Chemistry and Chemical Reactivity 6 th Edition 1












- Slides: 60

Chemistry and Chemical Reactivity 6 th Edition 1 John C. Kotz Paul M. Treichel Gabriela C. Weaver CHAPTER 3 Molecules, Ions and Their Compounds Lectures written by John Kotz © 2006 Brooks/Cole Thomson © 2006 Brooks/Cole - Thomson

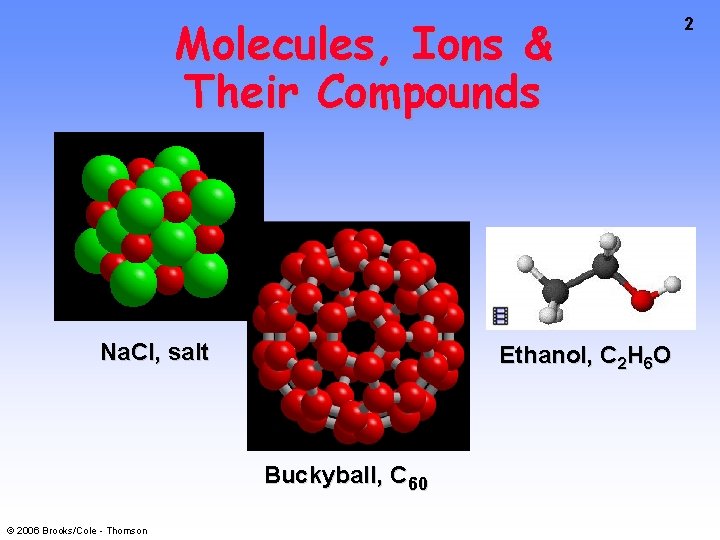
Molecules, Ions & Their Compounds Na. Cl, salt Ethanol, C 2 H 6 O Buckyball, C 60 © 2006 Brooks/Cole - Thomson 2

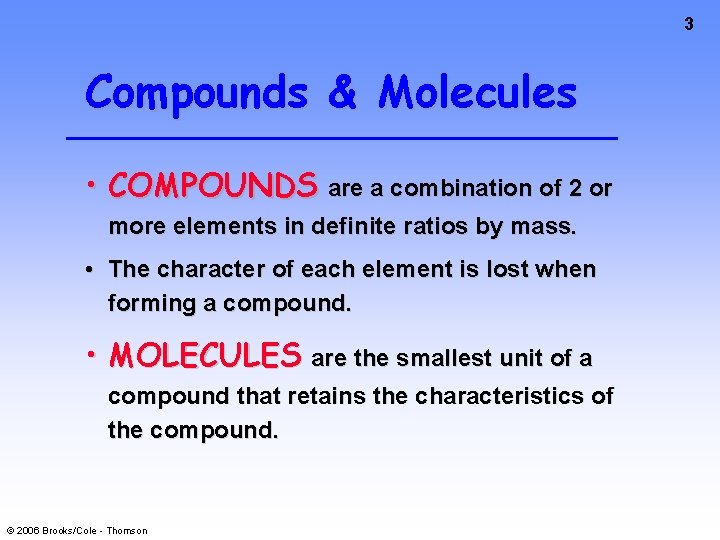
3 Compounds & Molecules • COMPOUNDS are a combination of 2 or more elements in definite ratios by mass. • The character of each element is lost when forming a compound. • MOLECULES are the smallest unit of a compound that retains the characteristics of the compound. © 2006 Brooks/Cole - Thomson

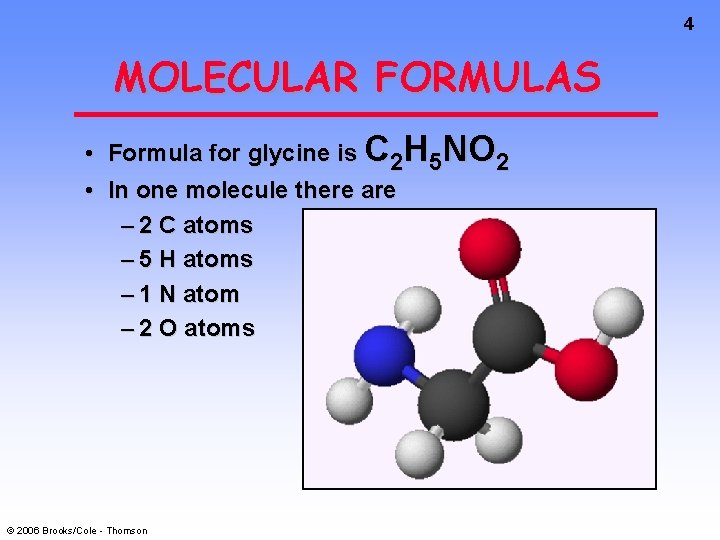
4 MOLECULAR FORMULAS • Formula for glycine is C 2 H 5 NO 2 • In one molecule there are – 2 C atoms – 5 H atoms – 1 N atom – 2 O atoms © 2006 Brooks/Cole - Thomson

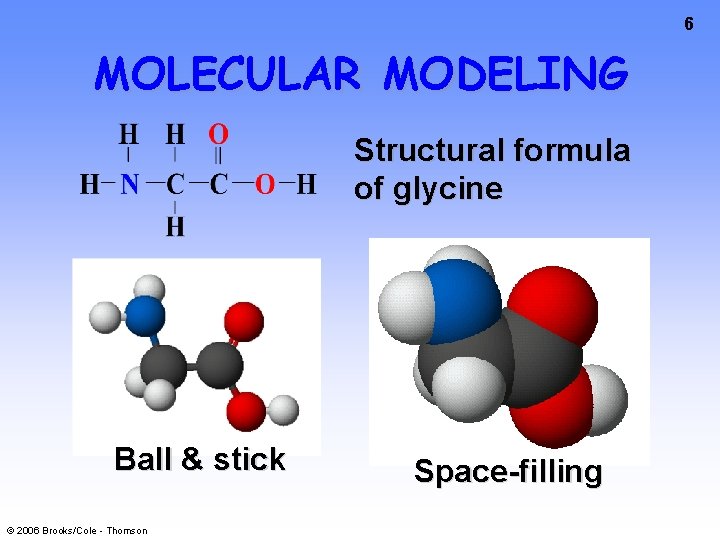
5 WRITING FORMULAS • Can also write glycine formula as –H 2 NCH 2 COOH to show atom ordering • or in the form of a structural © 2006 Brooks/Cole - Thomson formula

6 MOLECULAR MODELING Structural formula of glycine Ball & stick © 2006 Brooks/Cole - Thomson Space-filling

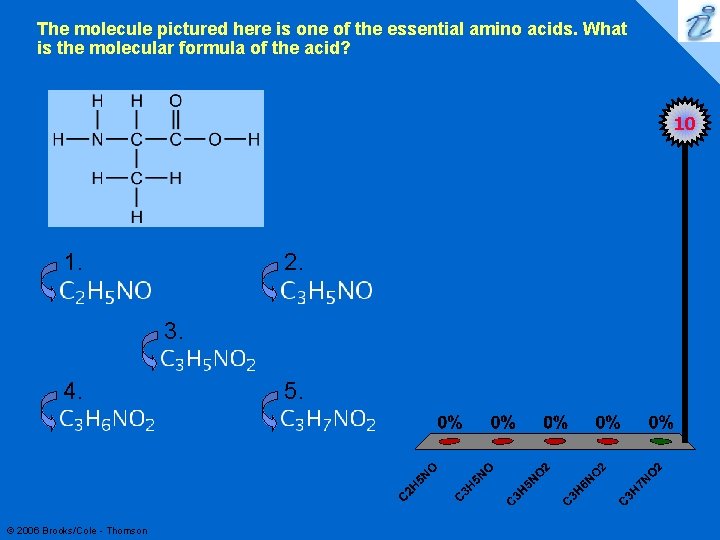
The molecule pictured here is one of the essential amino acids. What is the molecular formula of the acid? 7 10 1. 2. 3. 4. © 2006 Brooks/Cole - Thomson 5.

8 MOLECULAR WEIGHT AND MOLAR MASS Molecular weight = sum of the atomic weights of all atoms in the molecule. Molar mass = molecular weight in grams © 2006 Brooks/Cole - Thomson

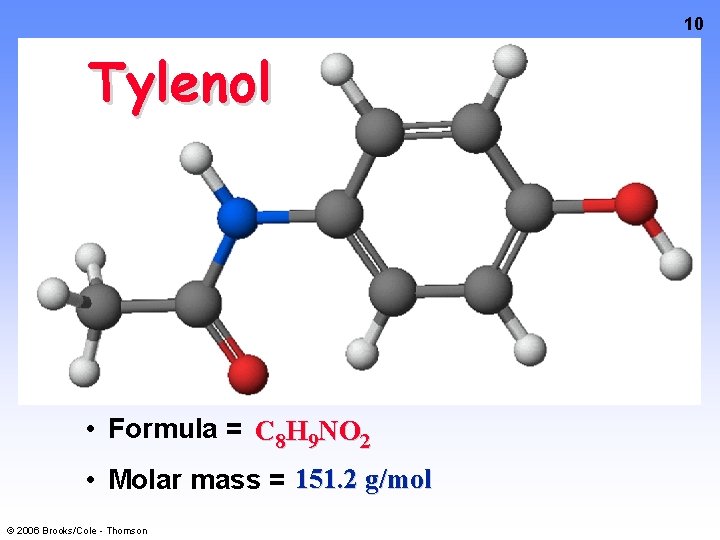
10 Tylenol • Formula = C 8 H 9 NO 2 • Molar mass = 151. 2 g/mol © 2006 Brooks/Cole - Thomson

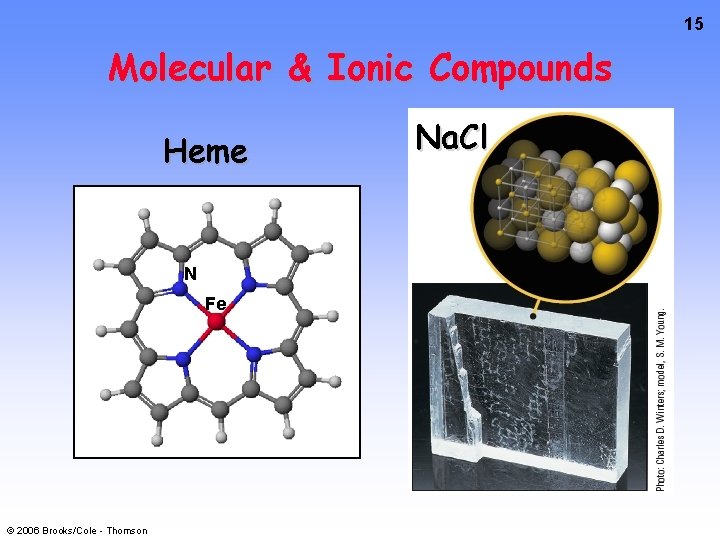
15 Molecular & Ionic Compounds Heme N Fe © 2006 Brooks/Cole - Thomson Na. Cl

16 ELEMENTS THAT EXIST AS MOLECULES Allotropes of C © 2006 Brooks/Cole - Thomson

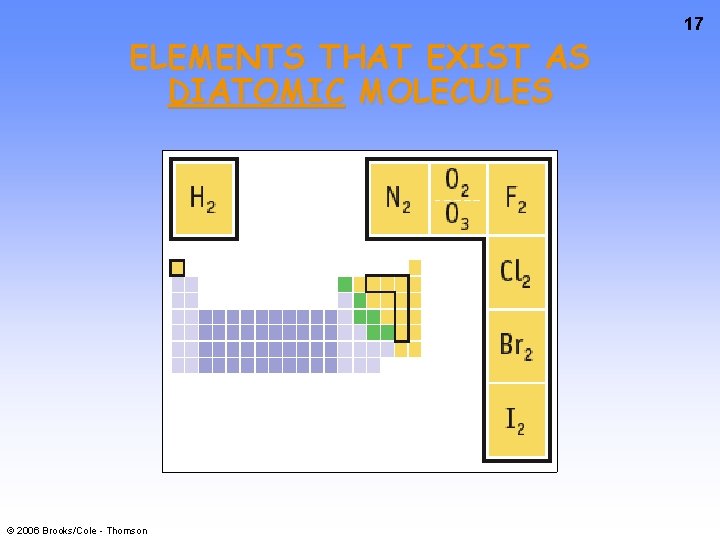
ELEMENTS THAT EXIST AS DIATOMIC MOLECULES © 2006 Brooks/Cole - Thomson 17

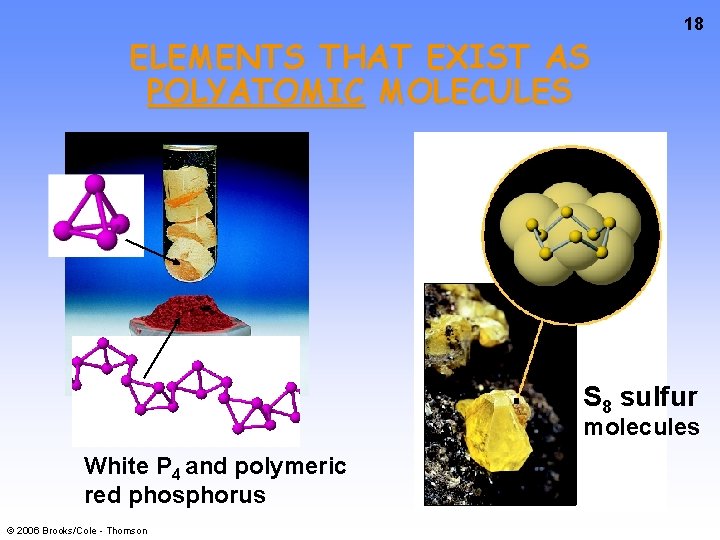
ELEMENTS THAT EXIST AS POLYATOMIC MOLECULES 18 S 8 sulfur molecules White P 4 and polymeric red phosphorus © 2006 Brooks/Cole - Thomson

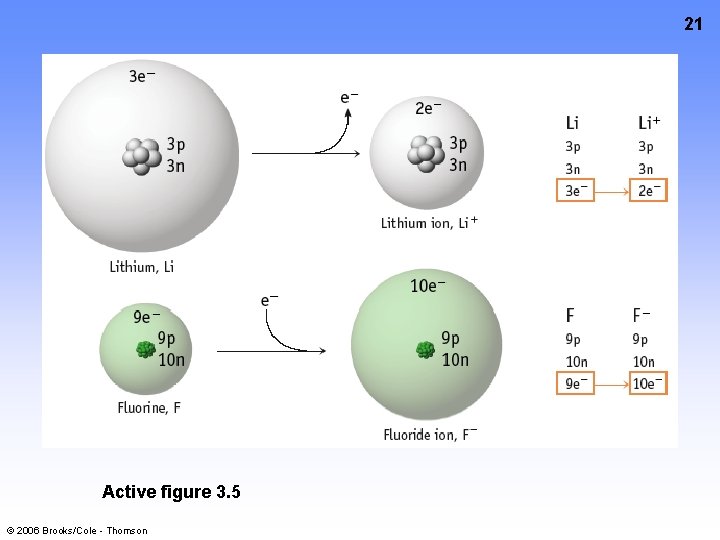
19 IONS AND IONIC COMPOUNDS see Screen 3. 5 • IONS are atoms or groups of atoms with a positive or negative charge. • Taking away an electron from an atom gives a CATION with a positive charge • Adding an electron to an atom gives an ANION with a negative charge. © 2006 Brooks/Cole - Thomson

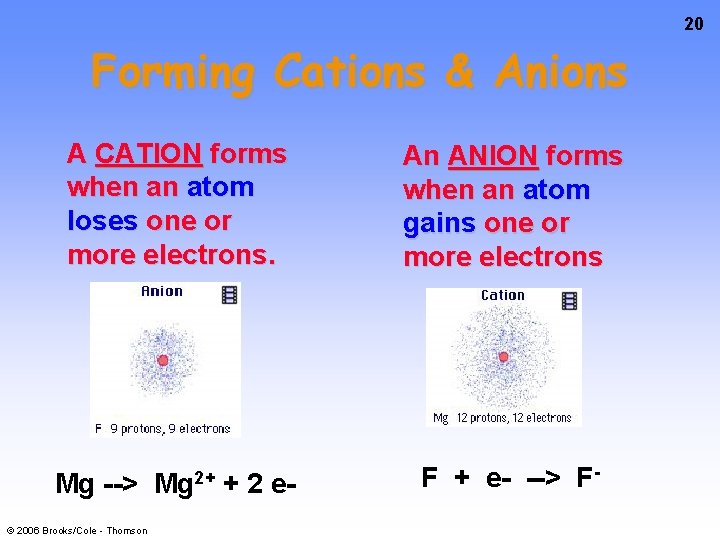
20 Forming Cations & Anions A CATION forms when an atom loses one or more electrons. An ANION forms when an atom gains one or more electrons Mg 2+ F + e- --> F- Mg --> © 2006 Brooks/Cole - Thomson + 2 e-

21 Active figure 3. 5 © 2006 Brooks/Cole - Thomson

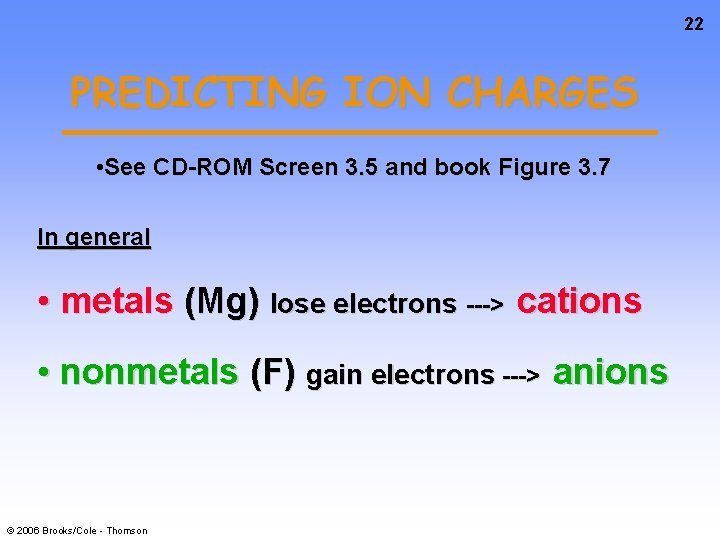
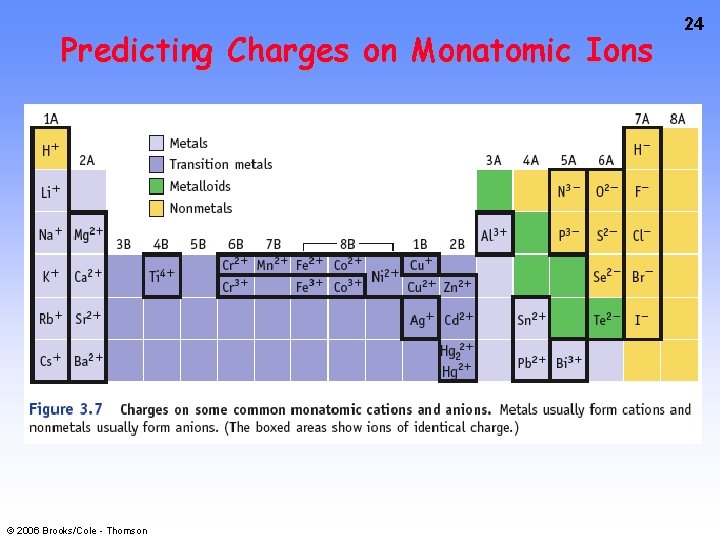
22 PREDICTING ION CHARGES • See CD-ROM Screen 3. 5 and book Figure 3. 7 In general • metals (Mg) lose electrons ---> cations • nonmetals (F) gain electrons ---> anions © 2006 Brooks/Cole - Thomson

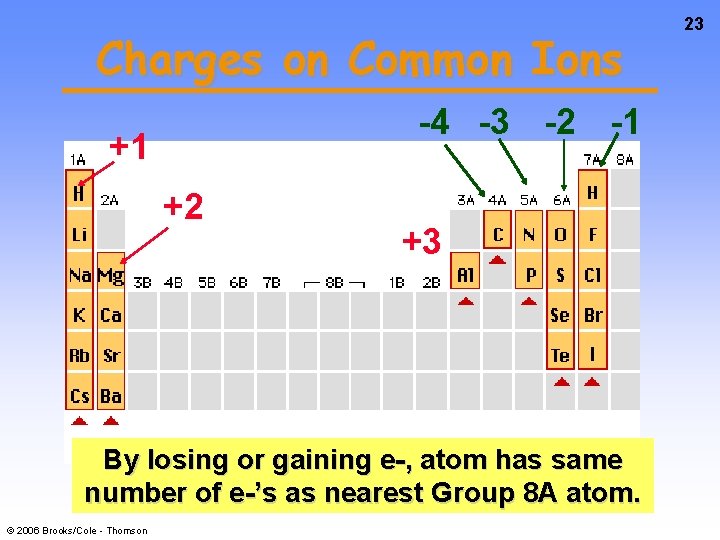
Charges on Common Ions -4 -3 -2 -1 +1 +2 +3 By losing or gaining e-, atom has same number of e-’s as nearest Group 8 A atom. © 2006 Brooks/Cole - Thomson 23

Predicting Charges on Monatomic Ions © 2006 Brooks/Cole - Thomson 24

Which ion in the following list is NOT likely to form? 1. 2. 3. 4. © 2006 Brooks/Cole - Thomson 5. 25

26 1. 2. 3. 4. 5. © 2006 Brooks/Cole - Thomson 25 protons and 25 electrons. 25 protons and 26 electrons. 25 protons and 24 electrons. 26 protons and 25 electrons. 26 protons and 26 electrons.

METALS M ---> n e- + Mn+ where n = periodic group Na+ sodium ion Mg 2+ magnesium ion Al 3+ aluminum ion Transition metals --> M 2+ or M 3+ are common Fe 2+ iron(II) ion Fe 3+ iron(III) ion © 2006 Brooks/Cole - Thomson 27

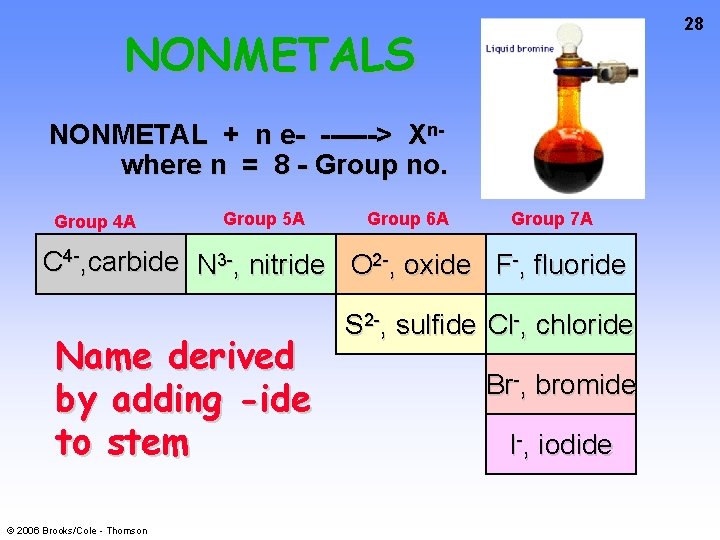
28 NONMETALS NONMETAL + n e- ------> Xnwhere n = 8 - Group no. Group 4 A Group 5 A Group 6 A Group 7 A C 4 -, carbide N 3 -, nitride O 2 -, oxide F-, fluoride Name derived by adding -ide to stem © 2006 Brooks/Cole - Thomson S 2 -, sulfide Cl-, chloride Br-, bromide I-, iodide

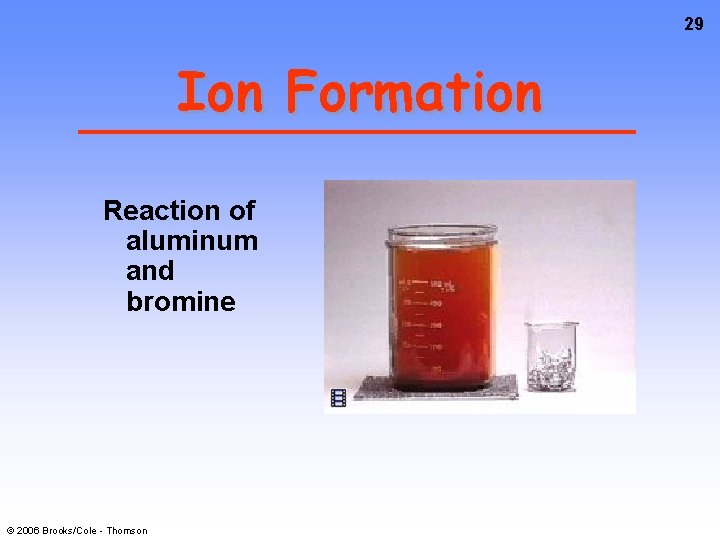
29 Ion Formation Reaction of aluminum and bromine © 2006 Brooks/Cole - Thomson

COMPOUNDS FORMED FROM IONS 30 CATION + ANION ---> COMPOUND Na+ + Cl- --> Na. Cl A neutral compd. requires equal number of + and - charges. © 2006 Brooks/Cole - Thomson

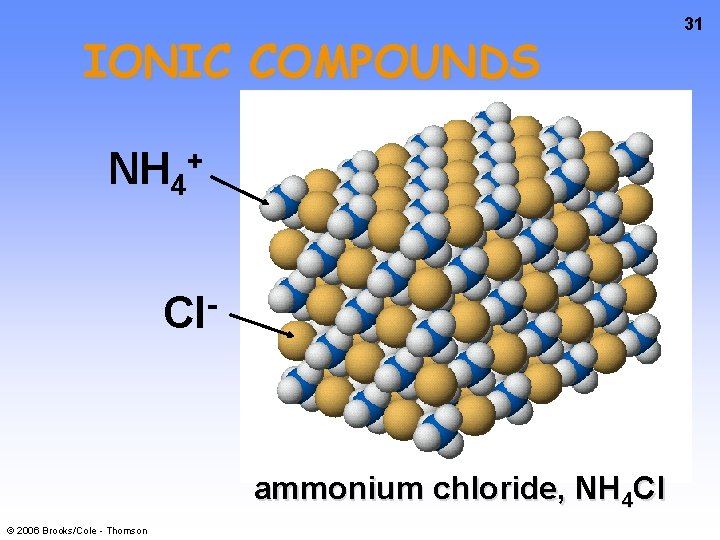
IONIC COMPOUNDS NH 4 + Cl ammonium chloride, NH 4 Cl © 2006 Brooks/Cole - Thomson 31

Some Ionic Compounds Ca 2+ + 2 F- ---> Ca. F 2 Mg 2+ + NO 3 - ----> Mg(NO 3)2 magnesium nitrate Fe 2+ + PO 43 - ----> calcium fluoride Fe 3(PO 4)2 iron(II) phosphate (See CD, Screen 3. 11 for naming practice) © 2006 Brooks/Cole - Thomson 32

Properties of Ionic Compounds Forming Na. Cl from Na and Cl 2 • A metal atom can transfer an electron to a nonmetal. • The resulting cation and anion are attracted to each other by electrostatic forces. © 2006 Brooks/Cole - Thomson 33

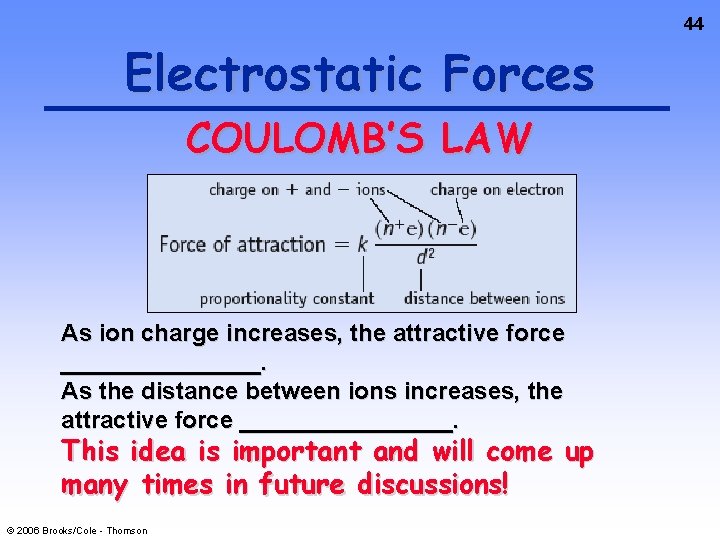
34 Electrostatic Forces The oppositely charged ions in ionic compounds are attracted to one another by ELECTROSTATIC FORCES. These forces are governed by COULOMB’S LAW. © 2006 Brooks/Cole - Thomson

35 POLYATOMIC IONS CD Screen 3. 6 Groups of atoms with a charge. MEMORIZE the names and formulas in Table 3. 1, page 107. © 2006 Brooks/Cole - Thomson

36 Note: many O containing anions have names ending in –ate (or -ite). © 2006 Brooks/Cole - Thomson

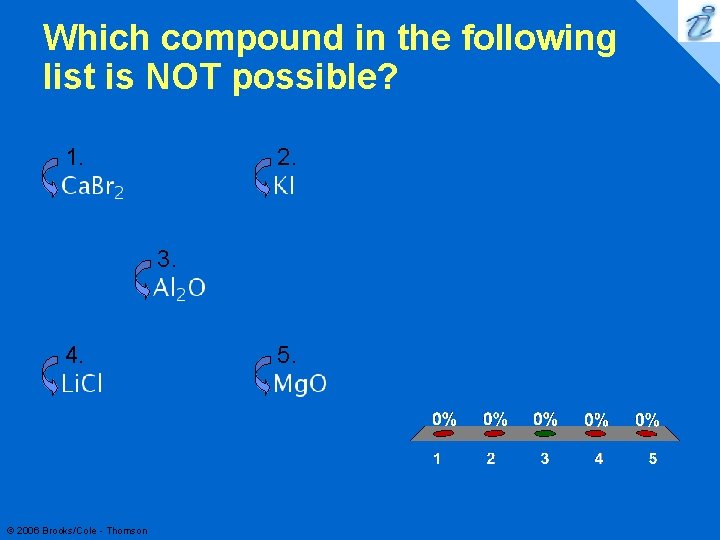
Which compound in the following list is NOT possible? 1. 2. 3. 4. © 2006 Brooks/Cole - Thomson 5. 37

38 Polyatomic Ions HNO 3 nitric acid © 2006 Brooks/Cole - Thomson NO 3 nitrate ion

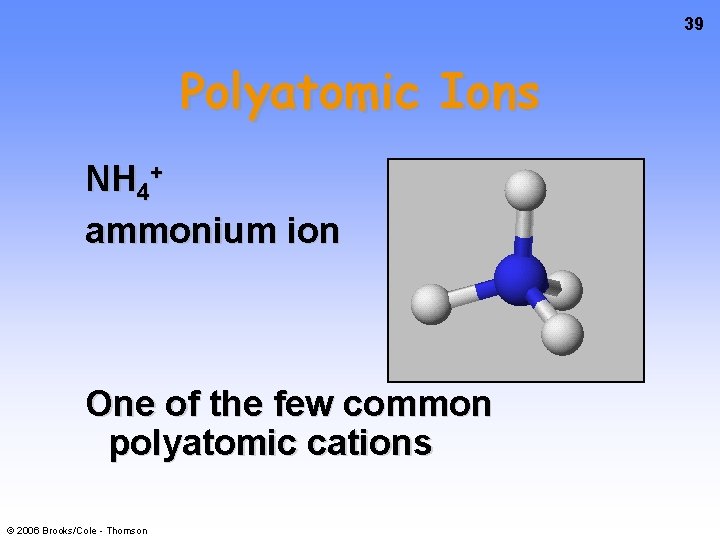
39 Polyatomic Ions NH 4+ ammonium ion One of the few common polyatomic cations © 2006 Brooks/Cole - Thomson

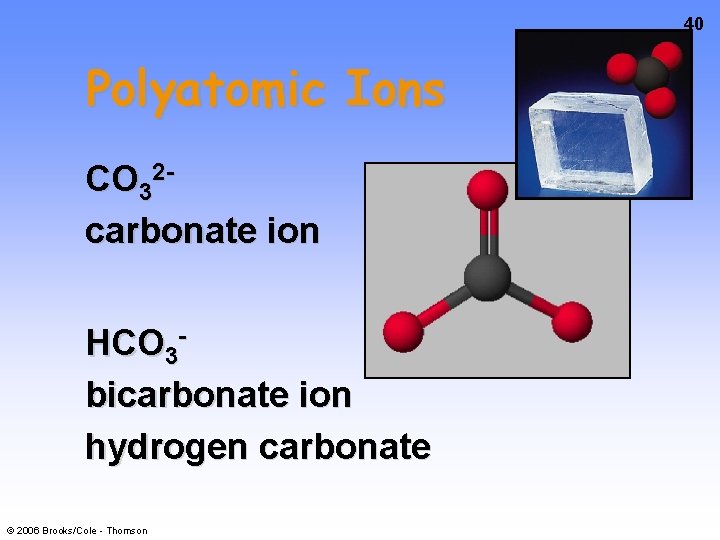
40 Polyatomic Ions CO 32 carbonate ion HCO 3 bicarbonate ion hydrogen carbonate © 2006 Brooks/Cole - Thomson

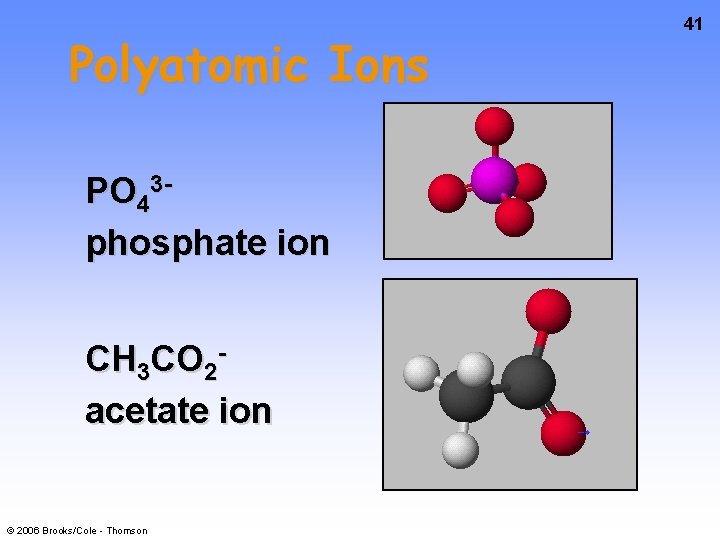
Polyatomic Ions PO 43 phosphate ion CH 3 CO 2 acetate ion © 2006 Brooks/Cole - Thomson 41

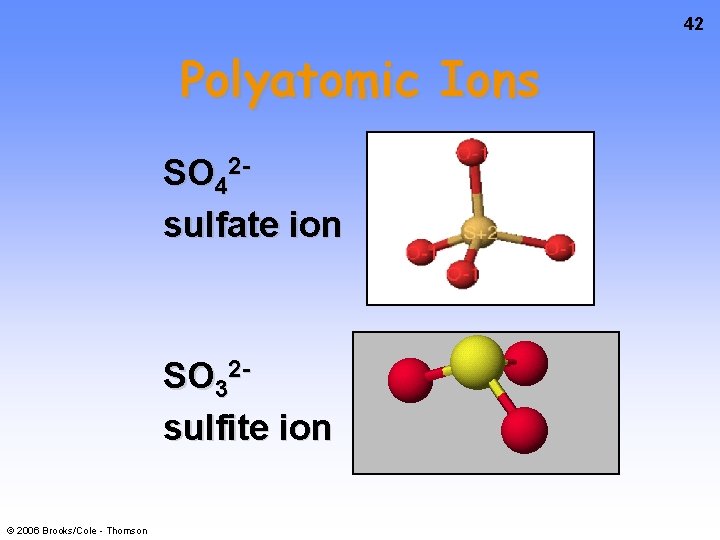
42 Polyatomic Ions SO 42 sulfate ion SO 32 sulfite ion © 2006 Brooks/Cole - Thomson

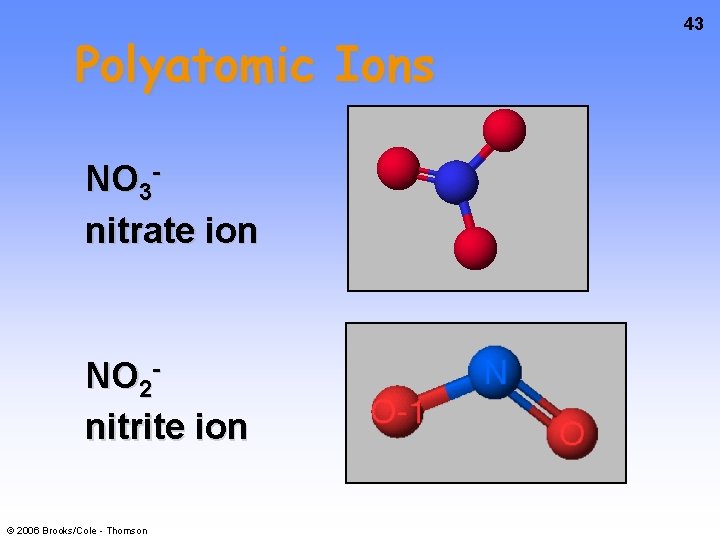
Polyatomic Ions NO 3 nitrate ion NO 2 nitrite ion © 2006 Brooks/Cole - Thomson 43

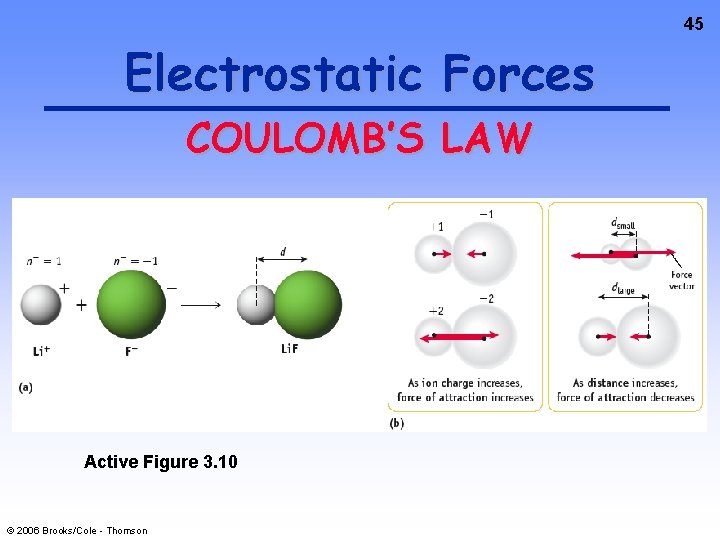
44 Electrostatic Forces COULOMB’S LAW As ion charge increases, the attractive force ________. As the distance between ions increases, the attractive force ________. This idea is important and will come up many times in future discussions! © 2006 Brooks/Cole - Thomson

45 Electrostatic Forces COULOMB’S LAW Active Figure 3. 10 © 2006 Brooks/Cole - Thomson

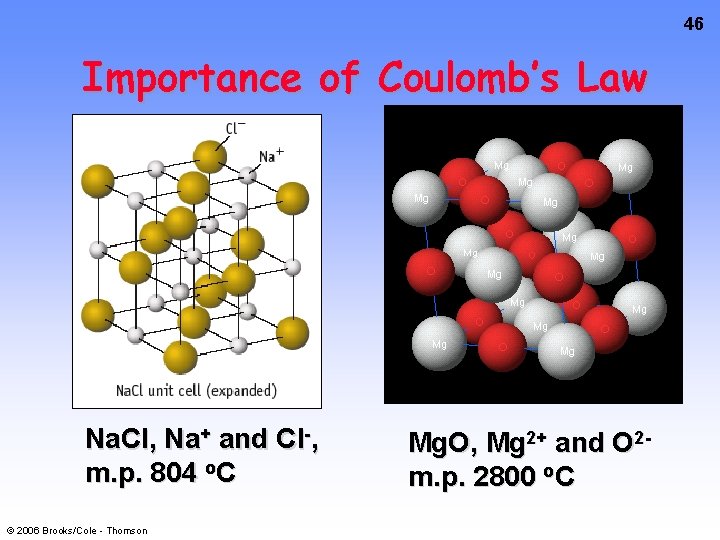
46 Importance of Coulomb’s Law Na. Cl, Na+ and Cl-, m. p. 804 o. C © 2006 Brooks/Cole - Thomson Mg. O, Mg 2+ and O 2 m. p. 2800 o. C

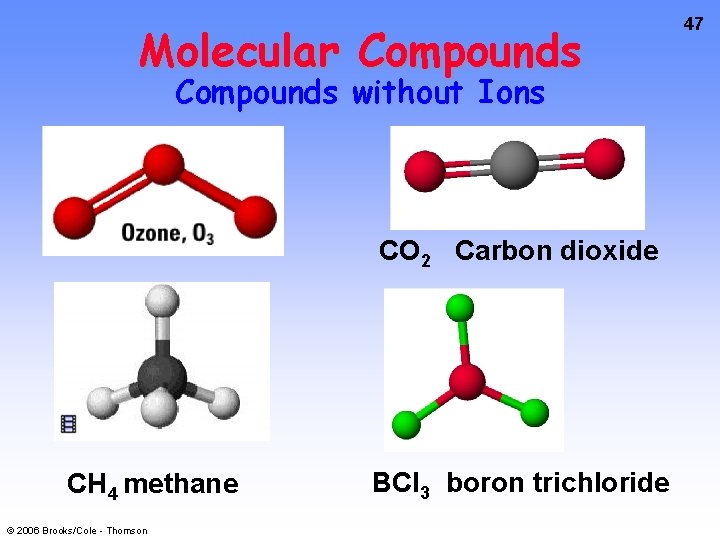
Molecular Compounds without Ions CO 2 Carbon dioxide CH 4 methane © 2006 Brooks/Cole - Thomson BCl 3 boron trichloride 47

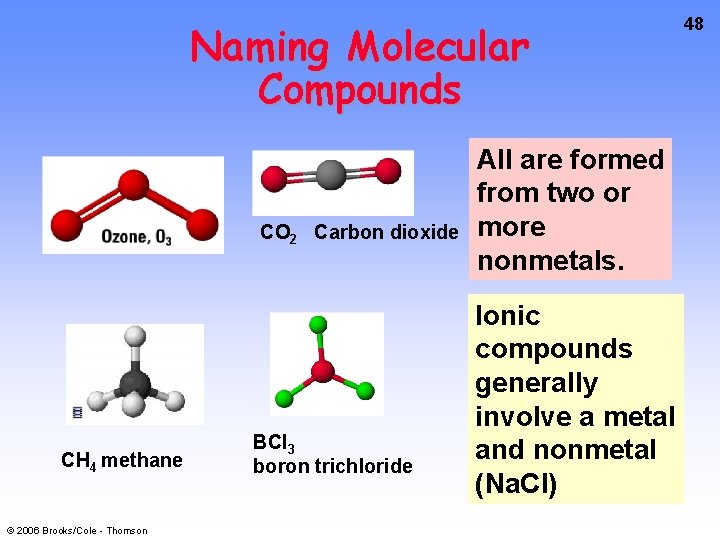
Naming Molecular Compounds CO 2 Carbon dioxide CH 4 methane © 2006 Brooks/Cole - Thomson BCl 3 boron trichloride All are formed from two or more nonmetals. Ionic compounds generally involve a metal and nonmetal (Na. Cl) 48

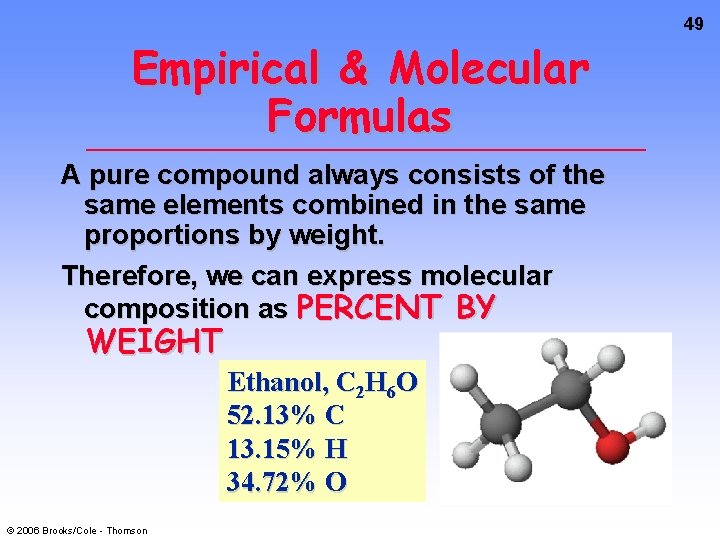
49 Empirical & Molecular Formulas A pure compound always consists of the same elements combined in the same proportions by weight. Therefore, we can express molecular composition as PERCENT BY WEIGHT Ethanol, C 2 H 6 O 52. 13% C 13. 15% H 34. 72% O © 2006 Brooks/Cole - Thomson

Percent Composition Consider some of the family of nitrogenoxygen compounds: NO 2, nitrogen dioxide and closely related, NO, nitrogen monoxide (or nitric oxide) Chemistry of NO, nitrogen monoxide Structure of NO 2 © 2006 Brooks/Cole - Thomson 50

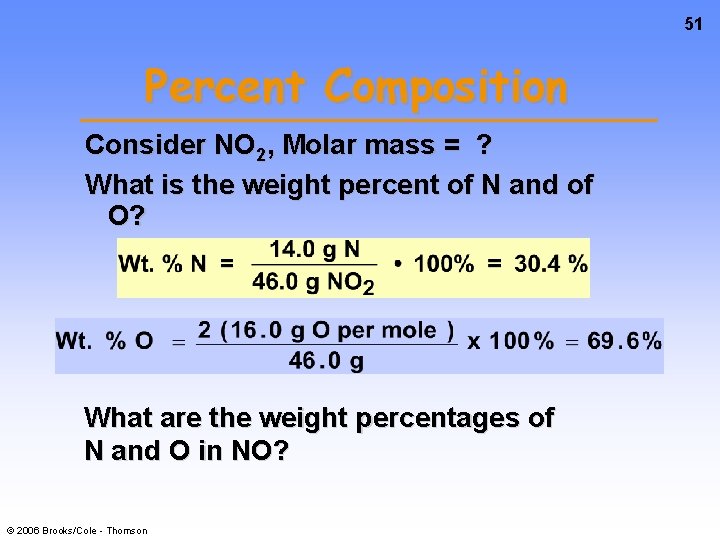
51 Percent Composition Consider NO 2, Molar mass = ? What is the weight percent of N and of O? What are the weight percentages of N and O in NO? © 2006 Brooks/Cole - Thomson

How to Determine a Formula? Mass spectrometer © 2006 Brooks/Cole - Thomson 52

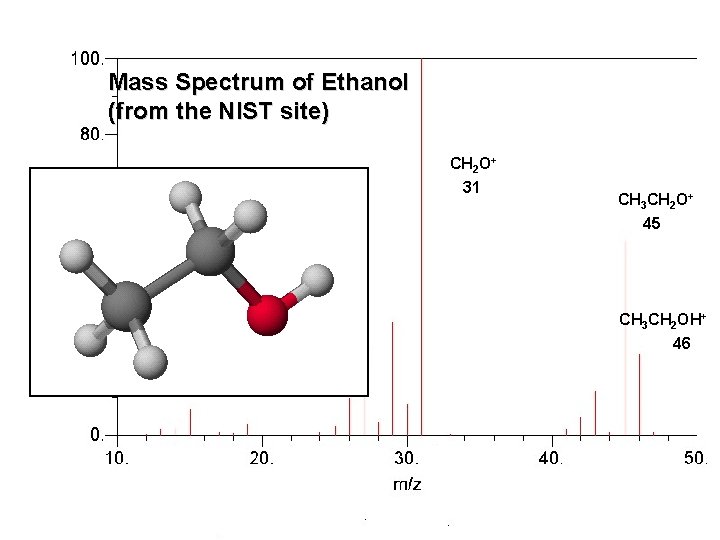
Mass Spectrum of Ethanol 53 (from the NIST site) CH 2 O+ 31 CH 3 CH 2 O+ 45 CH 3 CH 2 OH+ 46 © 2006 Brooks/Cole - Thomson

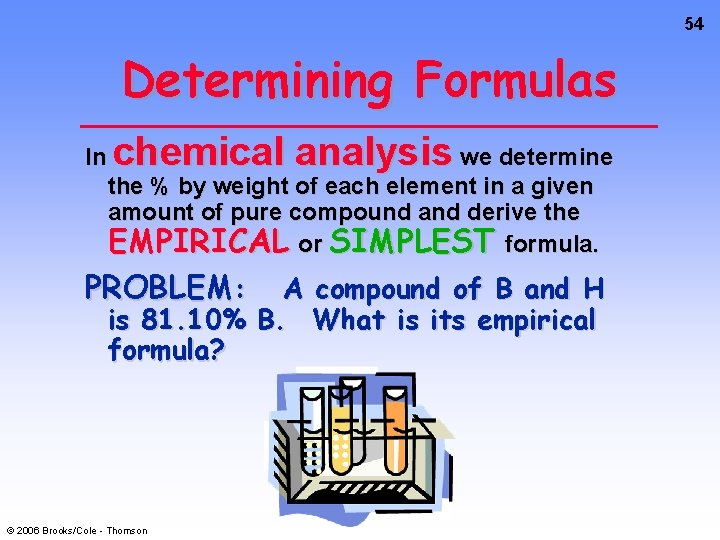
54 Determining Formulas In chemical analysis we determine the % by weight of each element in a given amount of pure compound and derive the EMPIRICAL or SIMPLEST formula. PROBLEM: A compound of B and H is 81. 10% B. What is its empirical formula? © 2006 Brooks/Cole - Thomson

55 A compound of B and H is 81. 10% B. What is its empirical formula? • Because it contains only B and H, it must contain 18. 90% H. • In 100. 0 g of the compound there are 81. 10 g of B and 18. 90 g of H. • Calculate the number of moles of each constitutent. © 2006 Brooks/Cole - Thomson

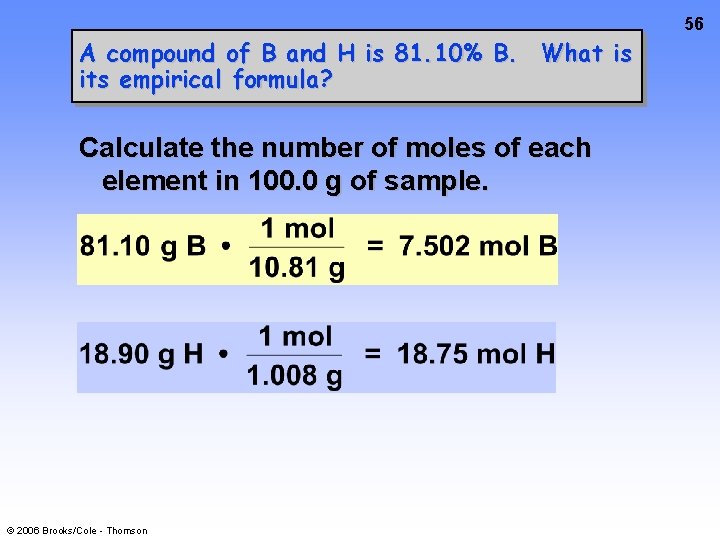
56 A compound of B and H is 81. 10% B. What is its empirical formula? Calculate the number of moles of each element in 100. 0 g of sample. © 2006 Brooks/Cole - Thomson

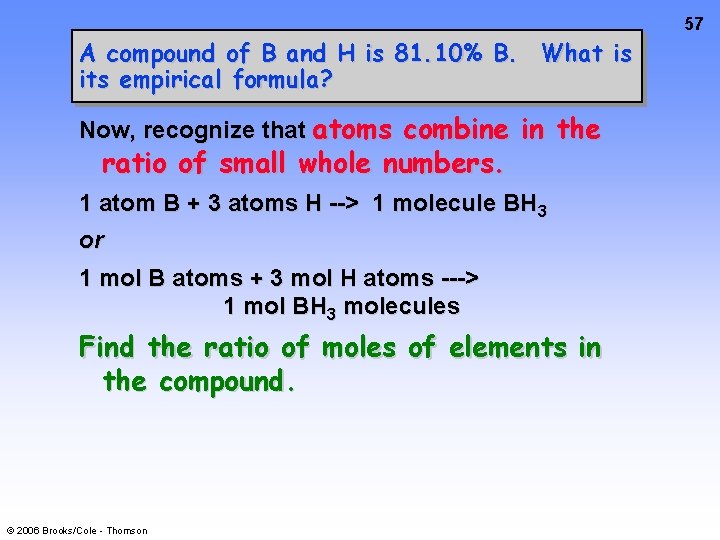
57 A compound of B and H is 81. 10% B. What is its empirical formula? Now, recognize that atoms combine in the ratio of small whole numbers. 1 atom B + 3 atoms H --> 1 molecule BH 3 or 1 mol B atoms + 3 mol H atoms ---> 1 mol BH 3 molecules Find the ratio of moles of elements in the compound. © 2006 Brooks/Cole - Thomson

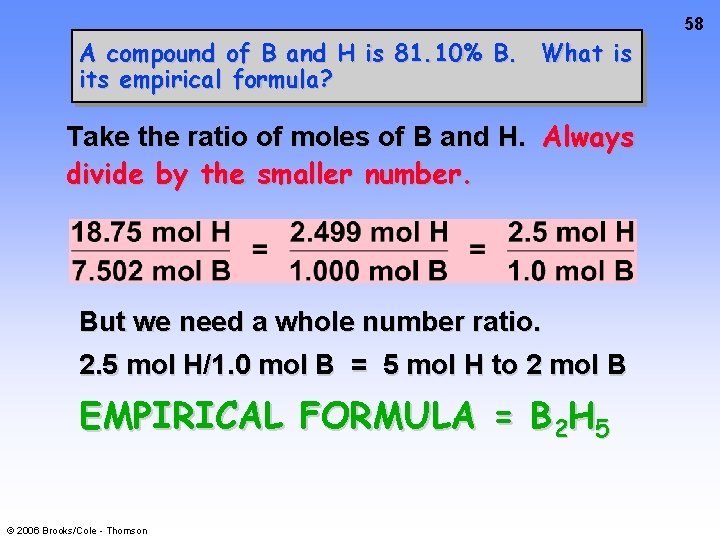
58 A compound of B and H is 81. 10% B. What is its empirical formula? Take the ratio of moles of B and H. Always divide by the smaller number. But we need a whole number ratio. 2. 5 mol H/1. 0 mol B = 5 mol H to 2 mol B EMPIRICAL FORMULA = B 2 H 5 © 2006 Brooks/Cole - Thomson

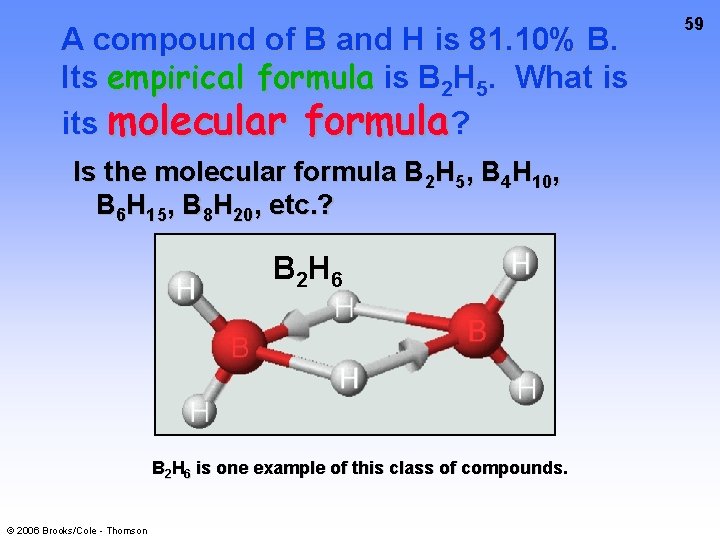
A compound of B and H is 81. 10% B. Its empirical formula is B 2 H 5. What is its molecular formula? Is the molecular formula B 2 H 5, B 4 H 10, B 6 H 15, B 8 H 20, etc. ? B 2 H 6 is one example of this class of compounds. © 2006 Brooks/Cole - Thomson 59

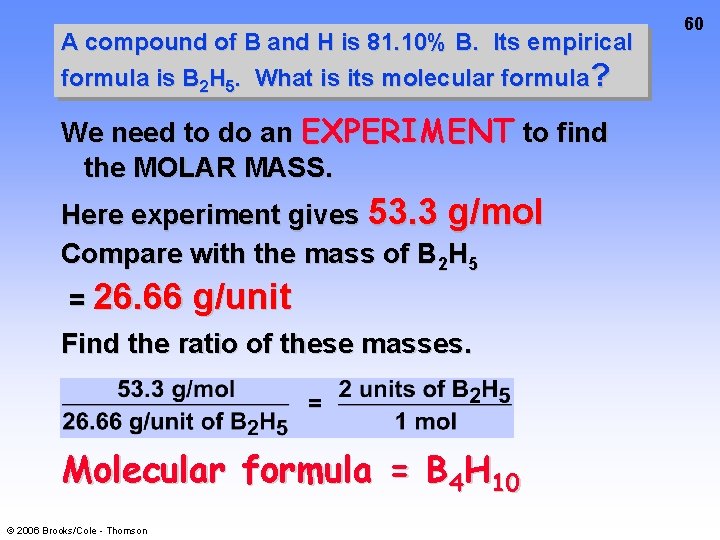
A compound of B and H is 81. 10% B. Its empirical formula is B 2 H 5. What is its molecular formula? We need to do an EXPERIMENT to find the MOLAR MASS. Here experiment gives 53. 3 g/mol Compare with the mass of B 2 H 5 = 26. 66 g/unit Find the ratio of these masses. Molecular formula = B 4 H 10 © 2006 Brooks/Cole - Thomson 60

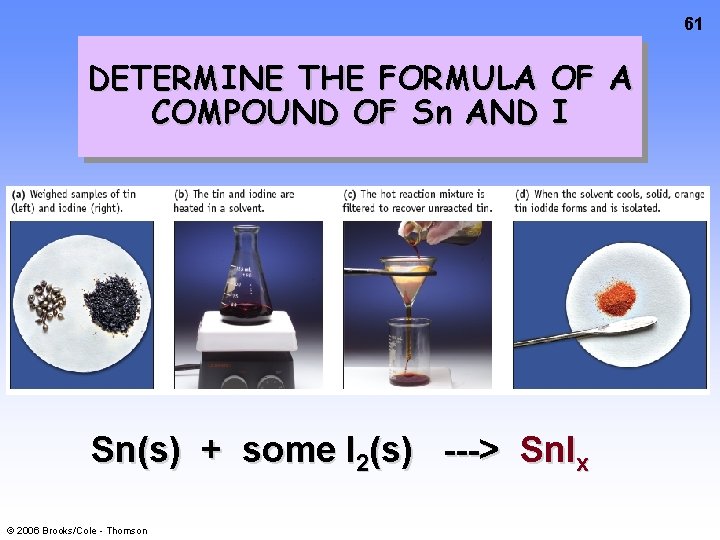
61 DETERMINE THE FORMULA OF A COMPOUND OF Sn AND I Sn(s) + some I 2(s) ---> Sn. Ix © 2006 Brooks/Cole - Thomson

62 Data to Determine the formula of a Sn—I Compound • Reaction of Sn and I 2 is done using excess Sn. • Mass of Sn in the beginning = 1. 056 g • Mass of iodine (I 2) used = 1. 947 g • Mass of Sn remaining = 0. 601 g • See p. 125 © 2006 Brooks/Cole - Thomson

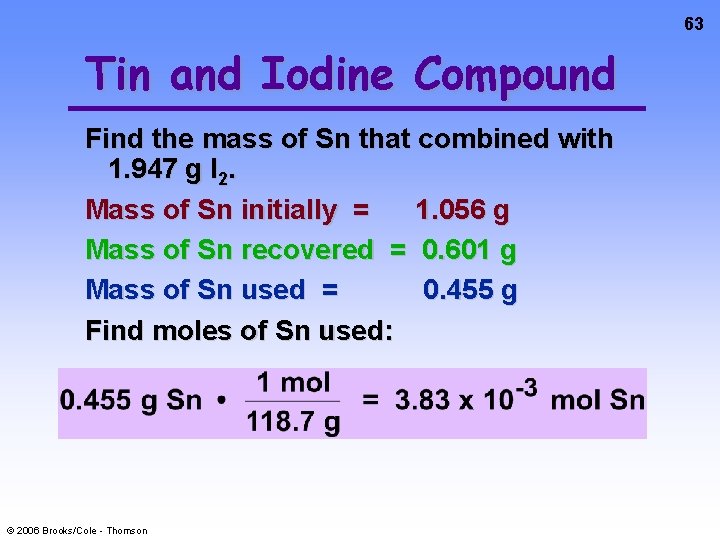
63 Tin and Iodine Compound Find the mass of Sn that combined with 1. 947 g I 2. Mass of Sn initially = 1. 056 g Mass of Sn recovered = 0. 601 g Mass of Sn used = 0. 455 g Find moles of Sn used: © 2006 Brooks/Cole - Thomson

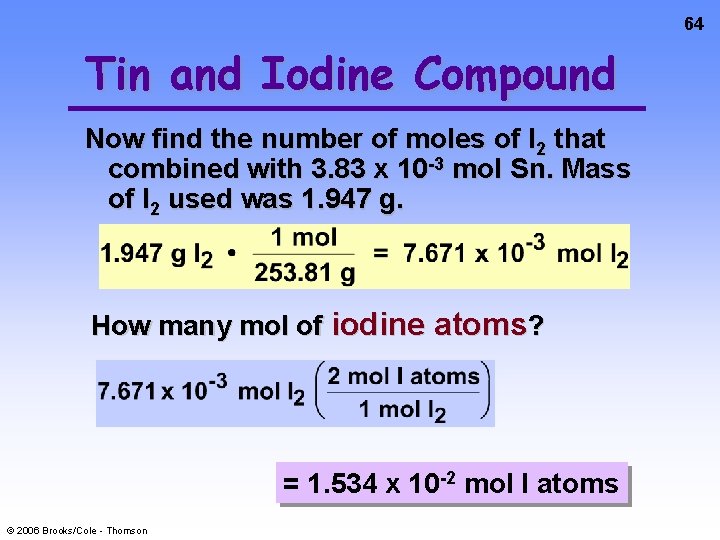
64 Tin and Iodine Compound Now find the number of moles of I 2 that combined with 3. 83 x 10 -3 mol Sn. Mass of I 2 used was 1. 947 g. How many mol of iodine atoms? = 1. 534 x 10 -2 mol I atoms © 2006 Brooks/Cole - Thomson

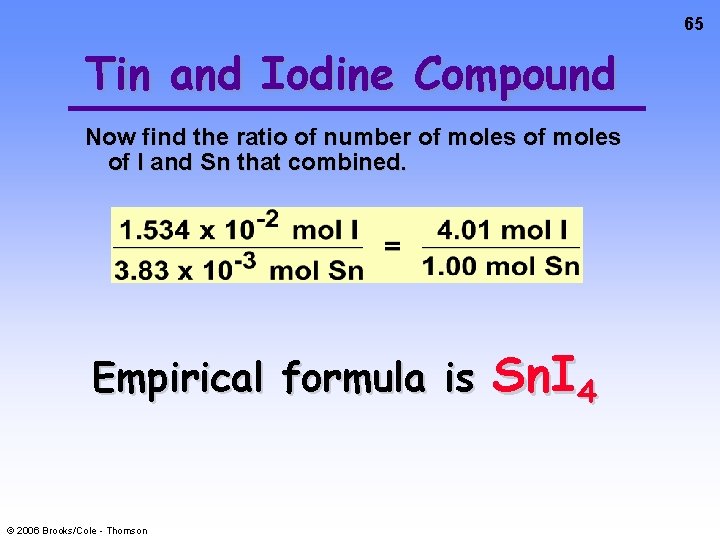
65 Tin and Iodine Compound Now find the ratio of number of moles of I and Sn that combined. Empirical formula is © 2006 Brooks/Cole - Thomson Sn. I 4
 Group one metals
Group one metals Difference between solution and suspension
Difference between solution and suspension Mis chapter 6
Mis chapter 6 Report
Report Chapter 8 review describing chemical reactions
Chapter 8 review describing chemical reactions Chemistry chapter 9 chemical names and formulas
Chemistry chapter 9 chemical names and formulas Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Pericyclic
Pericyclic Organic chemistry 2nd edition klein
Organic chemistry 2nd edition klein Introductory chemistry 4th edition
Introductory chemistry 4th edition Prefix multipliers
Prefix multipliers Introductory chemistry 5th edition answers
Introductory chemistry 5th edition answers Chemistry central science 14th edition
Chemistry central science 14th edition Carboxylic acid h3o+ reaction
Carboxylic acid h3o+ reaction Nomenclature of ethers
Nomenclature of ethers Ap chemistry notes zumdahl
Ap chemistry notes zumdahl Organic chemistry third edition david klein
Organic chemistry third edition david klein General chemistry 11th edition
General chemistry 11th edition Lesson 81 drop in molecular views answer key
Lesson 81 drop in molecular views answer key Chemistry by raymond chang 10th edition
Chemistry by raymond chang 10th edition Democritus atomic model diagram
Democritus atomic model diagram Halohydrin
Halohydrin Organic chemistry 2nd edition klein
Organic chemistry 2nd edition klein Organic chemistry
Organic chemistry Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Love chemical formula
Love chemical formula Functional groups ib chemistry
Functional groups ib chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Definition of chemical kinetics in chemistry
Definition of chemical kinetics in chemistry Pixl knowit gcse chemistry chemical changes
Pixl knowit gcse chemistry chemical changes Chemistry unit 4 grade 11
Chemistry unit 4 grade 11 A chemist shorthand way of representing chemical reaction
A chemist shorthand way of representing chemical reaction Chemistry chapter 10 chemical quantities
Chemistry chapter 10 chemical quantities Ap chemistry chemical equilibrium
Ap chemistry chemical equilibrium Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chapter 6 section 1 atoms elements and compounds answer key
Chapter 6 section 1 atoms elements and compounds answer key Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal Ketone reactivity
Ketone reactivity Reactivity series and displacement reactions
Reactivity series and displacement reactions Pyridine resonance
Pyridine resonance Reactivity trend periodic table
Reactivity trend periodic table Reactive periodic table
Reactive periodic table How to memorize activity series
How to memorize activity series Cathode reactivity series
Cathode reactivity series Ion arenium
Ion arenium Reactivity trend periodic table
Reactivity trend periodic table Periods of reactivity newborn
Periods of reactivity newborn Non metal examples
Non metal examples Clifford consulting and research
Clifford consulting and research Head circumference of newborn
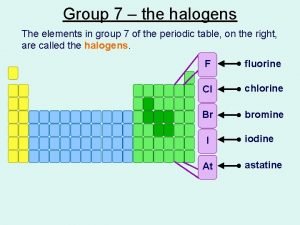
Head circumference of newborn Reactivity group 7
Reactivity group 7 What is halogen
What is halogen Halides in periodic table
Halides in periodic table Periods of reactivity newborn
Periods of reactivity newborn Carboxylic acid reactivity
Carboxylic acid reactivity Fischer esterification
Fischer esterification Trend in ionization energy across a period
Trend in ionization energy across a period Polonium reactivity
Polonium reactivity