The Reactivity Series Metal Reaction with dilute hydrochloric

- Slides: 3

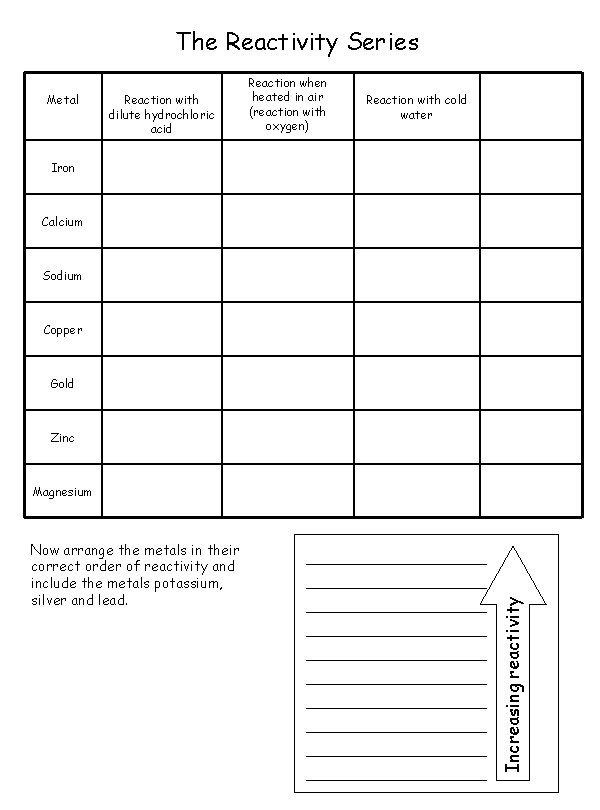
The Reactivity Series Metal Reaction with dilute hydrochloric acid Reaction when heated in air (reaction with oxygen) Reaction with cold water Iron Calcium Sodium Copper Gold Zinc Now arrange the metals in their correct order of reactivity and include the metals potassium, silver and lead. Increasing reactivity Magnesium

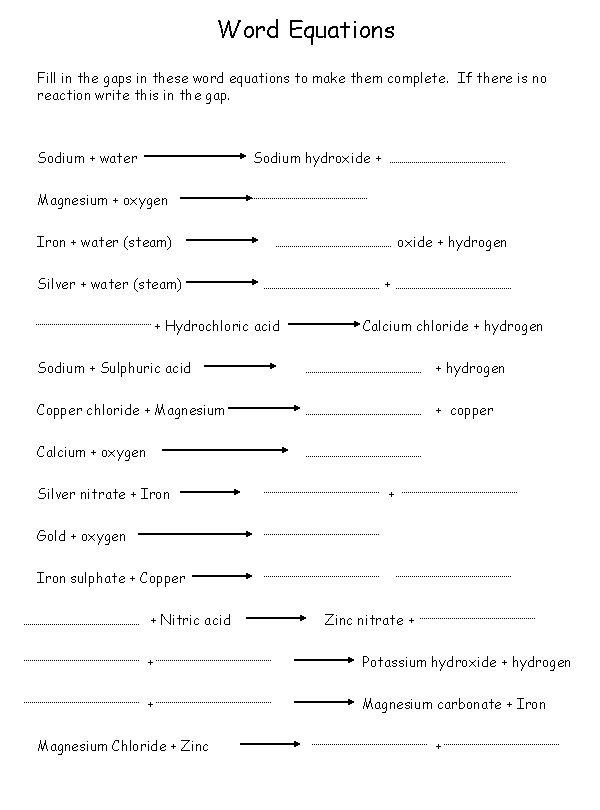
Word Equations Fill in the gaps in these word equations to make them complete. If there is no reaction write this in the gap. Sodium + water Sodium hydroxide + Magnesium + oxygen Iron + water (steam) Silver + water (steam) + Hydrochloric acid oxide + hydrogen + Calcium chloride + hydrogen Sodium + Sulphuric acid + hydrogen Copper chloride + Magnesium + copper Calcium + oxygen Silver nitrate + Iron + Gold + oxygen Iron sulphate + Copper + Nitric acid Zinc nitrate + + Potassium hydroxide + hydrogen + Magnesium carbonate + Iron Magnesium Chloride + Zinc +

Displacement Reactions A more reactive metal displaces a less reactive metal from its compounds. There are many ways to help you remember how displacement reactions work. Model 1 The metals football league: Potassium Rangers The metals nearer the top are stronger teams and they always beat the teams that are lower than them. Sodium City Magnesium United Zinc City Iron Town The league works like this: The teams play for a cup (the Chloride Cup). The team at home is always the cup holder. If the away team beats them then they take home the cup, if the home team wins then they keep the cup. Copper United Gold United Example: Home Away Winners Losers Zinc City Sodium City Zinc City Chloride cup Chloride Cup Reaction: Zinc Chloride + Sodium Chloride + Zinc Model 2 Another way is to think of the metals in the reactivity series as boys of varying strength. The strength (and temper) of the boys increases as you go up the table. + + Colin Copper and Suzie Sulphate. Peter Potassium is stronger than Colin Copper so fights him…………… ……and takes Suzy sulphate away. Colin Copper is all alone. Reaction: Copper Sulphate + Potassium Sulphate + Copper