In the time that it takes the music














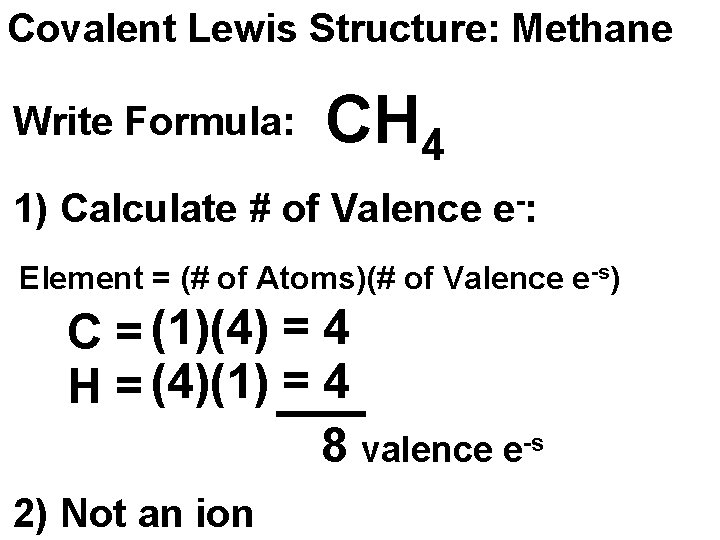






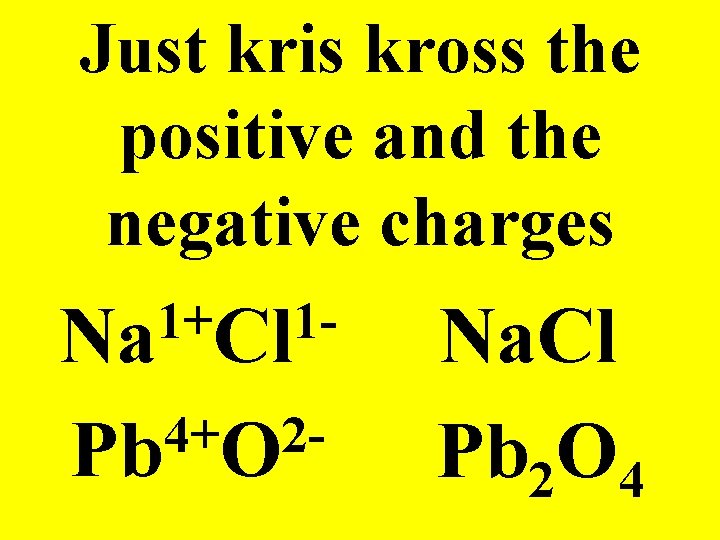


- Slides: 50

In the time that it takes the music to play…write as much as you know about bonds… Compound with the most correct wins!

What determines the type of bond that forms?

ELECTRONEGATIVITY The tendency of an atom to attract electrons to itself when it is bonded to another atom

BOND STRENGTH the energy needed to break the bonds between atoms in a compound

Bond Strength The greater the difference in electronegativities, the greater the bond strength

Misconception Alert! It does not require energy to make bonds. It requires energy to break bonds. “Breaking up is hard to do”

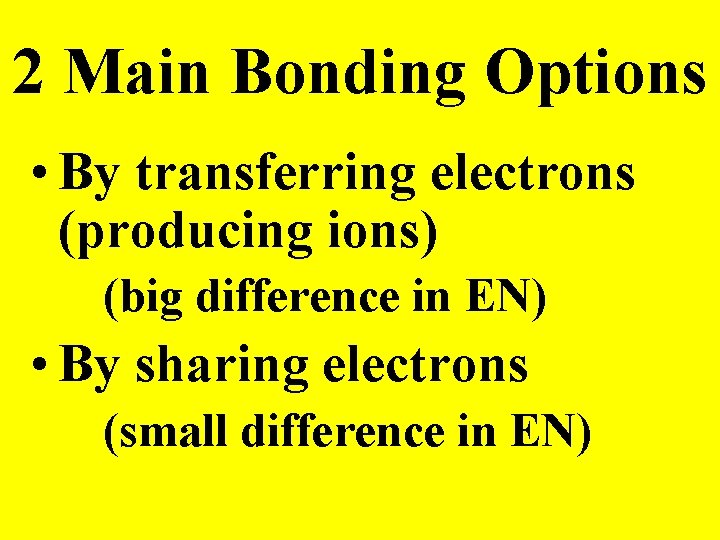
2 Main Bonding Options • By transferring electrons (producing ions) (big difference in EN) • By sharing electrons (small difference in EN)

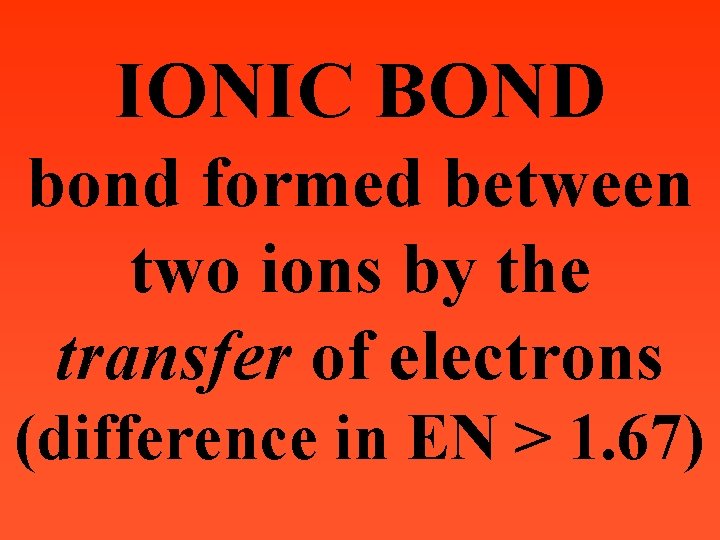
IONIC BOND bond formed between two ions by the transfer of electrons (difference in EN > 1. 67)

IONIC COMPOUND substance formed when electrons are transferred between 2 or more substances (making ions)

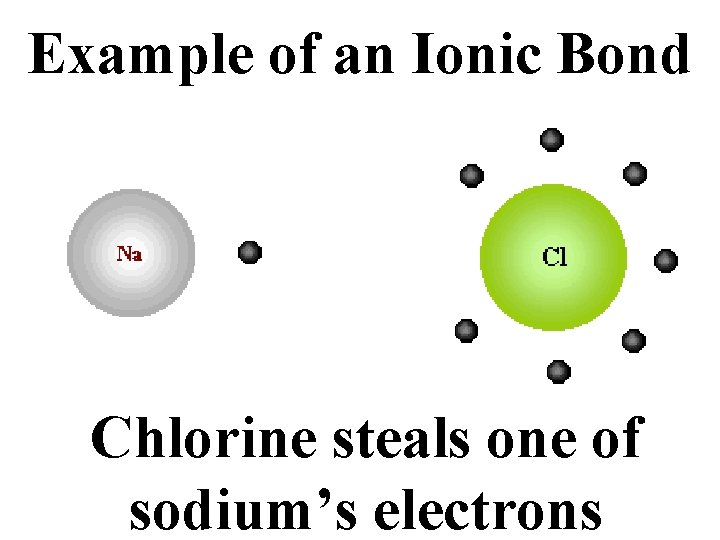
Example of an Ionic Bond Chlorine steals one of sodium’s electrons

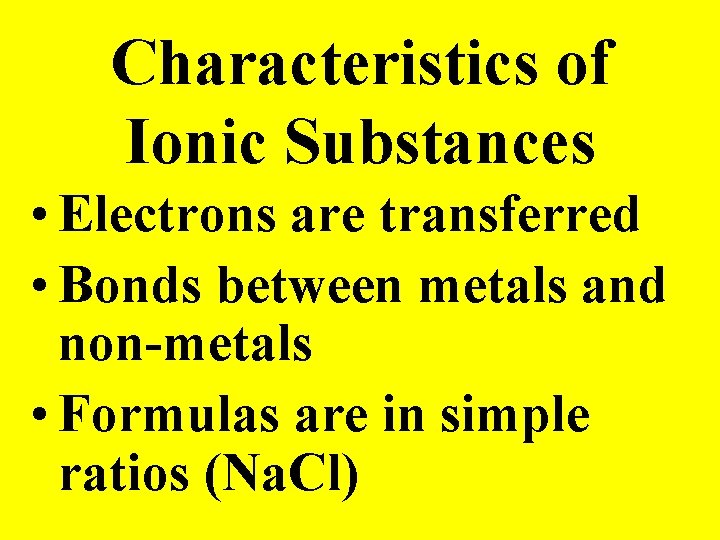
Characteristics of Ionic Substances • Electrons are transferred • Bonds between metals and non-metals • Formulas are in simple ratios (Na. Cl)

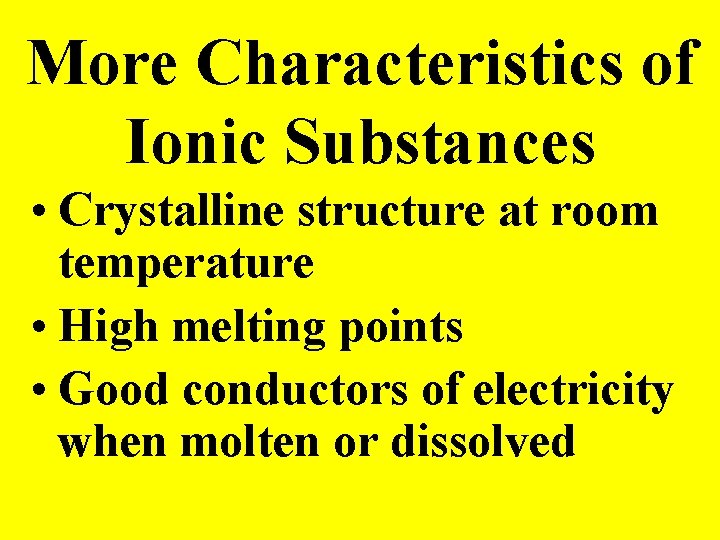
More Characteristics of Ionic Substances • Crystalline structure at room temperature • High melting points • Good conductors of electricity when molten or dissolved

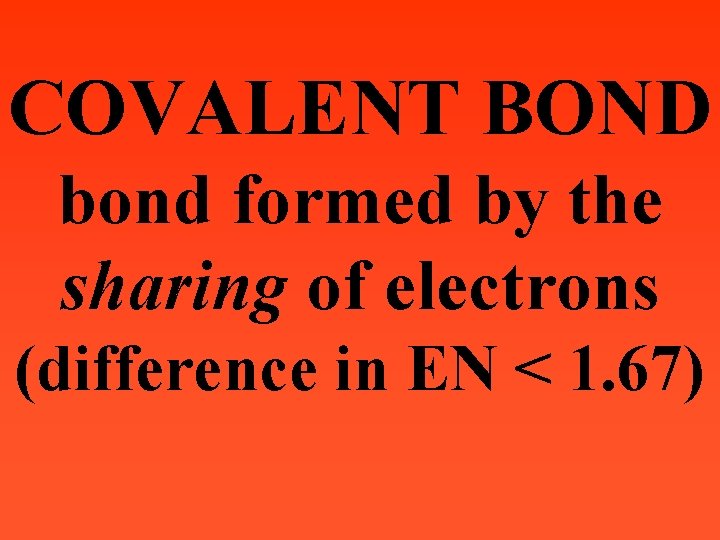
COVALENT BOND bond formed by the sharing of electrons (difference in EN < 1. 67)

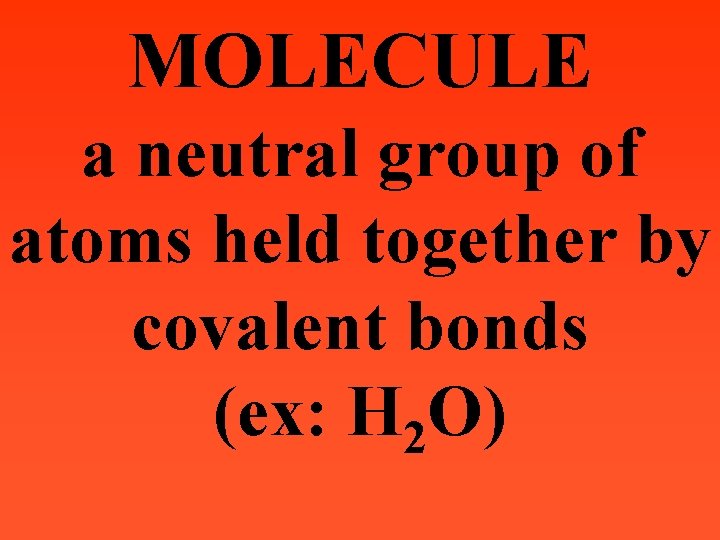
MOLECULE a neutral group of atoms held together by covalent bonds (ex: H 2 O)

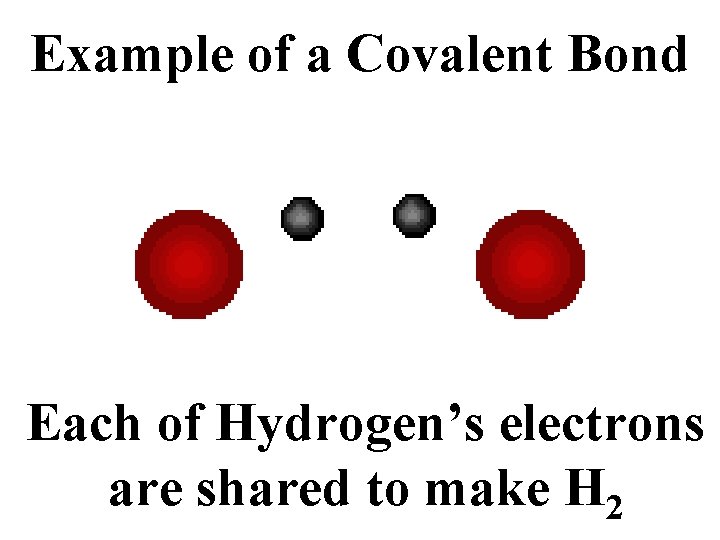
Example of a Covalent Bond Each of Hydrogen’s electrons are shared to make H 2

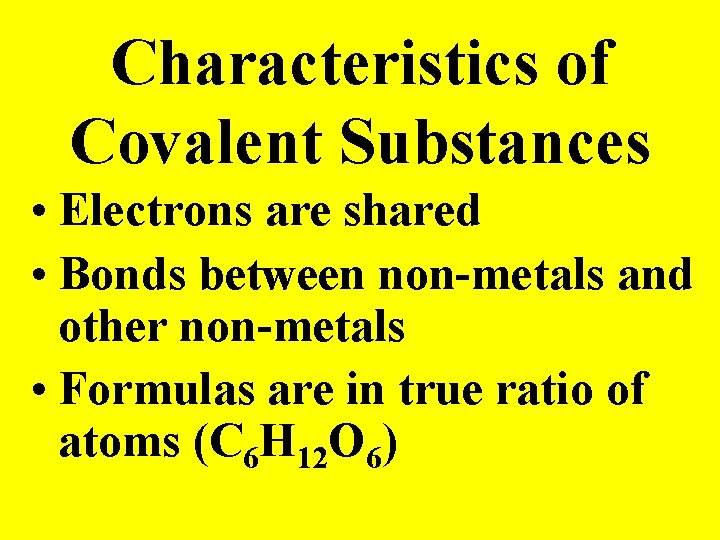
Characteristics of Covalent Substances • Electrons are shared • Bonds between non-metals and other non-metals • Formulas are in true ratio of atoms (C 6 H 12 O 6)

More Characteristics of Ionic Substances • Substances may exist in any state of matter at room temperature • Low melting points • Nonconductors of electricity

Bonds in all the polyatomic ions and diatomics are all covalent bonds

CONDUCTIVITY The ability to conduct an electrical current

Can we use conductivity to determine if a substance is ionic or covalent?

WHICH IS STRONGER? Ionic bonds are stronger than covalent

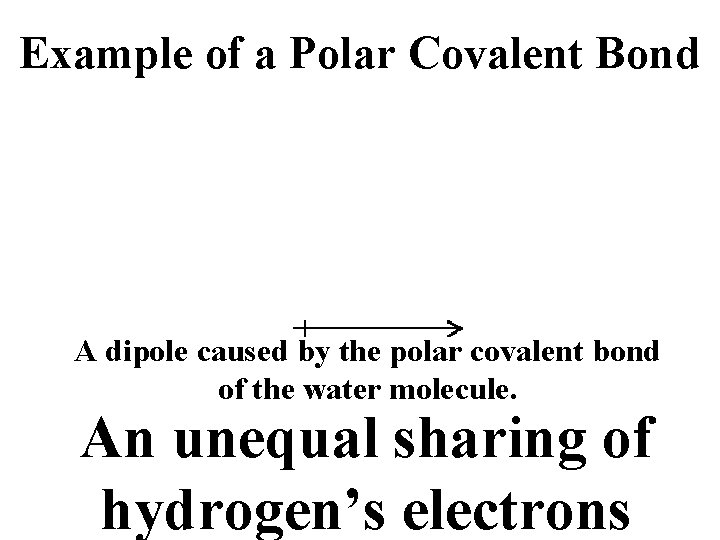
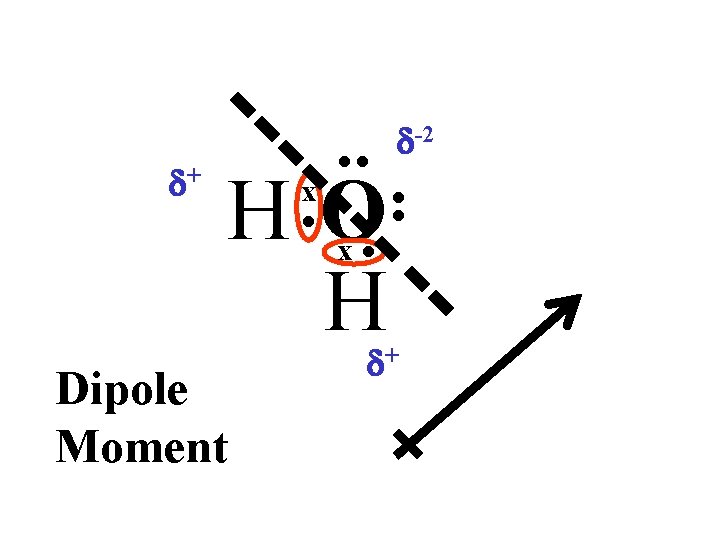
POLAR COVALENT BONDS when electrons are shared but shared unequally H 2 O

Example of a Polar Covalent Bond A dipole caused by the polar covalent bond of the water molecule. An unequal sharing of hydrogen’s electrons

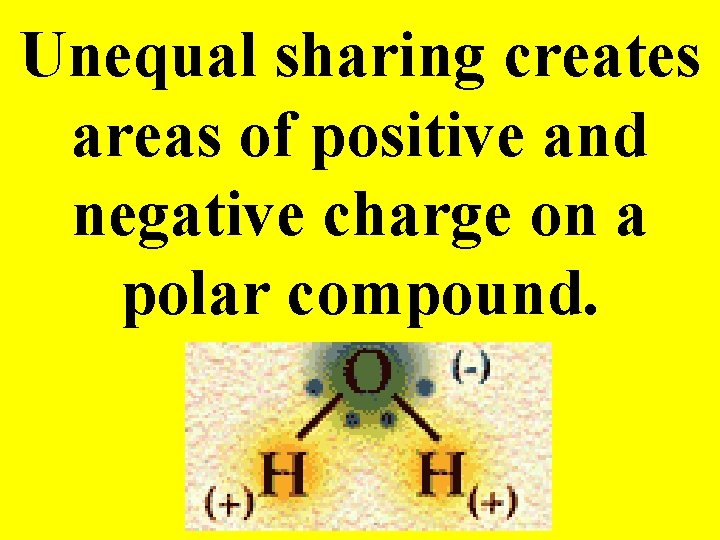
Unequal sharing creates areas of positive and negative charge on a polar compound.

d+ • • H • O • H x x Dipole Moment d-2 • • d+

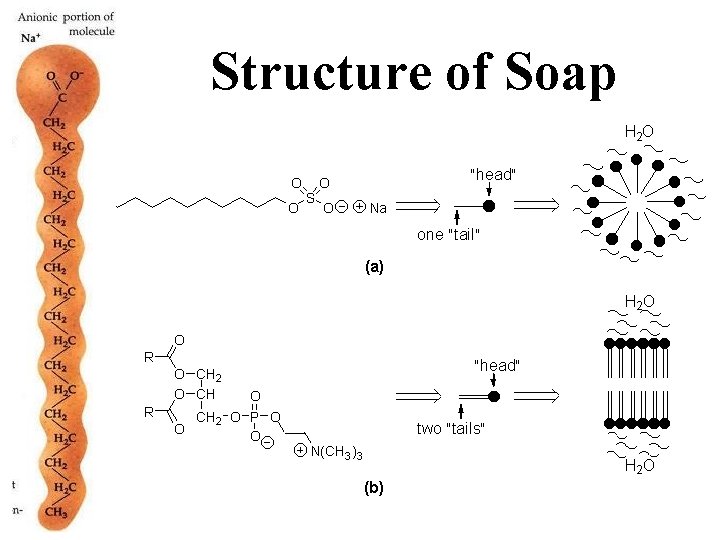
Structure of Soap

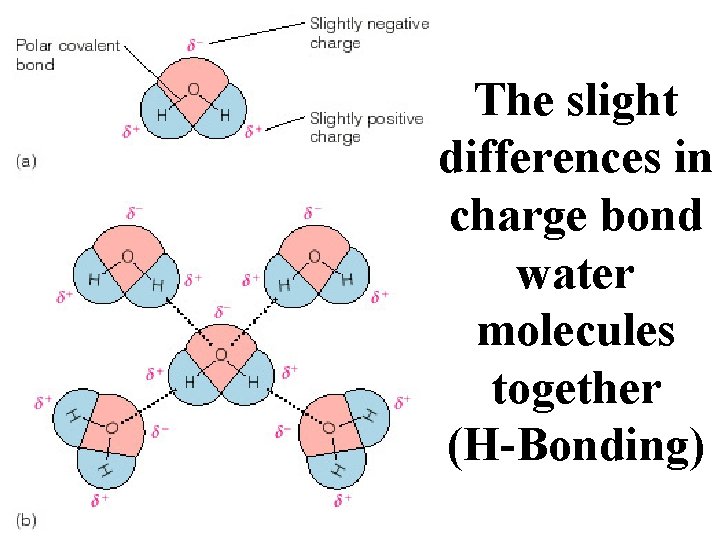
The slight differences in charge bond water molecules together (H-Bonding)

HOMEWORK Do problems 3 -4 on page 306 of the text. Pg. 304 #1, 2

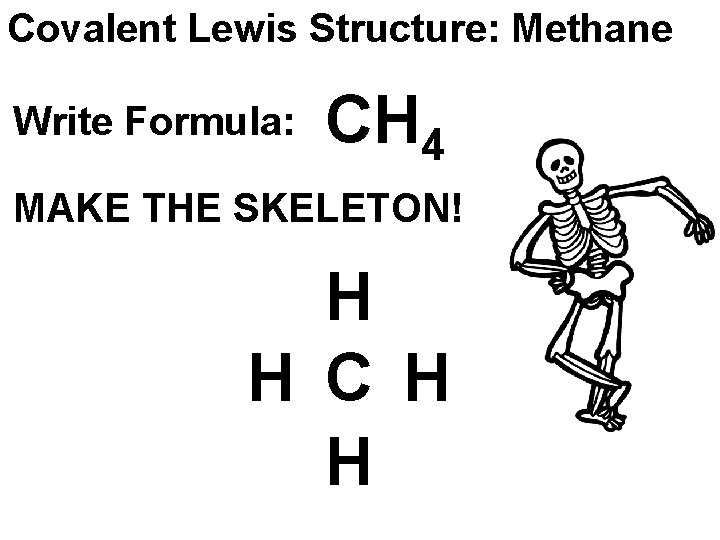
Covalent Lewis Structure: Methane Write Formula: CH 4 MAKE THE SKELETON! H H C H H
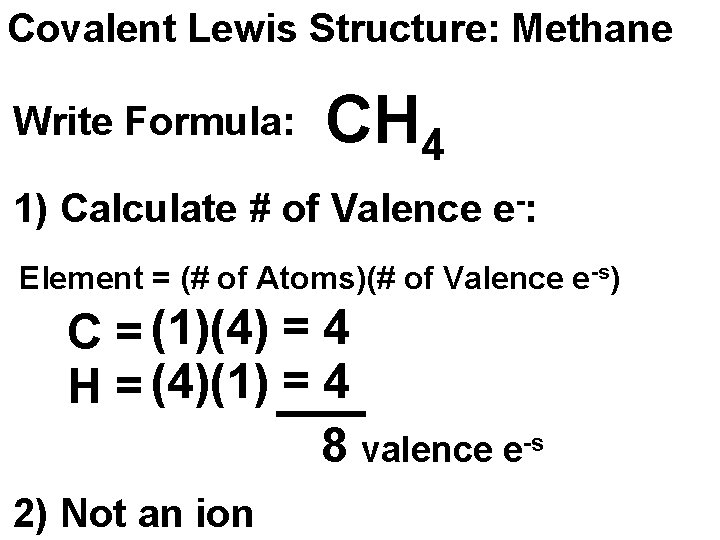
Covalent Lewis Structure: Methane Write Formula: CH 4 1) Calculate # of Valence e-: Element = (# of Atoms)(# of Valence e-s) C = (1)(4) = 4 H = (4)(1) = 4 8 valence e-s 2) Not an ion

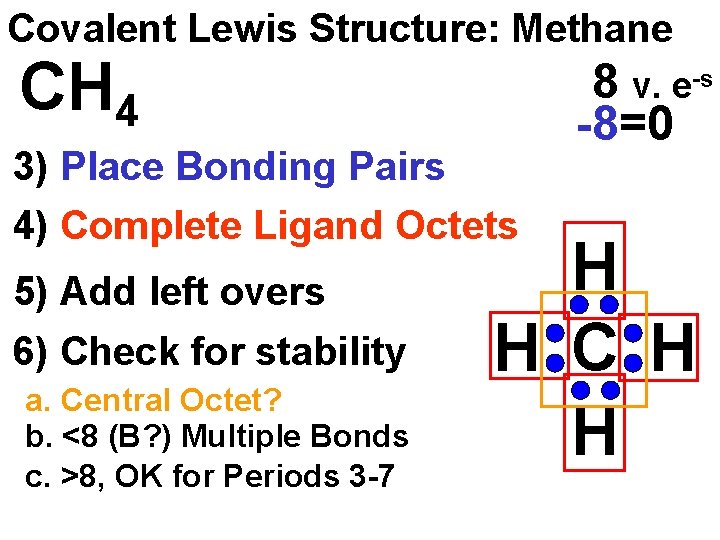
Covalent Lewis Structure: Methane CH 4 3) Place Bonding Pairs 4) Complete Ligand Octets 5) Add left overs 6) Check for stability a. Central Octet? b. <8 (B? ) Multiple Bonds c. >8, OK for Periods 3 -7 8 v. e-s -8=0 H H C H H

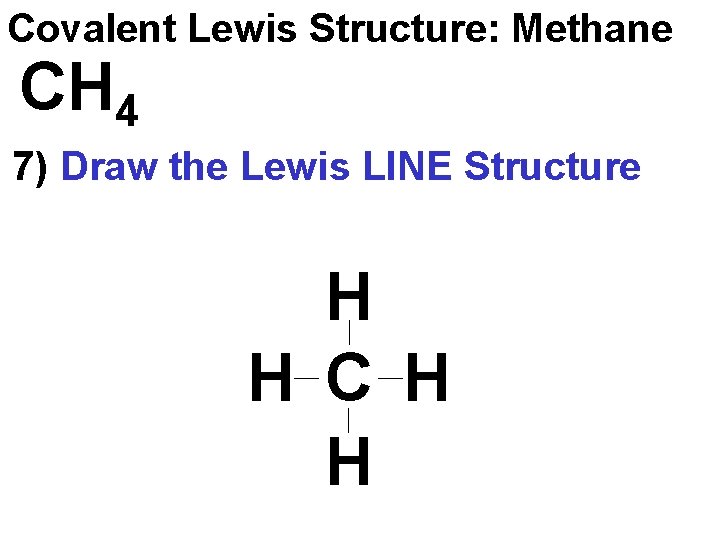
Covalent Lewis Structure: Methane CH 4 7) Draw the Lewis LINE Structure H H C H H

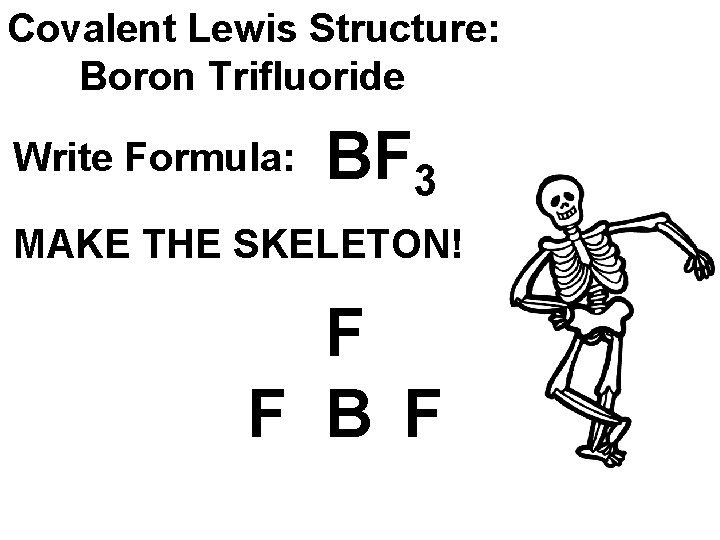
Covalent Lewis Structure: Boron Trifluoride Write Formula: BF 3 MAKE THE SKELETON! F F B F

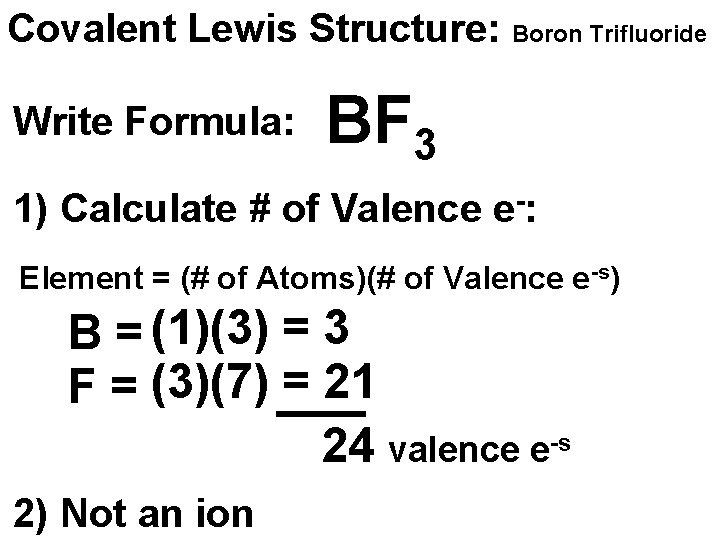
Covalent Lewis Structure: Boron Trifluoride Write Formula: BF 3 1) Calculate # of Valence e-: Element = (# of Atoms)(# of Valence e-s) B = (1)(3) = 3 F = (3)(7) = 21 24 valence e-s 2) Not an ion

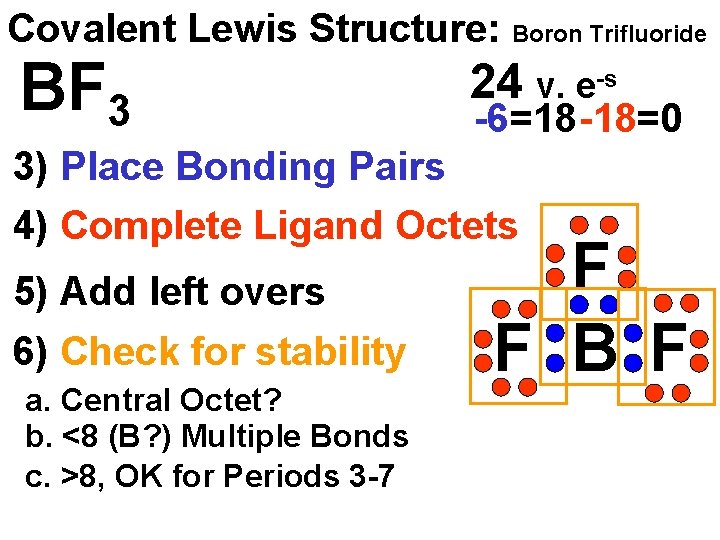
Covalent Lewis Structure: Boron Trifluoride BF 3 24 v. e-s -6=18 -18=0 3) Place Bonding Pairs 4) Complete Ligand Octets 5) Add left overs 6) Check for stability a. Central Octet? b. <8 (B? ) Multiple Bonds c. >8, OK for Periods 3 -7 F F B F

CARBON BONDS Carbon makes LOTS of bonds…but never more than 4


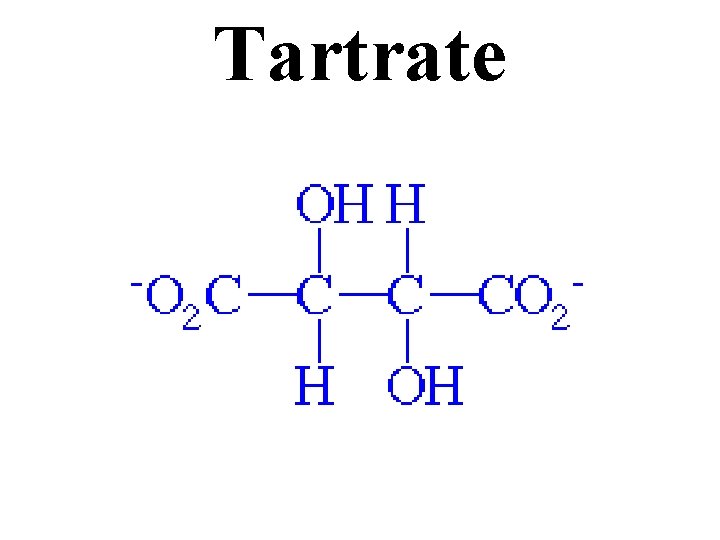
Tartrate


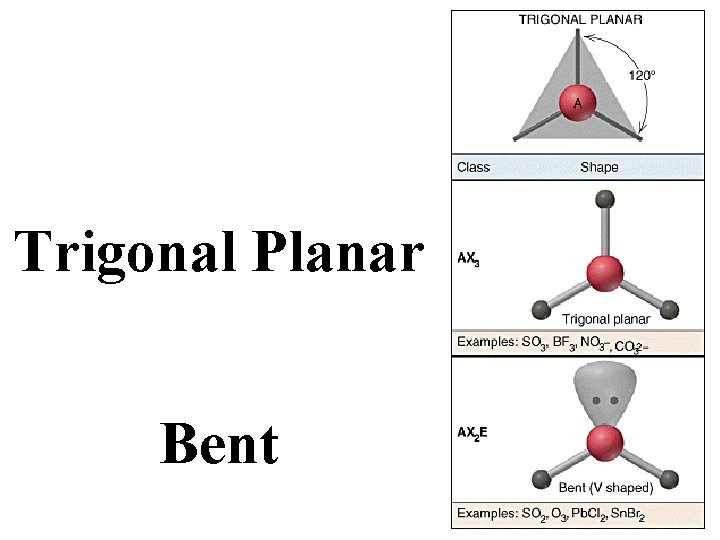
Trigonal Planar Bent

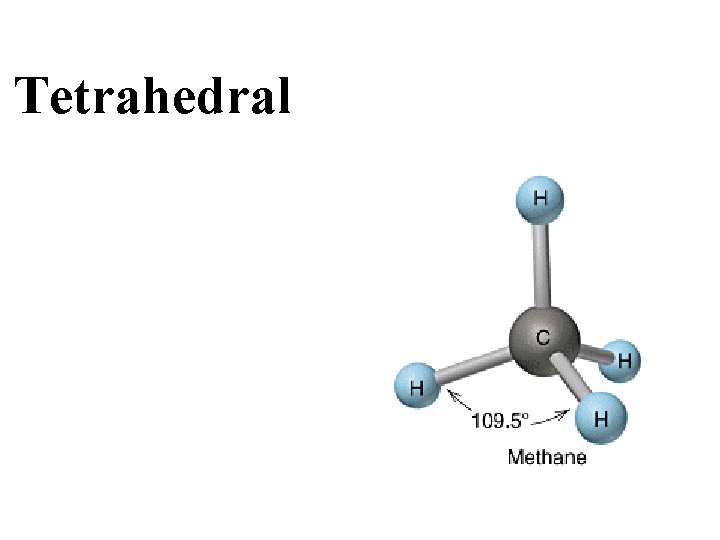
Tetrahedral

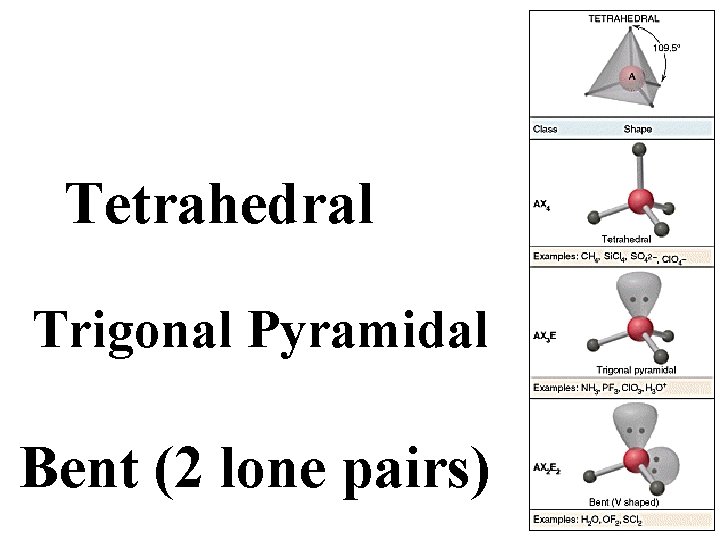
Tetrahedral Trigonal Pyramidal Bent (2 lone pairs)

IONIC COMPOUND substance formed when electrons are transferred between 2 or more substances (making ions)

How do you name ionic compounds?

How do you write ionic compounds?

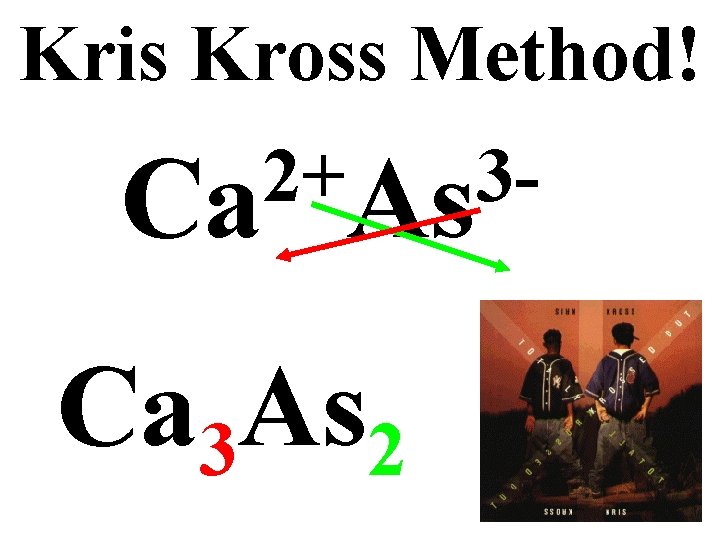
Kris Kross Method! 2+ 3 Ca As Ca 3 As 2
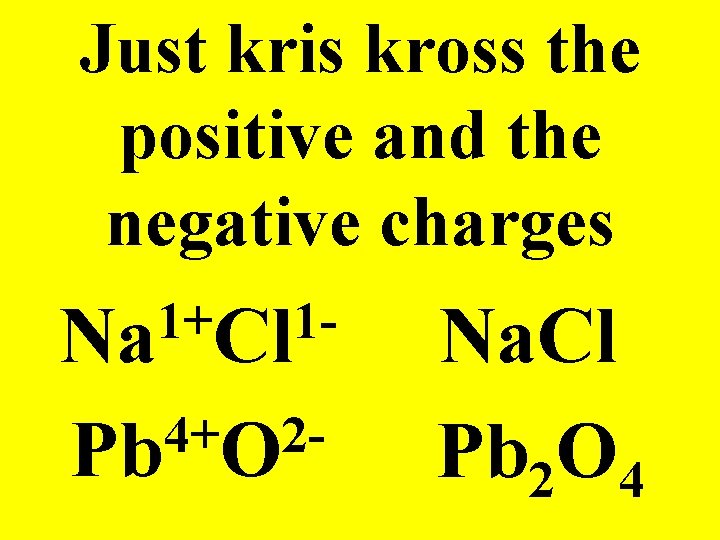
Just kris kross the positive and the negative charges 1+ 1 Na Cl 4+ 2 Pb O Na. Cl Pb 2 O 4

Which charge ALWAYS comes first? POSITIVE!!!

Are you sure?

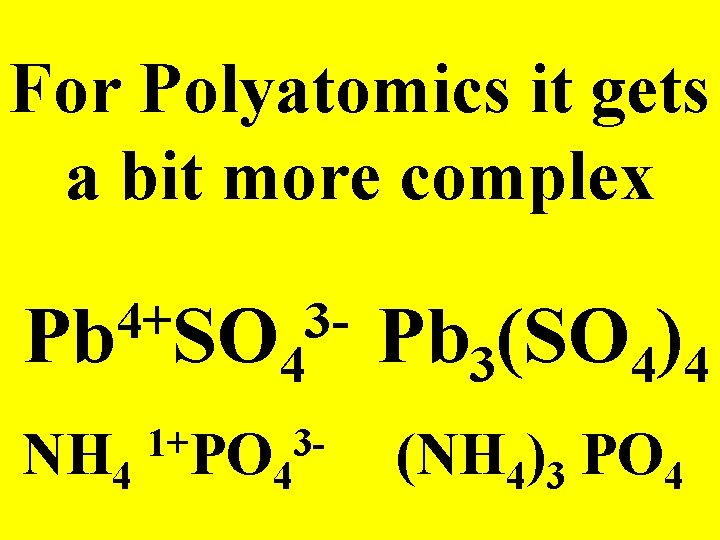
For Polyatomics it gets a bit more complex 4+ 3 Pb SO 4 1+ 3 NH 4 PO 4 Pb 3(SO 4)4 (NH 4)3 PO 4
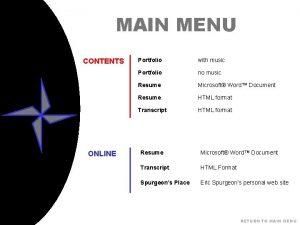
 Online music portfolio
Online music portfolio Phân độ lown
Phân độ lown Block xoang nhĩ độ 2
Block xoang nhĩ độ 2 Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Walmart thất bại ở nhật
Walmart thất bại ở nhật Tìm vết của đường thẳng
Tìm vết của đường thẳng Con hãy đưa tay khi thấy người vấp ngã
Con hãy đưa tay khi thấy người vấp ngã Tôn thất thuyết là ai
Tôn thất thuyết là ai Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Tucker turtle technique
Tucker turtle technique Tuck the turtle
Tuck the turtle When and where the story takes place
When and where the story takes place Tucker turtle
Tucker turtle Tucker turtle takes time to tuck and think
Tucker turtle takes time to tuck and think Tucker turtle takes time to tuck and think at home
Tucker turtle takes time to tuck and think at home What is the turtle technique
What is the turtle technique Emotional competence
Emotional competence Classical music vs romantic
Classical music vs romantic It refers to the number of individual musical lines
It refers to the number of individual musical lines What music that employs electronic music?
What music that employs electronic music? Ilocano name for the wind instrument
Ilocano name for the wind instrument What is elapsed time
What is elapsed time When a melodic idea is presented by one voice
When a melodic idea is presented by one voice Time periods of music
Time periods of music The first time forever
The first time forever Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Chụp tư thế worms-breton
Chụp tư thế worms-breton Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Môn thể thao bắt đầu bằng từ chạy
Môn thể thao bắt đầu bằng từ chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính thế năng
Công thức tính thế năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thế nào là giọng cùng tên? *
Thế nào là giọng cùng tên? * Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể