STARTER Do you remember the metal reactivity series

STARTER: Do you remember the metal reactivity series? Write down as many metals as you can think of with the most reactive at the top and the least reactive at the bottom: Try and do it WITHOUT looking at your Periodic table! Space is provided in your booklet to make your list!

Reactions of metals with acids • How much can you remember? • Have a conversation with your partner to revise what you know! (2 min) • Now, can you remember more? • Let’s carry out the experiment in your booklet! (15 min)
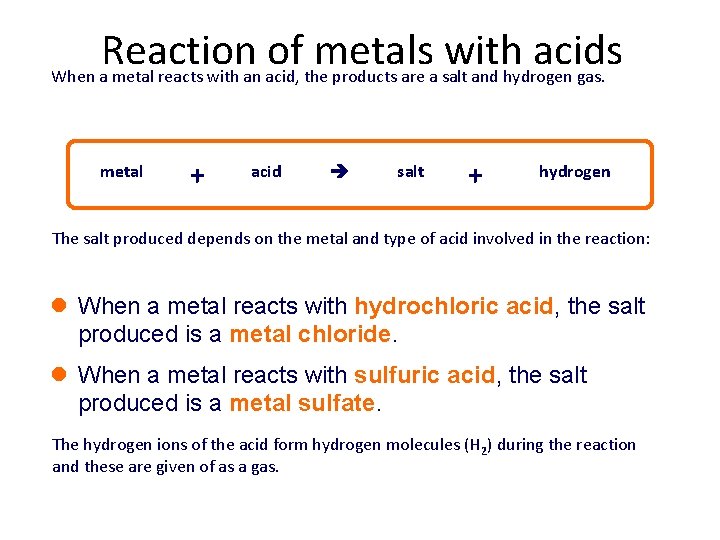
Reaction of metals with acids When a metal reacts with an acid, the products are a salt and hydrogen gas. metal + acid salt + hydrogen The salt produced depends on the metal and type of acid involved in the reaction: l When a metal reacts with hydrochloric acid, the salt produced is a metal chloride. l When a metal reacts with sulfuric acid, the salt produced is a metal sulfate. The hydrogen ions of the acid form hydrogen molecules (H 2) during the reaction and these are given of as a gas.
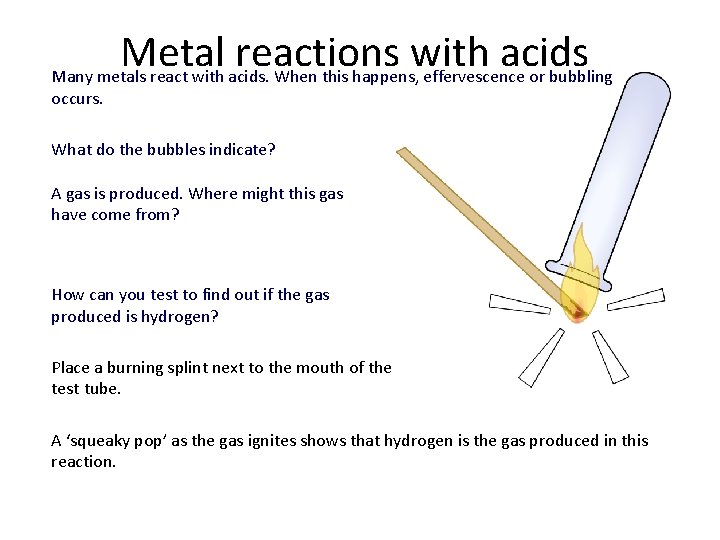
Metal reactions with acids Many metals react with acids. When this happens, effervescence or bubbling occurs. What do the bubbles indicate? A gas is produced. Where might this gas have come from? How can you test to find out if the gas produced is hydrogen? Place a burning splint next to the mouth of the test tube. A ‘squeaky pop’ as the gas ignites shows that hydrogen is the gas produced in this reaction.

Metals and acid – equations What are the products of each reaction? magnesium + hydrochloric acid magnesium chloride + hydrogen Mg + 2 HCl Mg. Cl 2 + H 2 aluminium + hydrochloric acid aluminium chloride + hydrogen 2 Al + 6 HCl 2 Al. Cl 3 + 3 H 2 zinc + sulfuric acid zinc sulfate + hydrogen Zn + H 2 SO 4 Zn. SO 4 + H 2

Now try the practice equations in your booklet! • Make sure EVERY page is completed -up to (and including) p 13!
- Slides: 6