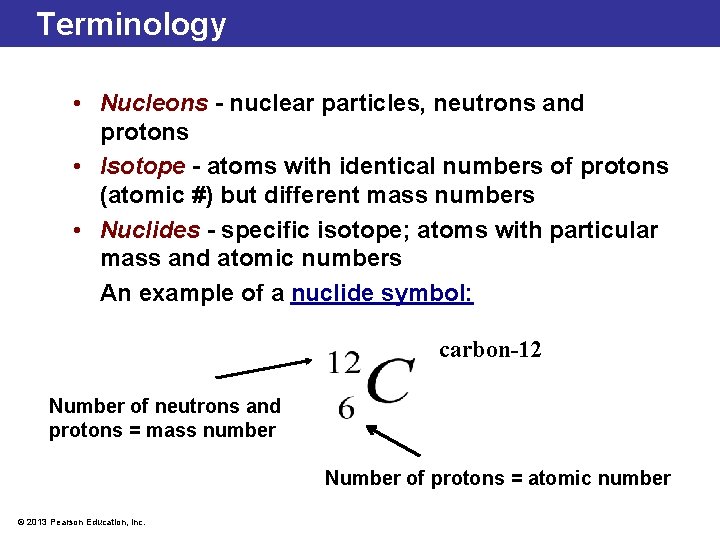
Terminology • Nucleons - nuclear particles, neutrons and protons • Isotope - atoms with identical numbers of protons (atomic #) but different mass numbers • Nuclides - specific isotope; atoms with particular mass and atomic numbers An example of a nuclide symbol: carbon-12 Number of neutrons and protons = mass number Number of protons = atomic number © 2013 Pearson Education, Inc.

Terminology • Nuclear reactions - chemical changes that involve nuclear particles and radioactive decay Radioactive decay shown by nuclear equation • Radioactive decay - nucleus of element spontaneously decomposes by emitting a nuclear particle, electron, positron, or electromagnetic radiation • Nuclear force - strong force of attraction between nuclear particles © 2013 Pearson Education, Inc.

Radioactivity • It is the spontaneous decay/disintegration of an unstable atomic nucleus. • The emission of radiation (energy) and/or matter (protons, neutrons, smaller isotopes, beta and/or alpha particles) • Occurs as a result of an unstable ratio of neutrons to protons in an atom’s nucleus • Radioactive rays: – can ionize matter, which • causes uncharged matter to become charged and • can be detected using a Geiger counter or an electroscope – have high energy – can penetrate matter – cause phosphorescent chemicals to glow • The ability of matter to glow after absorbing radiation is the basis of a scintillation counter © 2013 Pearson Education, Inc.

Radioactivity: Historical Overview • Becquerel: – discovered that certain minerals produced penetrating energy rays he called uranic rays • Similar to X-rays but were not related to fluorescence – determined that all the minerals that produced these uranic rays: • contained uranium • seemed to produce rays even though the mineral was not exposed to outside energy • Marie Curie: – used electroscope to detect uranic rays in other ore samples – discovered new elements by detecting their rays – radium named for its green phosphorescence – polonium named for her homeland, Poland • Her findings showed that these rays were no longer just a property of uranium and its ores. Based on her experimental work, renamed Becquerel’s uranic rays • radioactivity © 2013 Pearson Education, Inc.

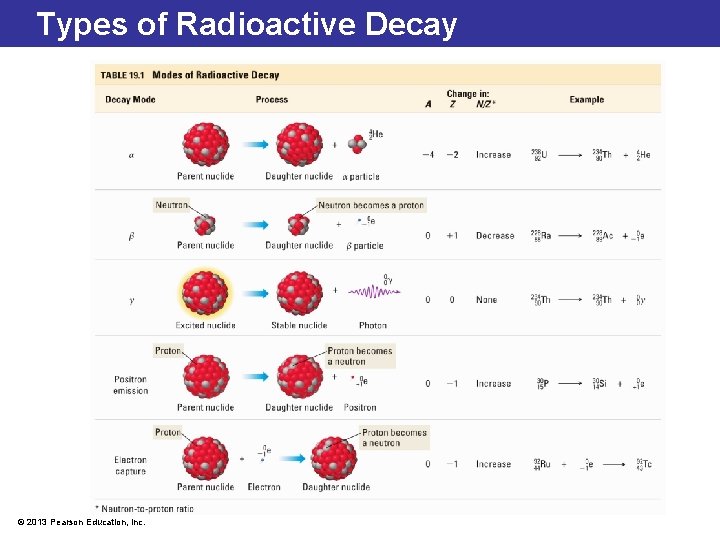
Predicting Types of Decay • Nuclides outside band of stability are often radioactive. • Compare N/Z ratio of nuclide with that of stable nucleus. • Nuclide with N/Z < stable nucleus: positron emission or electron capture (more common with heavier elements) • Nuclide with N/Z > stable nucleus: beta emission • Many elements with Z > 83 emit alpha particles. © 2013 Pearson Education, Inc.

Radioactive Decay Processes • Alpha emission ( ): emission of a helium-4 nucleus from unstable nucleus; has a +2 charge • Beta emission ( ): emission of a high-energy electron from unstable nucleus. Results from formation of proton from a neutron; has a -1 charge • Gamma emission ( ): emission of electromagnetic radiation, with wavelength of ~10 -12 m (excited nucleus). High ultra energy; nonvisible light that carries no electrical charge © 2013 Pearson Education, Inc.

Types of Radioactive Decay © 2013 Pearson Education, Inc.

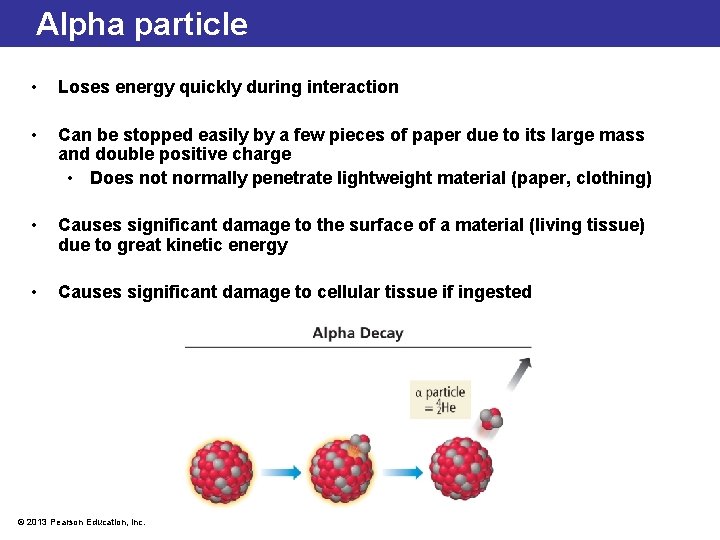
Alpha particle • Loses energy quickly during interaction • Can be stopped easily by a few pieces of paper due to its large mass and double positive charge • Does not normally penetrate lightweight material (paper, clothing) • Causes significant damage to the surface of a material (living tissue) due to great kinetic energy • Causes significant damage to cellular tissue if ingested © 2013 Pearson Education, Inc.

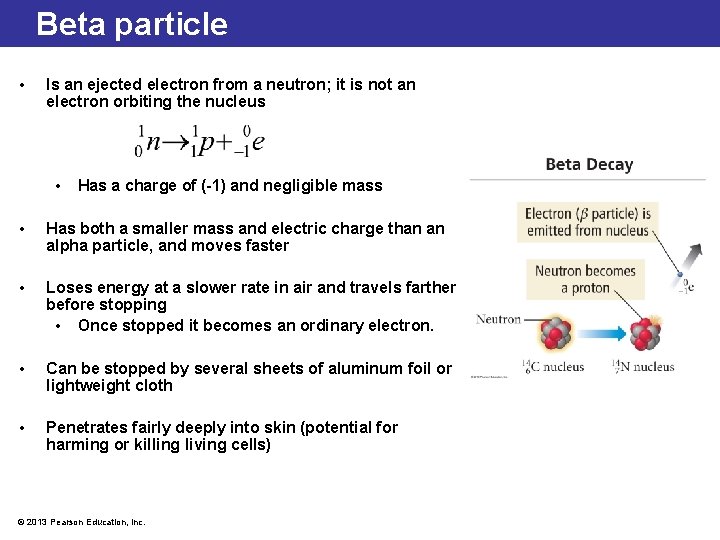
Beta particle • Is an ejected electron from a neutron; it is not an electron orbiting the nucleus • Has a charge of (-1) and negligible mass • Has both a smaller mass and electric charge than an alpha particle, and moves faster • Loses energy at a slower rate in air and travels farther before stopping • Once stopped it becomes an ordinary electron. • Can be stopped by several sheets of aluminum foil or lightweight cloth • Penetrates fairly deeply into skin (potential for harming or killing living cells) © 2013 Pearson Education, Inc.

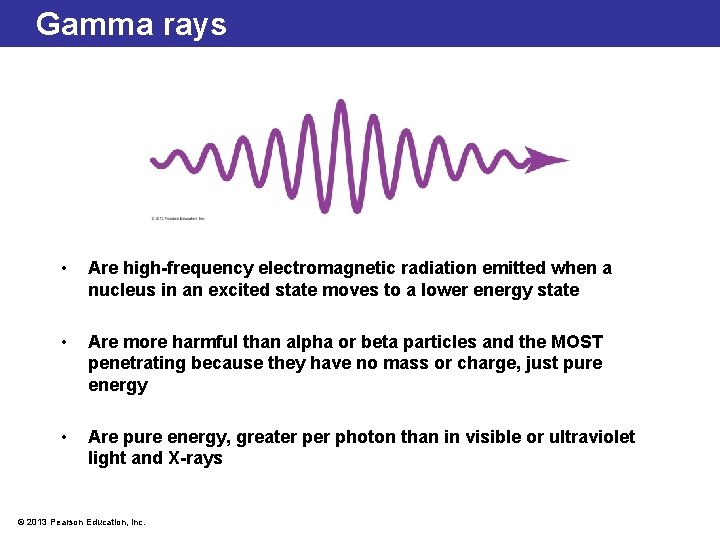
Gamma rays • Are high-frequency electromagnetic radiation emitted when a nucleus in an excited state moves to a lower energy state • Are more harmful than alpha or beta particles and the MOST penetrating because they have no mass or charge, just pure energy • Are pure energy, greater photon than in visible or ultraviolet light and X-rays © 2013 Pearson Education, Inc.

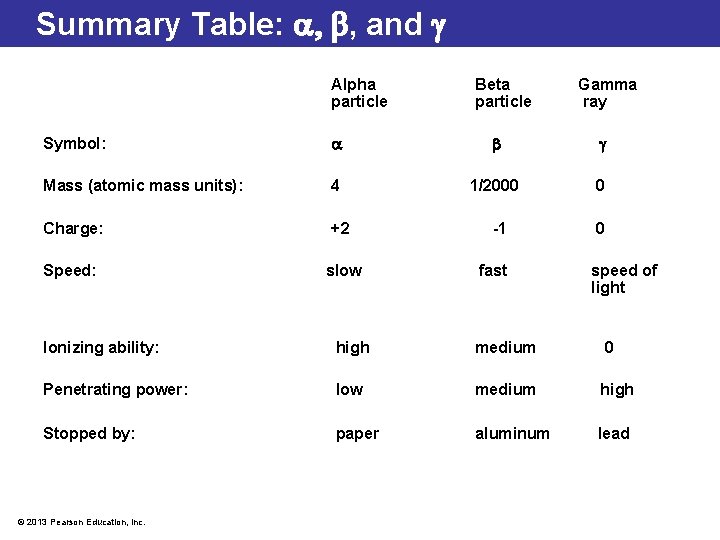
Summary Table: , and Alpha particle Beta particle Symbol: Mass (atomic mass units): 4 Charge: +2 Speed: slow Gamma ray 1/2000 -1 fast 0 0 speed of light Ionizing ability: high medium 0 Penetrating power: low medium high Stopped by: paper aluminum lead © 2013 Pearson Education, Inc.

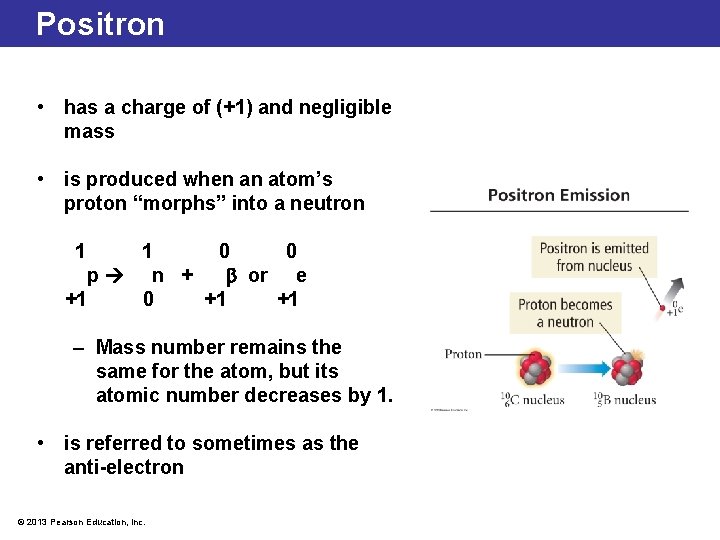
Positron • has a charge of (+1) and negligible mass • is produced when an atom’s proton “morphs” into a neutron 1 1 0 0 p n + or e +1 0 +1 +1 – Mass number remains the same for the atom, but its atomic number decreases by 1. • is referred to sometimes as the anti-electron © 2013 Pearson Education, Inc.

Electron Capture • is not a subatomic particle undergoing decay • No particle emission, but atom changes • occurs when an inner orbital electron is pulled into the nucleus • • Mass number stays the same. Atomic number decreases by one. – A similar result as seen with positron emission • When electron capture happens: – A proton in the nucleus combines with the electron to make a neutron. 1 0 p +1 © 2013 Pearson Education, Inc. + 1 e -1 n 0

Chemical Processes vs. Nuclear Processes • Chemical reactions involve changes in the electronic structure of the atom. – Atoms gain, lose, or share electrons. – No change in the nuclei occurs. • Nuclear reactions involve changes in the structure of the nucleus. – If the number of protons in the nucleus changes, the atom becomes a different element. – If the number of neutrons in the nucleus changes, the atom becomes an isotope. © 2013 Pearson Education, Inc.

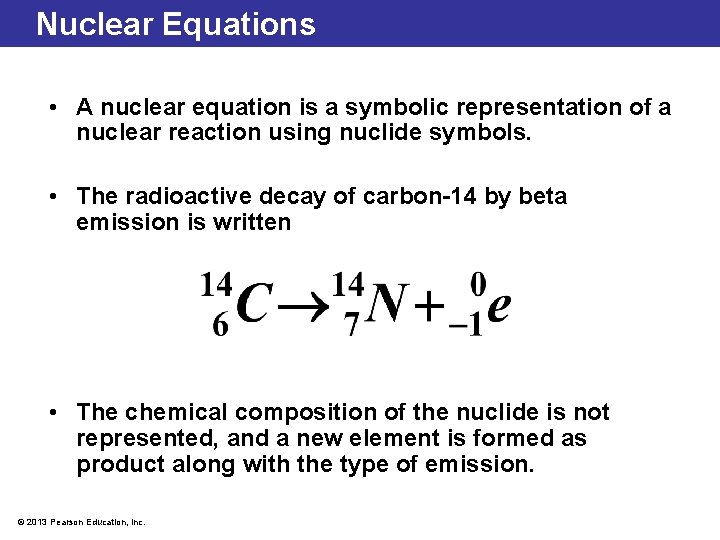
Nuclear Equations • A nuclear equation is a symbolic representation of a nuclear reaction using nuclide symbols. • The radioactive decay of carbon-14 by beta emission is written • The chemical composition of the nuclide is not represented, and a new element is formed as product along with the type of emission. © 2013 Pearson Education, Inc.

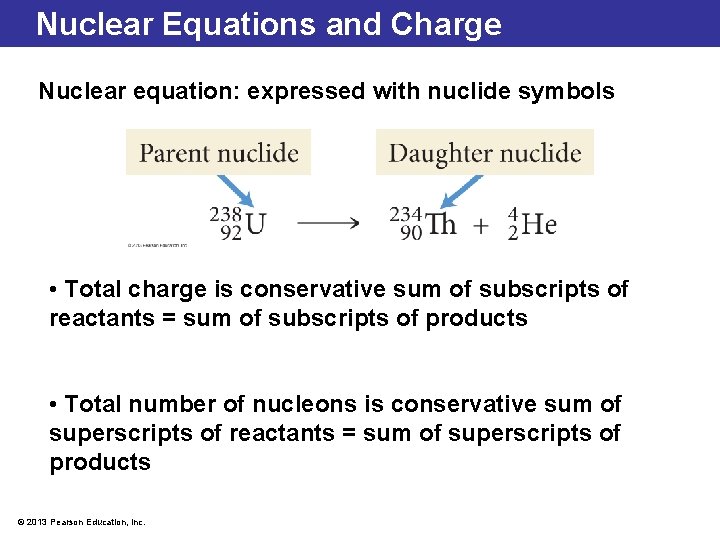
Nuclear Equations and Charge Nuclear equation: expressed with nuclide symbols • Total charge is conservative sum of subscripts of reactants = sum of subscripts of products • Total number of nucleons is conservative sum of superscripts of reactants = sum of superscripts of products © 2013 Pearson Education, Inc.

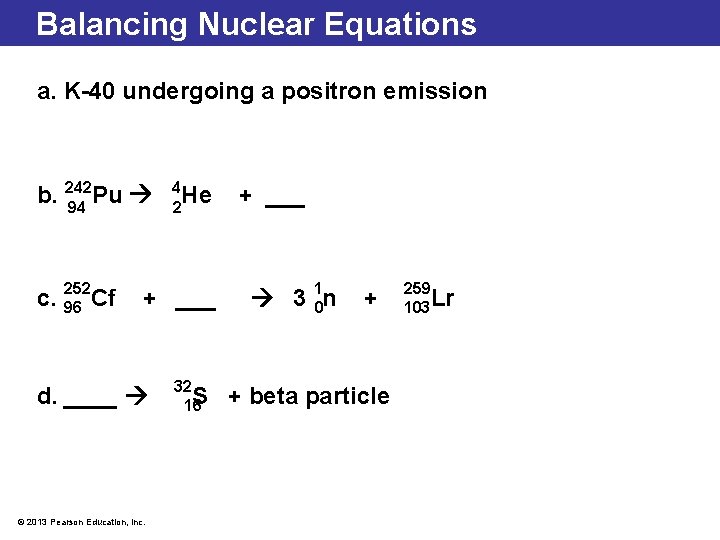
Balancing Nuclear Equations a. K-40 undergoing a positron emission b. 242 Pu 94 252 c. 96 Cf 4 He 2 + ___ d. ____ © 2013 Pearson Education, Inc. 32 S 16 + ___ 3 10 n + + beta particle 259 103 Lr

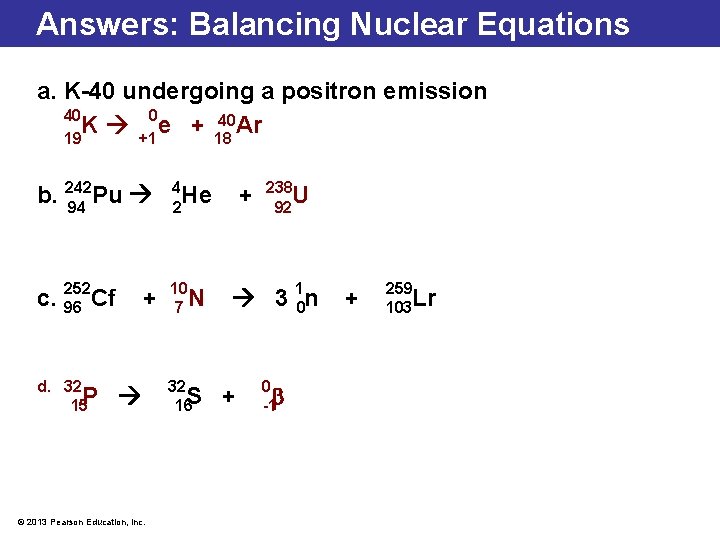
Answers: Balancing Nuclear Equations a. K-40 undergoing a positron emission 40 0 40 Ar K e + 19 +1 18 b. 242 Pu 94 4 He 2 252 c. 96 Cf 10 7 N d. 32 15 P + © 2013 Pearson Education, Inc. 32 16 S + 238 U 92 3 10 n + 0 -1 + 259 103 Lr

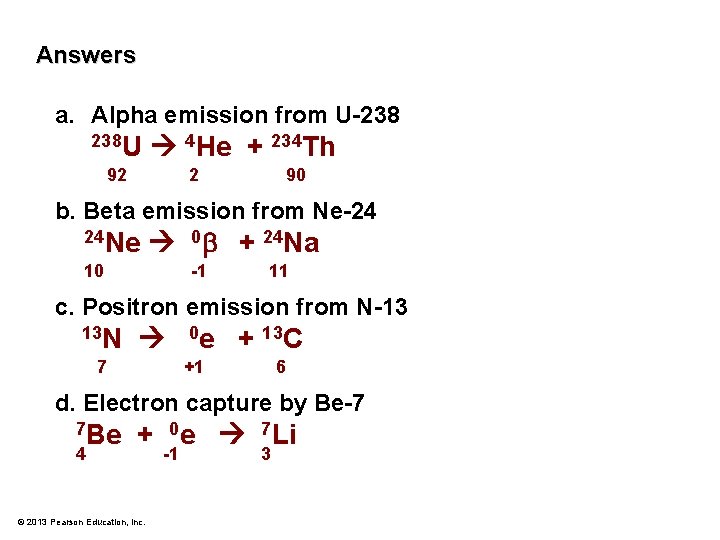
Problem: Write a nuclear equation for each of the following. a. Alpha emission from U-238 b. Beta emission from Ne-24 c. Positron emission from N-13 d. Electron capture by Be-7 © 2013 Pearson Education, Inc.

Answers a. Alpha emission from U-238 238 U 4 He + 234 Th 92 2 90 b. Beta emission from Ne-24 24 Ne 0 + 24 Na 10 -1 11 c. Positron emission from N-13 13 N 7 0 e + 13 C +1 6 d. Electron capture by Be-7 7 Be 4 + 0 e 7 Li © 2013 Pearson Education, Inc. -1 3

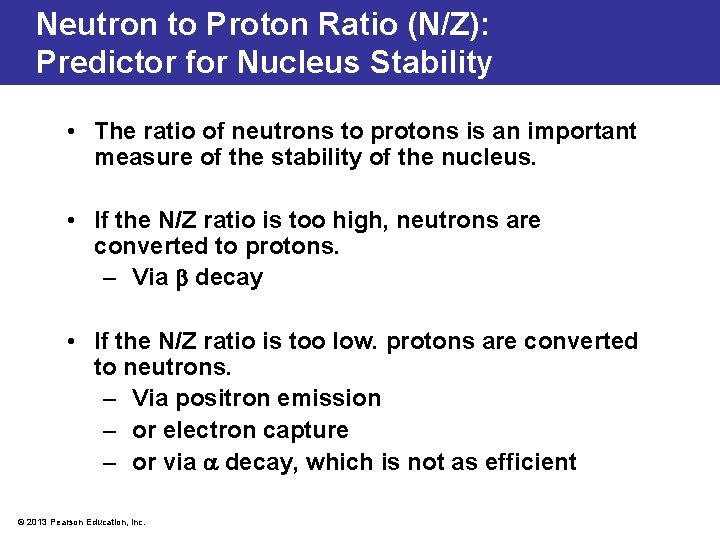
Neutron to Proton Ratio (N/Z): Predictor for Nucleus Stability • The ratio of neutrons to protons is an important measure of the stability of the nucleus. • If the N/Z ratio is too high, neutrons are converted to protons. – Via decay • If the N/Z ratio is too low. protons are converted to neutrons. – Via positron emission – or electron capture – or via decay, which is not as efficient © 2013 Pearson Education, Inc.

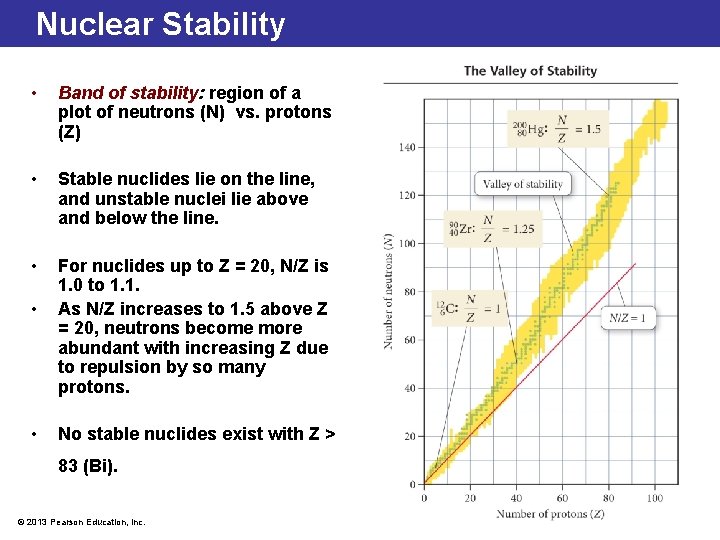
Nuclear Stability • Band of stability: region of a plot of neutrons (N) vs. protons (Z) • Stable nuclides lie on the line, and unstable nuclei lie above and below the line. • For nuclides up to Z = 20, N/Z is 1. 0 to 1. 1. As N/Z increases to 1. 5 above Z = 20, neutrons become more abundant with increasing Z due to repulsion by so many protons. • • No stable nuclides exist with Z > 83 (Bi). © 2013 Pearson Education, Inc.

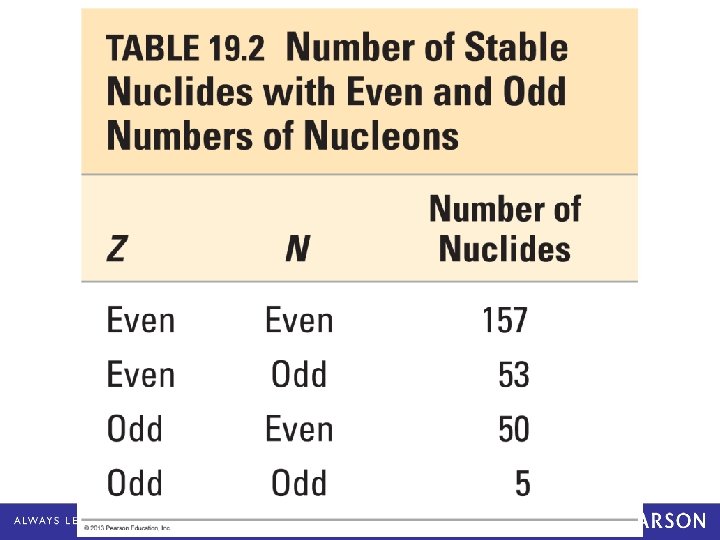
Magic Numbers and Nucleus Stability The actual numbers of protons and neutrons affect the stability of the nucleus. • Most stable nuclei have even numbers of protons and neutrons. • Only a few have odd numbers of protons and neutrons. • If the total number of nucleons adds to a magic number, the nucleus is more stable. – Most stable when N or Z = 2, 8, 20, 28, 50, 82; or N = 126 • “Magic numbers” are similar to concept regarding electrons and the “full shell” electron configuration resulting in a more stable atom. © 2013 Pearson Education, Inc.


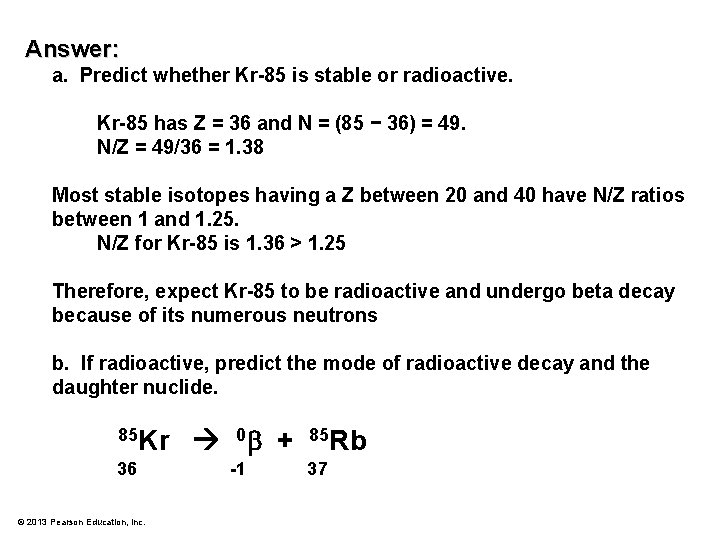
Problem: a. Predict whether Kr-85 is stable or radioactive. b. If radioactive, predict the mode of radioactive decay and the daughter nuclide. . © 2013 Pearson Education, Inc.

Answer: a. Predict whether Kr-85 is stable or radioactive. Kr-85 has Z = 36 and N = (85 − 36) = 49. N/Z = 49/36 = 1. 38 Most stable isotopes having a Z between 20 and 40 have N/Z ratios between 1 and 1. 25. N/Z for Kr-85 is 1. 36 > 1. 25 Therefore, expect Kr-85 to be radioactive and undergo beta decay because of its numerous neutrons b. If radioactive, predict the mode of radioactive decay and the daughter nuclide. 85 Kr 36 © 2013 Pearson Education, Inc. 0 + -1 85 Rb 37

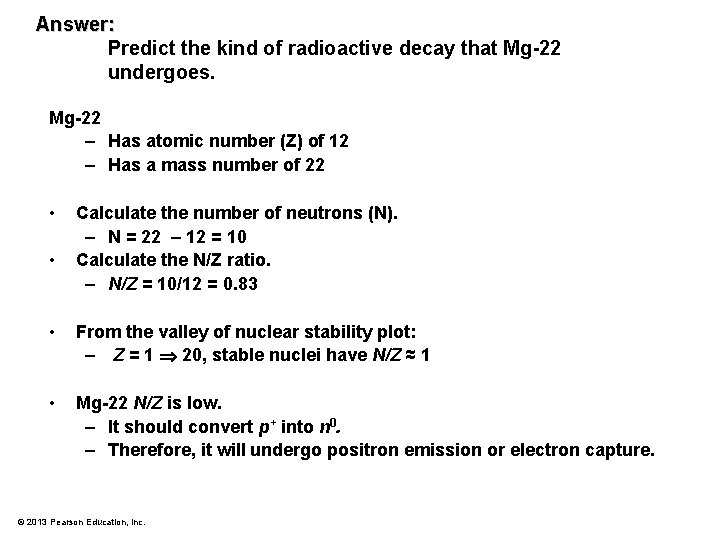
Problem: Predict the kind of radioactive decay that Mg-22 undergoes. © 2013 Pearson Education, Inc.

Answer: Predict the kind of radioactive decay that Mg-22 undergoes. Mg-22 – Has atomic number (Z) of 12 – Has a mass number of 22 • • Calculate the number of neutrons (N). – N = 22 – 12 = 10 Calculate the N/Z ratio. – N/Z = 10/12 = 0. 83 • From the valley of nuclear stability plot: – Z = 1 20, stable nuclei have N/Z ≈ 1 • Mg-22 N/Z is low. – It should convert p+ into n 0. – Therefore, it will undergo positron emission or electron capture. © 2013 Pearson Education, Inc.

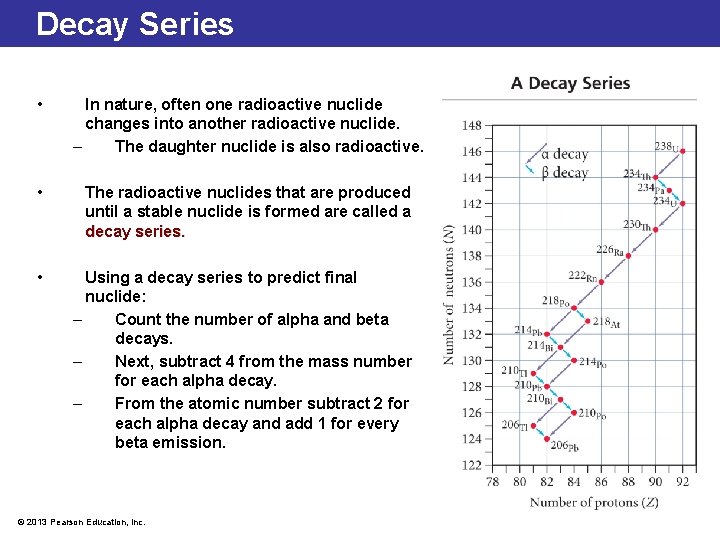
Decay Series • In nature, often one radioactive nuclide changes into another radioactive nuclide. – The daughter nuclide is also radioactive. • The radioactive nuclides that are produced until a stable nuclide is formed are called a decay series. • Using a decay series to predict final nuclide: – Count the number of alpha and beta decays. – Next, subtract 4 from the mass number for each alpha decay. – From the atomic number subtract 2 for each alpha decay and add 1 for every beta emission. © 2013 Pearson Education, Inc.

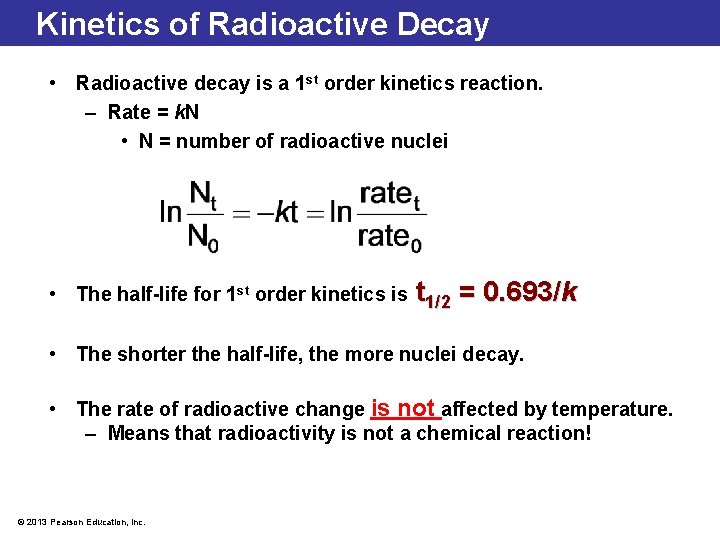
Radioactive Decay is a 1 st Order Half-Life Kinetic Problem • It is the rate of decay associated with a radioactive isotope. • It is characteristic to the isotope. • Each radioactive isotope has its own half-life. • It is the time required for half of an original quantity of an element to decay. • The shorter the half-life, the faster the decay. • The decay can be particular in nature (beta, alpha, etc. ) or energy (gamma, x-rays, etc. ) • It is constant and independent of any physical or chemical change the atom may undergo. • It can be calculated at any given moment by measuring the rate of decay of a known quantity using a radiation detector. © 2013 Pearson Education, Inc.

Kinetics of Radioactive Decay • Radioactive decay is a 1 st order kinetics reaction. – Rate = k. N • N = number of radioactive nuclei • The half-life for 1 st order kinetics is t 1/2 = 0. 693/k • The shorter the half-life, the more nuclei decay. • The rate of radioactive change is not affected by temperature. – Means that radioactivity is not a chemical reaction! © 2013 Pearson Education, Inc.

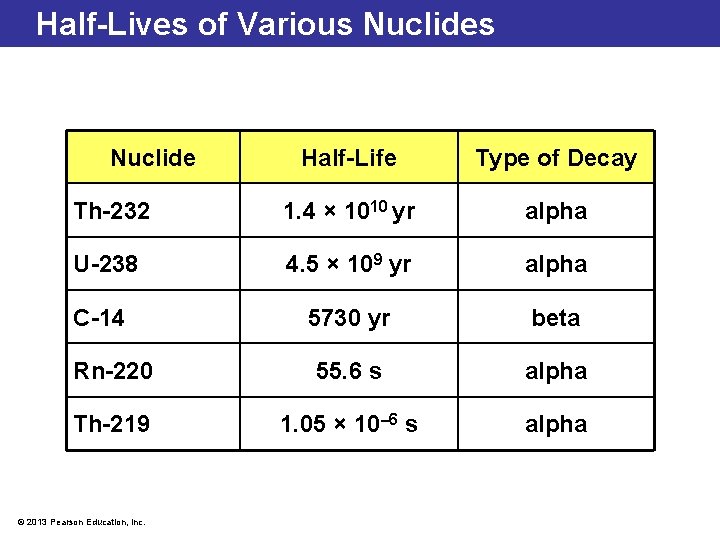
Half-Lives of Various Nuclide Half-Life Type of Decay Th-232 1. 4 × 1010 yr alpha U-238 4. 5 × 109 yr alpha C-14 5730 yr beta Rn-220 55. 6 s alpha Th-219 1. 05 × 10– 6 s alpha © 2013 Pearson Education, Inc.

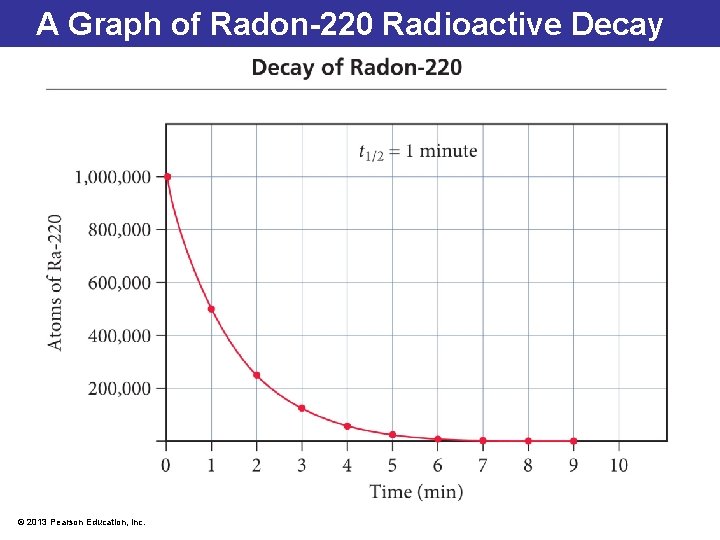
A Graph of Radon-220 Radioactive Decay © 2013 Pearson Education, Inc.

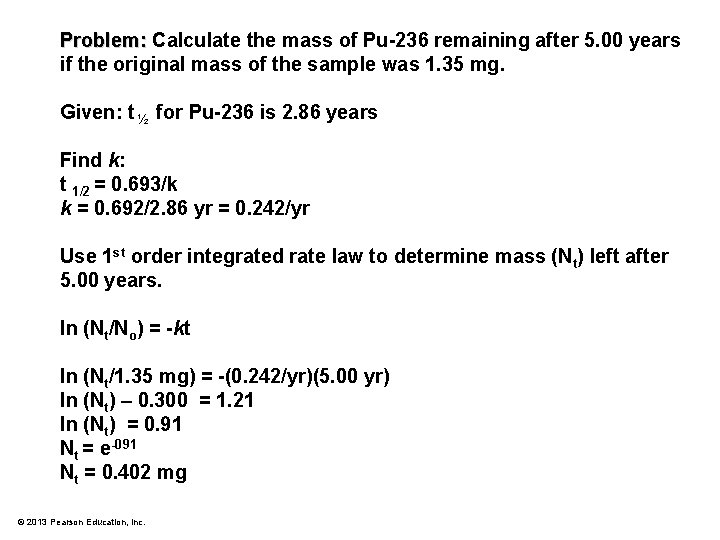
Problem: Calculate the mass of Pu-236 remaining after 5. 00 years if the original mass of the sample was 1. 35 mg. © 2013 Pearson Education, Inc.

Problem: Calculate the mass of Pu-236 remaining after 5. 00 years if the original mass of the sample was 1. 35 mg. Given: t ½ for Pu-236 is 2. 86 years Find k: t 1/2 = 0. 693/k k = 0. 692/2. 86 yr = 0. 242/yr Use 1 st order integrated rate law to determine mass (Nt) left after 5. 00 years. ln (Nt/No) = -kt ln (Nt/1. 35 mg) = -(0. 242/yr)(5. 00 yr) ln (Nt) – 0. 300 = 1. 21 ln (Nt) = 0. 91 Nt = e-091 Nt = 0. 402 mg © 2013 Pearson Education, Inc.

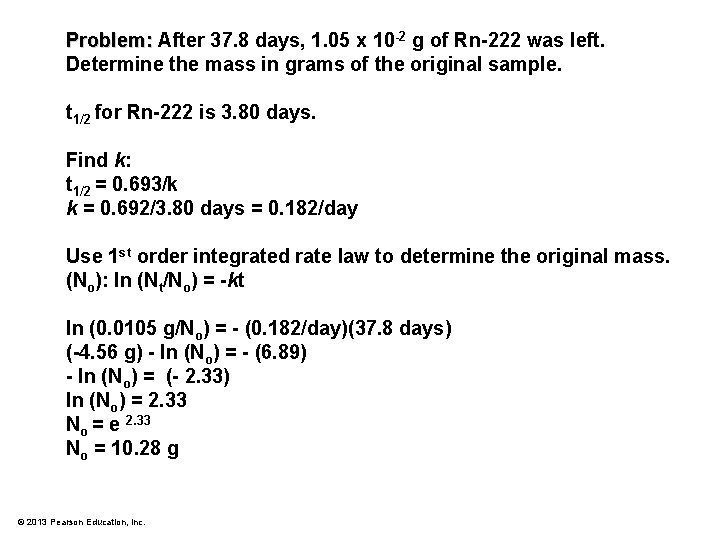
Problem: After 37. 8 days, 1. 05 x 10 -2 g of Rn-222 was left. Determine the mass in grams of the original sample. t 1/2 for Rn-222 is 3. 80 days. © 2013 Pearson Education, Inc.

Problem: After 37. 8 days, 1. 05 x 10 -2 g of Rn-222 was left. Determine the mass in grams of the original sample. t 1/2 for Rn-222 is 3. 80 days. Find k: t 1/2 = 0. 693/k k = 0. 692/3. 80 days = 0. 182/day Use 1 st order integrated rate law to determine the original mass. (No): ln (Nt/No) = -kt ln (0. 0105 g/No) = - (0. 182/day)(37. 8 days) (-4. 56 g) - ln (No) = - (6. 89) - ln (No) = (- 2. 33) ln (No) = 2. 33 No = e 2. 33 No = 10. 28 g © 2013 Pearson Education, Inc.

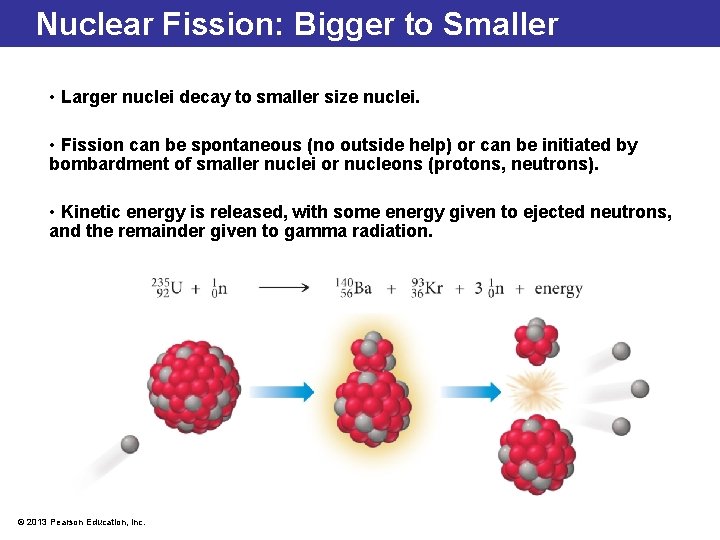
Nuclear Fission: Bigger to Smaller • Larger nuclei decay to smaller size nuclei. • Fission can be spontaneous (no outside help) or can be initiated by bombardment of smaller nuclei or nucleons (protons, neutrons). • Kinetic energy is released, with some energy given to ejected neutrons, and the remainder given to gamma radiation. © 2013 Pearson Education, Inc.

Nuclear Fission: Chain Reactions • Chain reaction is a self-sustaining reaction in which the products of one reaction event stimulate further reaction events. • Critical mass: • the minimum mass of fissionable material in a reactor or nuclear bomb that will sustain a chain reaction • a mass large enough to sustain fission • At or above critical mass, in a large quantity of atoms, an enormous explosion can occur. © 2013 Pearson Education, Inc.

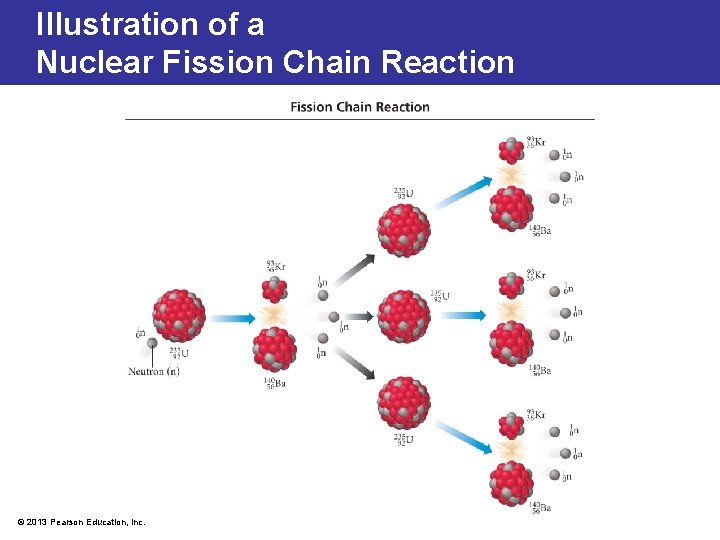
Illustration of a Nuclear Fission Chain Reaction © 2013 Pearson Education, Inc.

Nuclear Fusion: Smaller to Bigger Thermonuclear fusion: • is produced by high temperature resulting in more tightly bound nuclei • decreases mass as energy is released • This is analogous to chemical combustion requiring a high temperature, where the end result is energy release and a tightly bound molecule. • A solution is still being sought for reactions to occur under controlled conditions to provide an enormous amount of sustained energy. © 2013 Pearson Education, Inc.

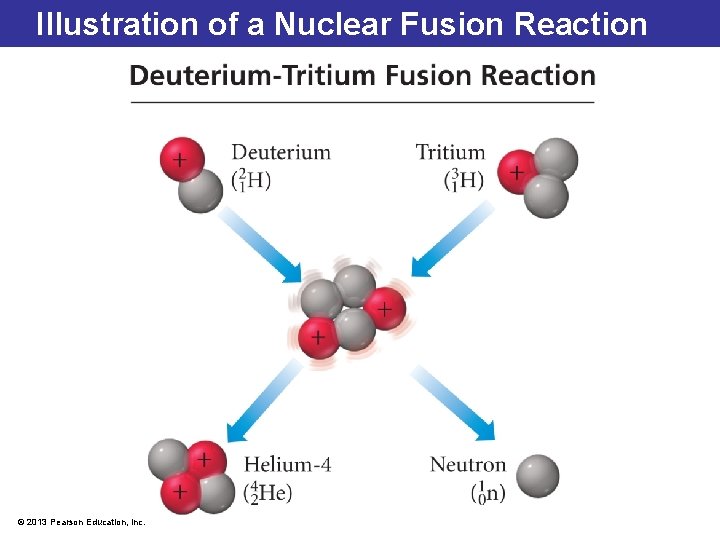
Illustration of a Nuclear Fusion Reaction © 2013 Pearson Education, Inc.

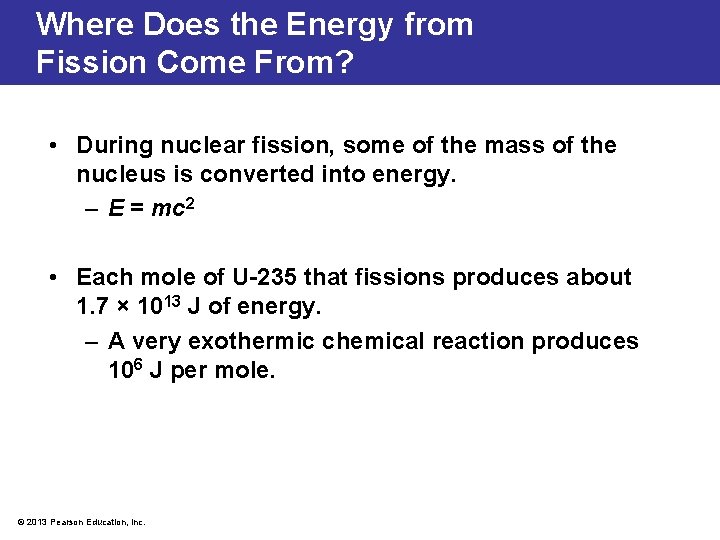
Where Does the Energy from Fission Come From? • During nuclear fission, some of the mass of the nucleus is converted into energy. – E = mc 2 • Each mole of U-235 that fissions produces about 1. 7 × 1013 J of energy. – A very exothermic chemical reaction produces 106 J per mole. © 2013 Pearson Education, Inc.

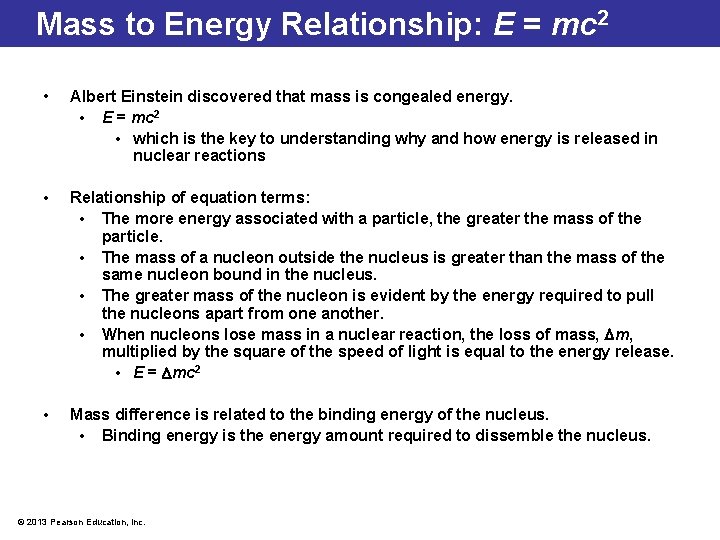
Mass to Energy Relationship: E = mc 2 • Albert Einstein discovered that mass is congealed energy. • E = mc 2 • which is the key to understanding why and how energy is released in nuclear reactions • Relationship of equation terms: • The more energy associated with a particle, the greater the mass of the particle. • The mass of a nucleon outside the nucleus is greater than the mass of the same nucleon bound in the nucleus. • The greater mass of the nucleon is evident by the energy required to pull the nucleons apart from one another. • When nucleons lose mass in a nuclear reaction, the loss of mass, m, multiplied by the square of the speed of light is equal to the energy release. • E = mc 2 • Mass difference is related to the binding energy of the nucleus. • Binding energy is the energy amount required to dissemble the nucleus. © 2013 Pearson Education, Inc.

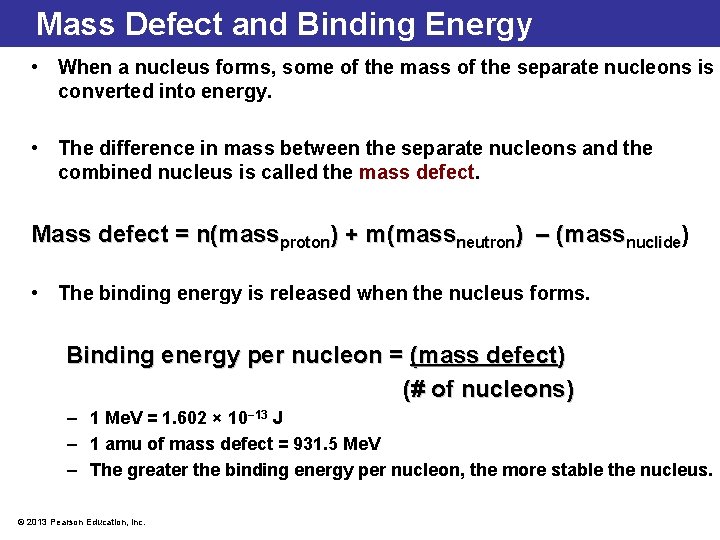
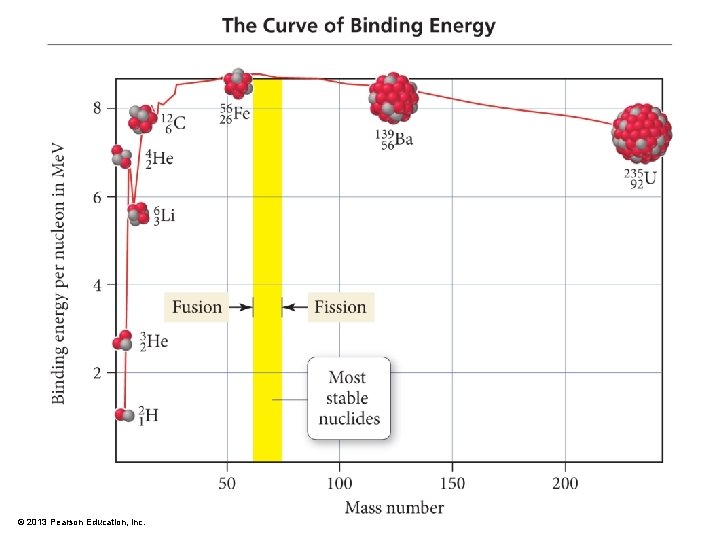
Mass Defect and Binding Energy • When a nucleus forms, some of the mass of the separate nucleons is converted into energy. • The difference in mass between the separate nucleons and the combined nucleus is called the mass defect. Mass defect = n(massproton) + m(massneutron) – (massnuclide) • The binding energy is released when the nucleus forms. Binding energy per nucleon = (mass defect) (# of nucleons) – 1 Me. V = 1. 602 × 10− 13 J – 1 amu of mass defect = 931. 5 Me. V – The greater the binding energy per nucleon, the more stable the nucleus. © 2013 Pearson Education, Inc.

© 2013 Pearson Education, Inc.

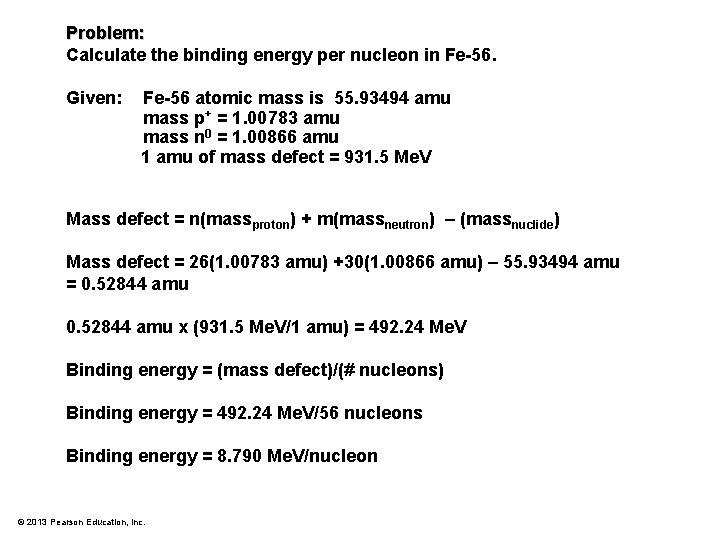
Problem: Calculate the binding energy per nucleon in Fe 56. Given: Fe-56 atomic mass is 55. 93494 amu mass p+ = 1. 00783 amu mass n 0 = 1. 00866 amu 1 amu of mass defect = 931. 5 Me. V © 2013 Pearson Education, Inc.

Problem: Calculate the binding energy per nucleon in Fe-56. Given: Fe-56 atomic mass is 55. 93494 amu mass p+ = 1. 00783 amu mass n 0 = 1. 00866 amu 1 amu of mass defect = 931. 5 Me. V Mass defect = n(massproton) + m(massneutron) – (massnuclide) Mass defect = 26(1. 00783 amu) +30(1. 00866 amu) – 55. 93494 amu = 0. 52844 amu x (931. 5 Me. V/1 amu) = 492. 24 Me. V Binding energy = (mass defect)/(# nucleons) Binding energy = 492. 24 Me. V/56 nucleons Binding energy = 8. 790 Me. V/nucleon © 2013 Pearson Education, Inc.

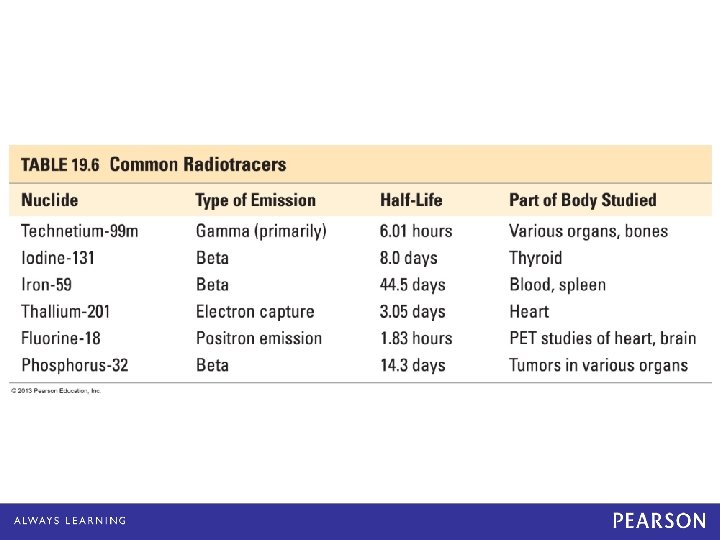
Uses of Nuclear Chemistry/Physics • Energy Geological/Archeological • Weaponry Miscellaneous » Smoke detectors • Medical • Food preservation • Security – Detection of explosives © 2013 Pearson Education, Inc.

Geological and Archeological Radioactive Dating • Mineral geology compares: – the amount of U-238 to Pb-206 or – The amount of K-40 to Ar-40 • Archaeological (past to present living materials) compares: – the amount of C-14 to C-12 • C-14 radioactive with half-life = 5730 yr – while substance living, C-14 © 2013 Pearson Education, Inc.

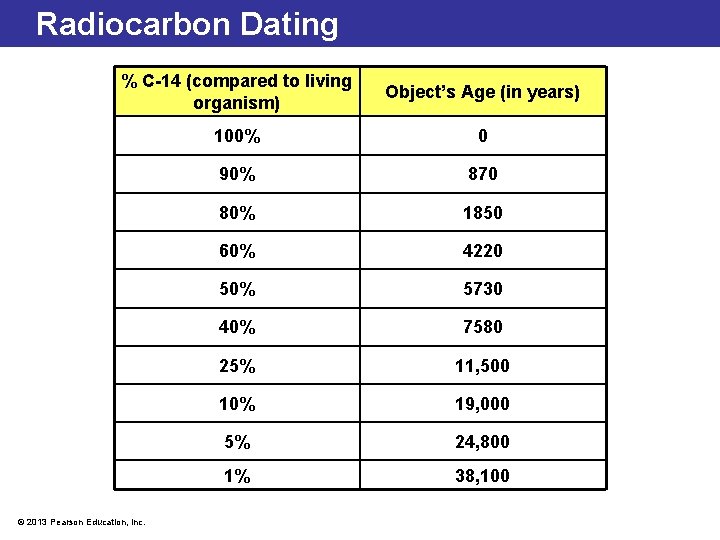
Radiocarbon Dating % C-14 (compared to living organism) Object’s Age (in years) 100% 0 90% 870 80% 1850 60% 4220 50% 5730 40% 7580 25% 11, 500 10% 19, 000 5% 24, 800 1% 38, 100 © 2013 Pearson Education, Inc.

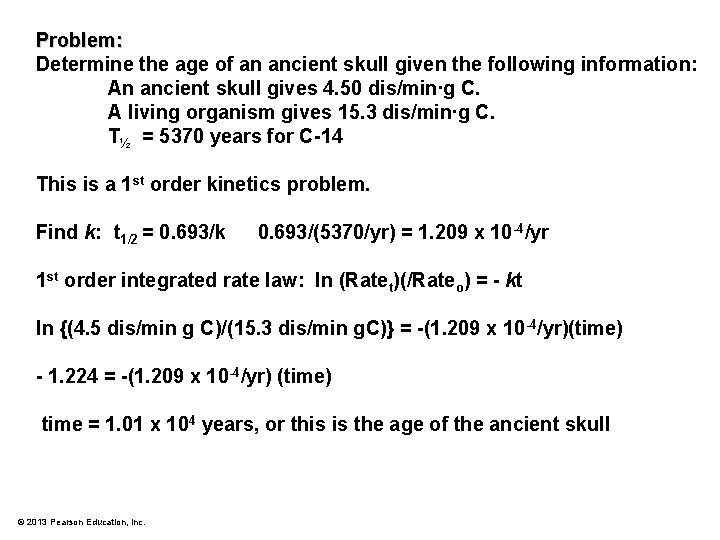
Problem: Determine the age of an ancient skull given the following information: An ancient skull gives 4. 50 dis/min∙g C. A living organism gives 15. 3 dis/min∙g C. t½ for C-14 = 5370 years © 2013 Pearson Education, Inc.

Problem: Determine the age of an ancient skull given the following information: An ancient skull gives 4. 50 dis/min∙g C. A living organism gives 15. 3 dis/min∙g C. T½ = 5370 years for C-14 This is a 1 st order kinetics problem. Find k: t 1/2 = 0. 693/k 0. 693/(5370/yr) = 1. 209 x 10 -4/yr 1 st order integrated rate law: ln (Ratet)(/Rateo) = - kt ln {(4. 5 dis/min g C)/(15. 3 dis/min g. C)} = -(1. 209 x 10 -4/yr)(time) - 1. 224 = -(1. 209 x 10 -4/yr) (time) time = 1. 01 x 104 years, or this is the age of the ancient skull © 2013 Pearson Education, Inc.

Nuclear Power © 2013 Pearson Education, Inc.

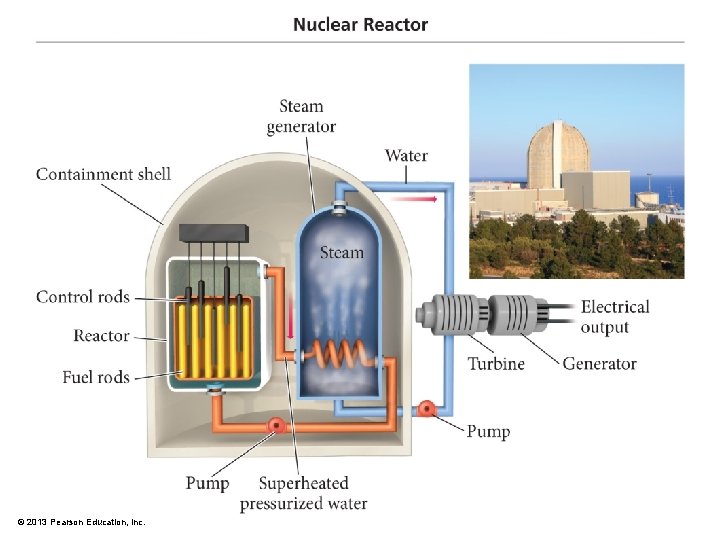
Basics of Nuclear Power Plant • The fissionable material is stored in long tubes, called fuel rods, arranged in a matrix. – Subcritical • Between the fuel rods are control rods made of neutron-absorbing material. – B and/or Cd – Neutrons needed to sustain the chain reaction • The rods are placed in a material to slow down the ejected neutrons, called a moderator. – Allows chain reaction to occur below critical mass © 2013 Pearson Education, Inc.

© 2013 Pearson Education, Inc.

Medical: Isotope Usage Criteria • Tissue or organ to be targeted – Size (thickness) • The more penetrating the ionization energy, the greater the effect. – Want to choose radionuclide that is specific to the tissue • Half-life length of: – Radionuclide – Biological half-life or element • Type of radiation emitted – Internal vs. external use of radiation • Physical state (solid, liquid) of radionuclide to be administered © 2013 Pearson Education, Inc.

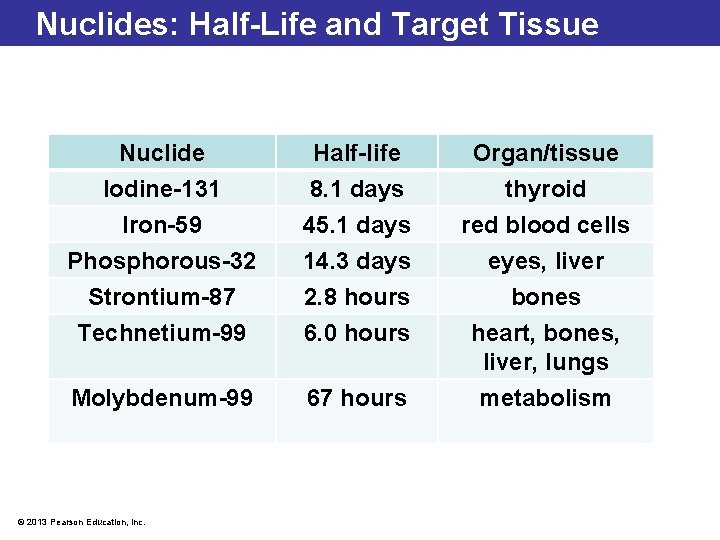
Nuclides: Half-Life and Target Tissue Nuclide Half-life Organ/tissue Iodine-131 8. 1 days thyroid Iron-59 45. 1 days red blood cells Phosphorous-32 14. 3 days eyes, liver Strontium-87 2. 8 hours bones Technetium-99 6. 0 hours heart, bones, liver, lungs Molybdenum-99 67 hours metabolism © 2013 Pearson Education, Inc.


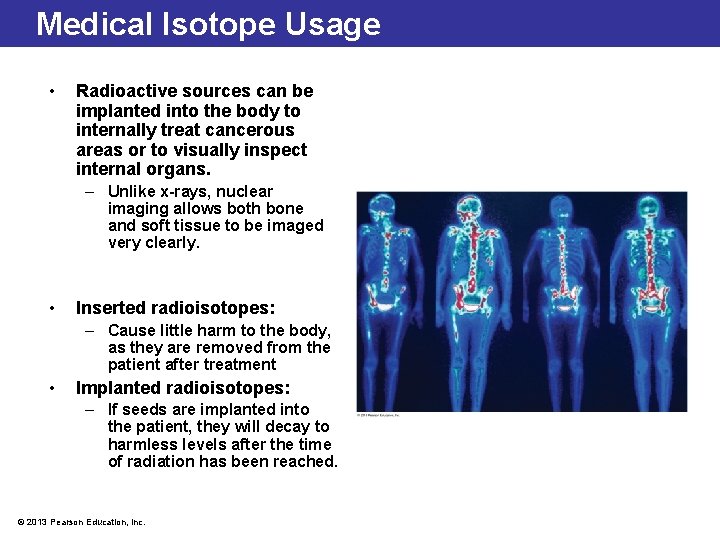
Medical Isotope Usage • Radioactive sources can be implanted into the body to internally treat cancerous areas or to visually inspect internal organs. – Unlike x-rays, nuclear imaging allows both bone and soft tissue to be imaged very clearly. • Inserted radioisotopes: – Cause little harm to the body, as they are removed from the patient after treatment • Implanted radioisotopes: – If seeds are implanted into the patient, they will decay to harmless levels after the time of radiation has been reached. © 2013 Pearson Education, Inc.

Biological Effects of Radiation • Ionizing radiation: – can break molecular bonds and/or – form radicals (molecules or ions that have had an electron “knock” out) – can damage biological molecules and cause malfunction of the cell • High levels of radiation over a short period of time: – can kill large numbers of cells – cause the immune system to weakened – decrease the cell’s ability to absorb nutrients from food • May result in death, usually from infection © 2013 Pearson Education, Inc.

Medical Use of Isotopes • • Biological dosages – REM (roentgen equivalent man): a weighed absorbed amount of radiation – RAD (radiation-absorbed amount) is measure of radiation absorbed by ONE gram of tissue • 1 REM = 1 RAD • A single dose of 500 REM or greater is fatal for most individuals. Biological effects – Nuclear emissions have enough energy to cause cellular damage. – High-energy radiation damage referred to as ionizing radiation • Radiation knocks an electron from biological molecule, forming a radical or ion molecule + radiation + electron – Radiation knocks electron from biological receptor molecule © 2013 Pearson Education, Inc.

Chronic Health Effects Caused by Exposure to Radiation • Low doses of radiation over a period of time show an increased risk for the development of cancer. – Radiation damages DNA that may not get repaired properly. • Low doses over time may damage reproductive organs, which may lead to sterility. • Damage to reproductive cells may lead to genetic defects in offspring. © 2013 Pearson Education, Inc.

Measuring Radiation Exposure: Units • The curie (Ci) is an exposure of 3. 7 × 1010 events per second. – Type of radiation not an issue • The gray (Gy) measures the amount of energy absorbed by body tissue from radiation. – 1 Gy = 1 J/kg body tissue • The rad also measures the amount of energy absorbed by body tissue from radiation. – 1 rad = 0. 01 Gy • A correction factor is used to account for a number of factors that affect the result of the exposure—this biological effectiveness factor is the RBE, and the result is the dose in rems. – rads × RBE = rems – rem = roentgen equivalent man © 2013 Pearson Education, Inc.

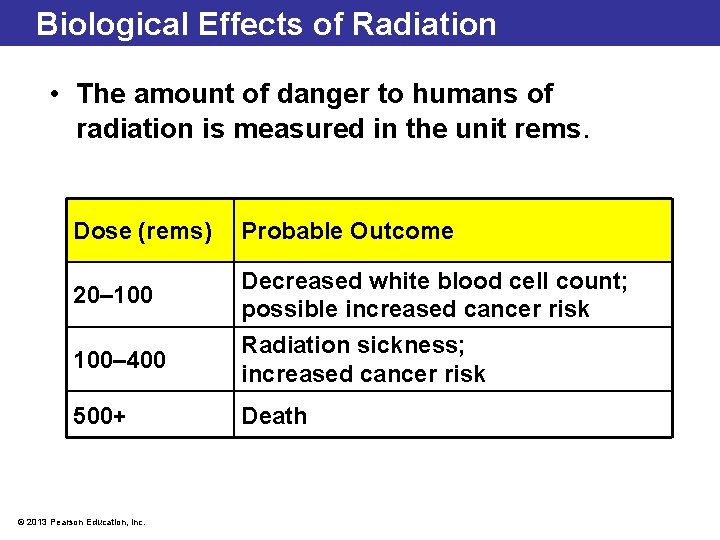
Biological Effects of Radiation • The amount of danger to humans of radiation is measured in the unit rems. Dose (rems) Probable Outcome 20– 100 Decreased white blood cell count; possible increased cancer risk 100– 400 Radiation sickness; increased cancer risk 500+ Death © 2013 Pearson Education, Inc.

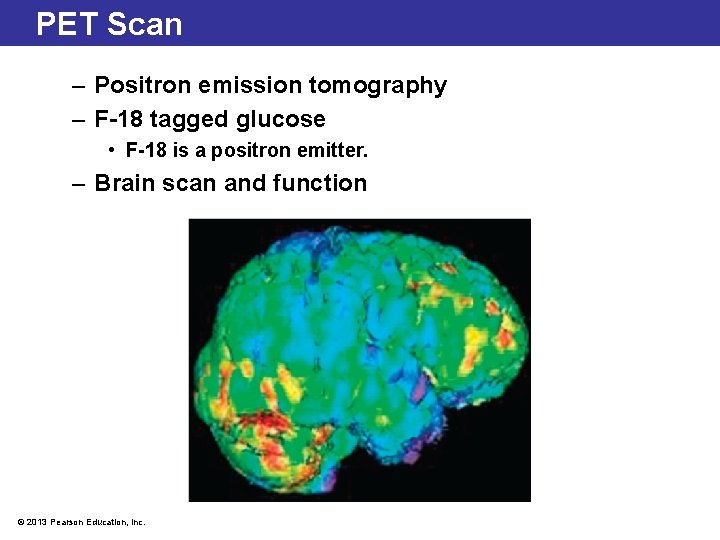
PET Scan – Positron emission tomography – F-18 tagged glucose • F-18 is a positron emitter. – Brain scan and function © 2013 Pearson Education, Inc.