Chemistry 161 General Chemistry 1 Your Instructor is






































































- Slides: 102

Chemistry& 161 General Chemistry 1 Your Instructor is Dr Lee West Welcome !!

First of all lets get the formalities out of the way…. Instructor: Lee West Office: LSC-114 Phone: (253) 864 -3353 Email: lwest@pierce. ctc. edu Office Hours: Mon-Thurs 12: 00 PM– 1: 30 PM

Class Times: Mon-Thurs. 11: 00 - 11: 50 AM (LSC 244) Lab Time: Friday (9 AM 7066 or 12 PM 7067) (LSC 103) Textbook: “Chemistry” by Open Stax Calculator: Students must have a basic scientific, calculator. “Carbonless Copy” Bound Lab Notebook: Access to Sapling online HW system

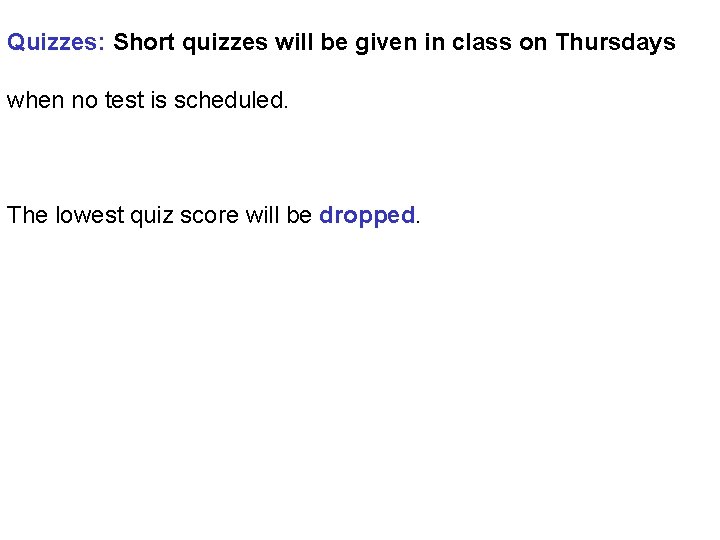
Quizzes: Short quizzes will be given in class on Thursdays when no test is scheduled. The lowest quiz score will be dropped.

Homework: Will be given for each chapter. Homework will be peer evaluated in the first ten minutes of class. Homework will also be assigned through Sapling.

Tests: Three tests will be given. Final Exam: The final exam is comprehensive and will cover all material presented during the quarter.

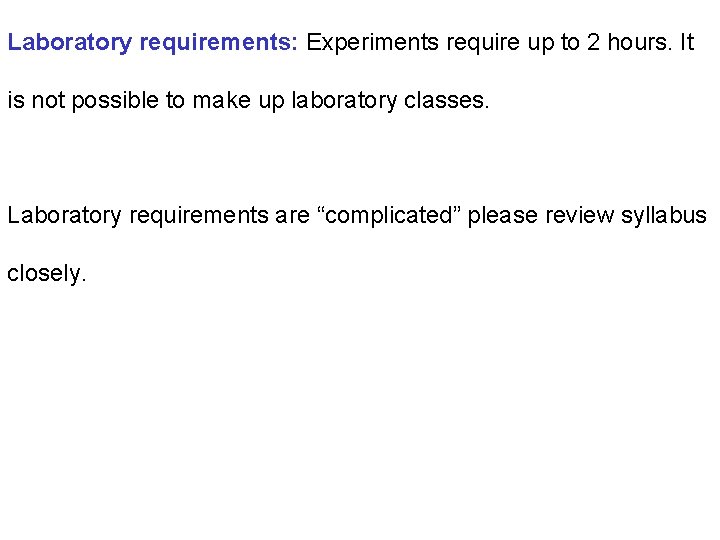
Laboratory requirements: Experiments require up to 2 hours. It is not possible to make up laboratory classes. Laboratory requirements are “complicated” please review syllabus closely.

Essential Ideas 1. 1 Chemistry in Context 1. 2 Phases and Classification of Matter 1. 3 Physical and Chemical Properties 1. 4 Measurements 1. 5 Measurement Uncertainty, Accuracy and Precision 1. 6 Mathematical treatment of Measurement Results

Essential Ideas 1. 1 Chemistry in Context

What is Chemistry? Boring…. Difficult… The object of my hatred…. The stupid class I have to repeat…. Chemistry can be defined as: “The scientific study of matter its properties and transformations”

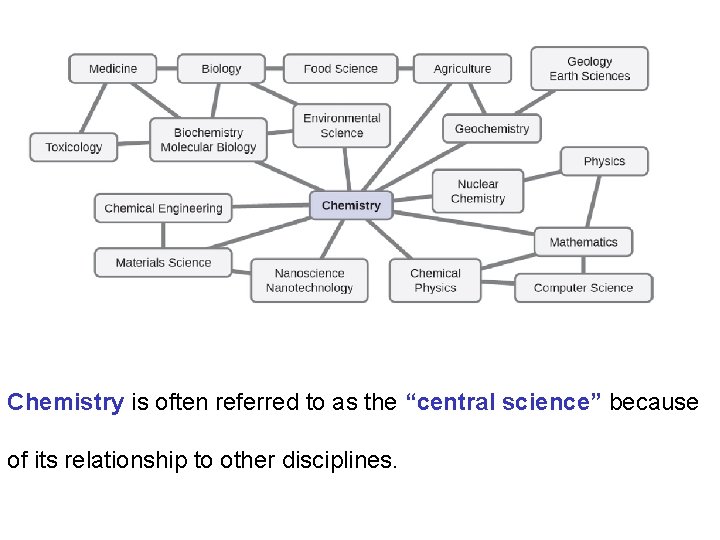
Chemistry is often referred to as the “central science” because of its relationship to other disciplines.

“The scientific study of matter and its transformations” What is Science?

Negative views of science • Unethical • Anti-religious • The enemy

Positive views of science • • • A source of technology A solution to global hunger Our only hope !!!

What does the dictionary say? “knowledge or a system of knowledge covering general truths or the operation of general laws especially as obtained and tested through scientific method. ” (Merriam-Webster Dictionary ) What ? !?

Science can be more simply defined as: “An approach to the study of The Universe” In science an assumption is made that: “The Universe obeys laws of natural origin” The role of a scientist is to: “identify and understand these laws using the scientific method”

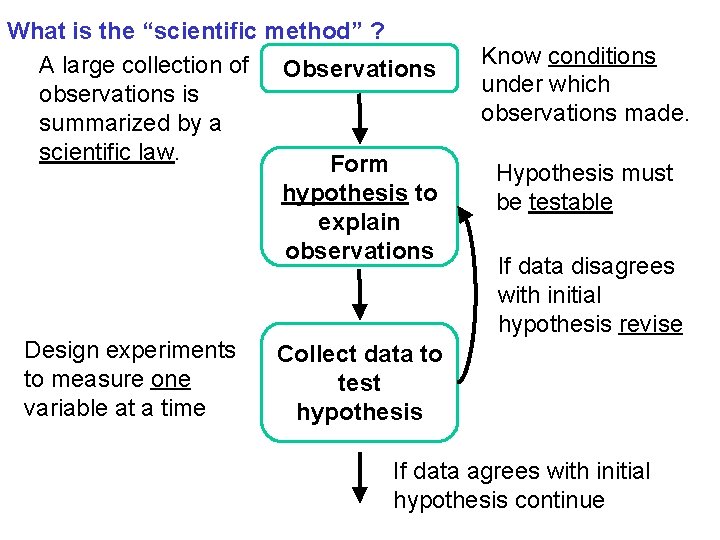
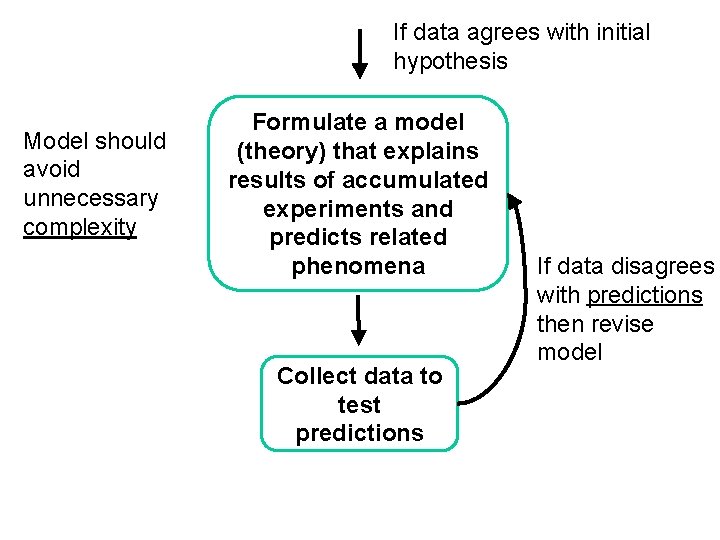
What is the “scientific method” ? A large collection of Observations observations is summarized by a scientific law. Form hypothesis to explain observations Design experiments to measure one variable at a time Know conditions under which observations made. Hypothesis must be testable If data disagrees with initial hypothesis revise Collect data to test hypothesis If data agrees with initial hypothesis continue

If data agrees with initial hypothesis Model should avoid unnecessary complexity Formulate a model (theory) that explains results of accumulated experiments and predicts related phenomena Collect data to test predictions If data disagrees with predictions then revise model

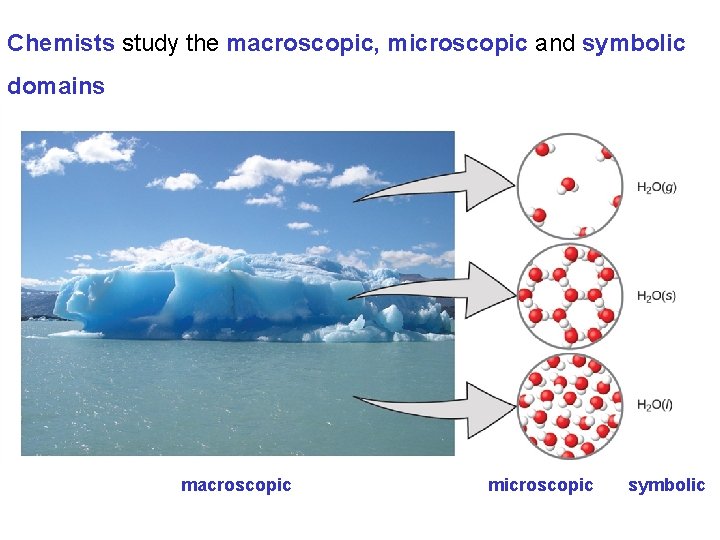
Chemists study the macroscopic, microscopic and symbolic domains macroscopic microscopic symbolic

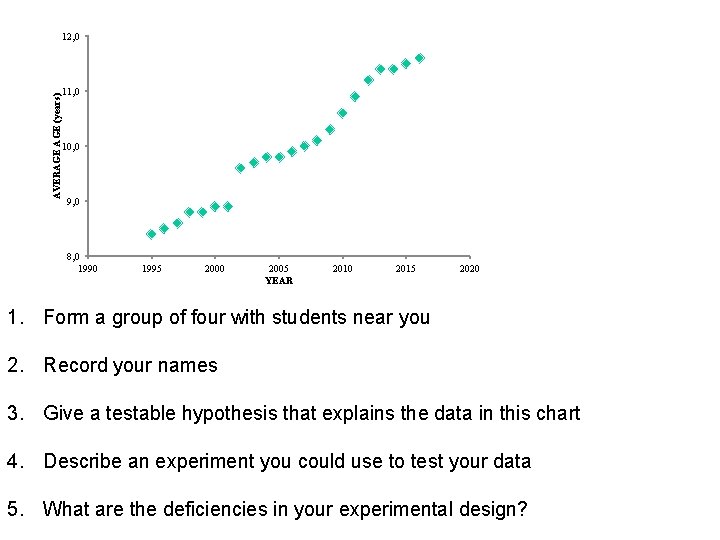
AVERAGE (years) 12, 0 11, 0 10, 0 9, 0 8, 0 1995 2000 2005 YEAR 2010 2015 2020 1. Form a group of four with students near you 2. Record your names 3. Give a testable hypothesis that explains the data in this chart 4. Describe an experiment you could use to test your data 5. What are the deficiencies in your experimental design?

Essential Ideas 1. 1 Chemistry in Context Chemists apply the scientific method to the study of matter and its transformations. The scientific method attempts to explain the results of observations through hypothesis (tentative explanations) and ultimately theories (well tested explanations). Scientific Laws summarize a large collection of observations. Chemists study and describe matter across the macroscopic, microscopic and symbolic domains.

Essential Ideas 1. 2 Phases and Classification of Matter

“The scientific study of matter and its transformations” What is Matter? “The physical material of the universe. ” “Anything that occupies space and has mass”

Mass is the quantity that describes the amount of matter in an object. • The mass of an object is constant at every place in the universe. • Mass is measured using a balance.

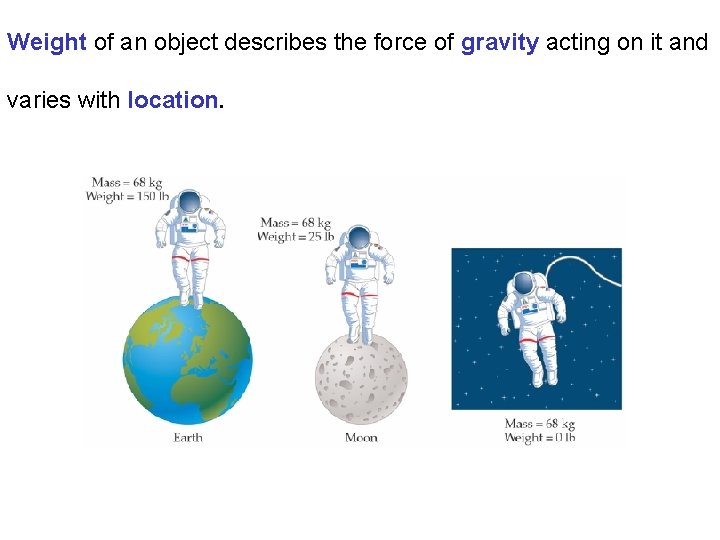
Weight of an object describes the force of gravity acting on it and varies with location.

It useful to distinguish matter from energy. Energy can be defined as the capacity to do work. • Work is defined as moving an object against a force For example work is done when an object is lifted up against the force of gravity and this requires energy.


Matter commonly exists in one of three states: § Solid § Liquid § Gas How is temperature related to the state of a substance? How is pressure related to the state of a substance?

Gases are characterized by: § Shape adapts to that of the container. § Fill whatever container they are placed in (have variable volume). Is the density of a gas fixed? Can gases be readily compressed?

In liquids the particles are closer to each other than in gases so: § The density of liquids is greater than that of gases. § Liquids adopt the shape of the container into which they are placed. § Unlike gases liquids have a fixed volume. Is the density of a liquid fixed? Can liquids be readily compressed?

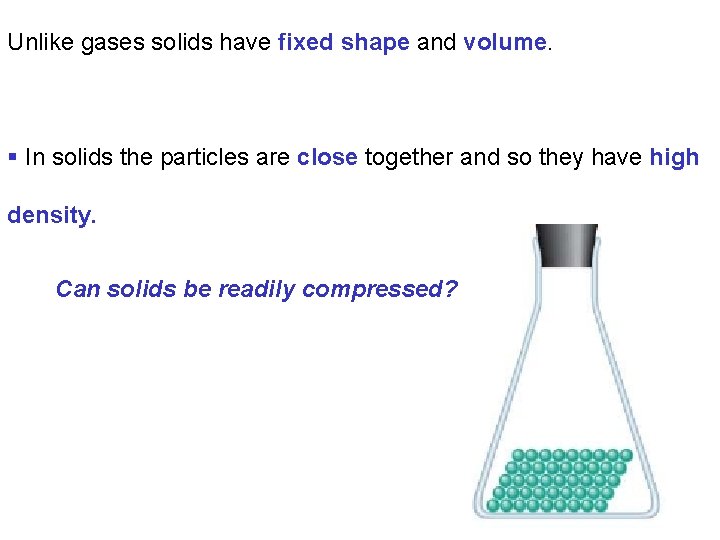
Unlike gases solids have fixed shape and volume. § In solids the particles are close together and so they have high density. Can solids be readily compressed?

In modern chemistry we take an atomistic view of matter. We describe matter as being composed of particles called atoms that are indivisible by either physical or chemical methods. What would be the alternate hypothesis?

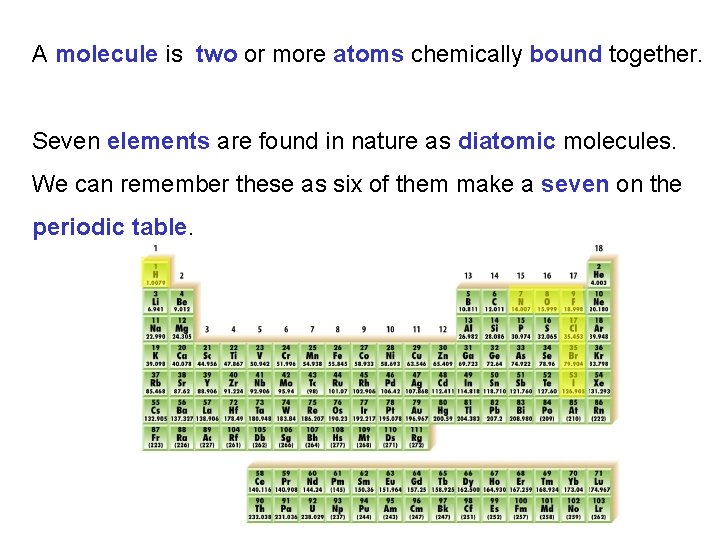
There are 118 known kinds of atoms. Each has its own symbol. The first character in an atoms symbol is an uppercase letter. Most atoms have a second character in their symbol which is always a lowercase letter. e. g. Ca K Sr Sn Ti W Na

A molecule is two or more atoms chemically bound together. Seven elements are found in nature as diatomic molecules. We can remember these as six of them make a seven on the periodic table.

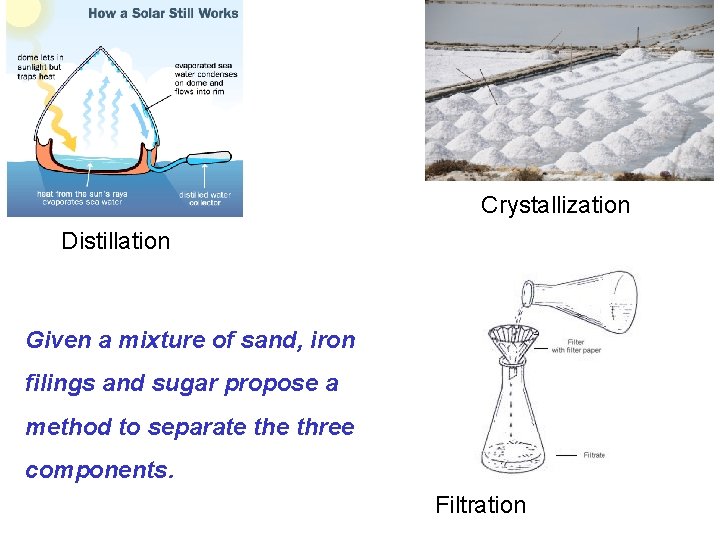
A sample of matter can be characterized as either a pure substance or a mixture. A mixture is a physical combination of two or more pure substances. • The components of a mixture may be separated by physical means e. g. filtration, distillation etc. Pure substances cannot be separated into other kinds of matter by any physical means.

Crystallization Distillation Given a mixture of sand, iron filings and sugar propose a method to separate three components. Filtration

Mixtures can have a variable composition. e. g. The strength of a cup of coffee can be varied. The amount of mixer you add to an alcoholic beverage can be changed (depending on how stressful your students are). The color of paint depends on how much pigment is added.

Mixtures may be either homogenous or heterogeneous. Heterogeneous mixtures have: • Visibly different phases • A sample of one region may have a different composition from a sample from a different region Can you think of any examples of heterogeneous mixtures?

Sand Vegetable Soup

In a Homogenous mixture a sample of any part of the mixture is identical to any other sample. e. g. sugar dissolved in water, alloys (bronze, brass)

Pure substances can be classified as either elements or compounds. • Elements cannot be broken down into simpler components. They are pure substances composed of one type of atom. • Compounds can be chemically broken down into simpler components. They are pure substances composed of more than one type of atom.

A compound is two or more different atoms chemically bound together. The elements present in a compound are always there in the same ratio for a given compound. e. g. NH 3, will always have 3 hydrogen atoms for every nitrogen atom. The elements present in a compound can be separated only by chemical methods.


Essential Ideas 1. 2 Phases and Classification of Matter has both mass and volume. Pure substances cannot be broken down into simpler substances by physical means. They have constant composition. Elements cannot be broken down into simpler substances by chemical means, compounds can. Mixtures are composed of two or more pure substances physically combined. They can be separated into pure substances by physical means. Homogenous mixtures have the same composition throughout. Heterogeneous mixtures have a variable composition.

Essential Ideas 1. 3 Physical and Chemical Properties

Two types of Properties distinguish different kinds of matter: § Chemical properties § Physical properties are those that can be observed without changing or trying to change the composition of the sample.

Can anyone think of any physical properties ? Color Luster State Shape Hardness Size Melting point Solubility Boiling point Density Mass

Chemical properties describe what happens when attempts are made to change matter into other kinds of matter. Determining chemical properties results in the composition of the sample changing.

Can anyone think of any chemical properties ? Flammability Explosiveness Reactivity Corrosiveness

Properties can also be classified as either extensive or intensive. Extensive properties depend on the amount of the substance i. e. mass, volume Intensive properties are independent on the amount of the substance. i. e. density, color, temperature

Physical changes are those that do not result in a change in the composition of the sample e. g. § Subdivision (dividing into smaller pieces) § Dissolution (dissolving in a solvent) § Changing shape § Changes of state § Boiling (liquid to gas), § Melting (solid to liquid) § Sublimation (solid to gas)

Chemical changes are those that do result in a change in the composition of the sample e. g. § Combustion (burning in oxygen) § Decomposition (one substance forming 2 or more new substances) § Combination (two or more substances combing to form a single substance)

Essential Ideas 1. 3 Physical and Chemical Properties of matter allow us to distinguish one sample of matter form another. Physical properties can be observed without attempting to change the composition of a sample of matter. Chemical changes are observed when attempts are made to change the composition of a sample. Properties may also be classified as being either extensive (dependent on the amount of substance) or intensive (not dependent on the amount of substance). Chemical changes result in a change in composition, physical changes do not.

Essential Ideas 1. 4 Measurements

All measurements have at least two parts a number (the magnitude) and units. A measurement may also include such things as the direction, e. g. “I drove at 30 mph east. ” or an estimate of the variance in the measurement. e. g. “today’s maximum temperature was 27. 5 ± 0. 2 o. C”

In chemistry we use the international system of units. This is a modern version of the metric system. Unfortunately this system of units is not widely used in everyday life in the USA. Being able to use conversion factors and formulas to transform measurements between systems of units is extremely important.

There have been many serious incidents that have resulted from errors in converting between systems of units. Air Canada Flight 143 (You. Tube has the “air crash investigation” episode about this)

$193 million Mars Climate Orbiter. Lost in Space.

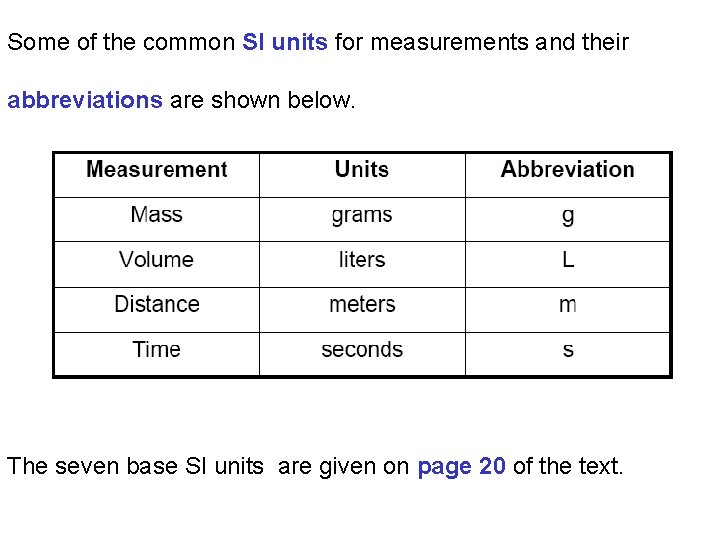
Some of the common SI units for measurements and their abbreviations are shown below. The seven base SI units are given on page 20 of the text.

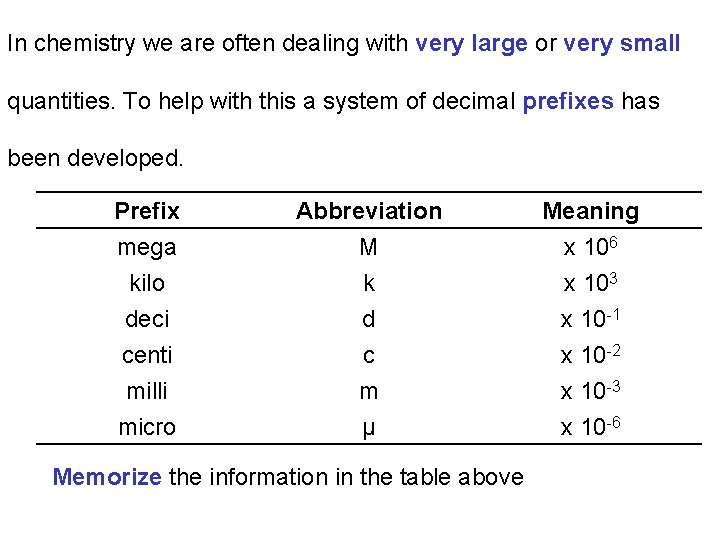
In chemistry we are often dealing with very large or very small quantities. To help with this a system of decimal prefixes has been developed. Prefix mega kilo deci Abbreviation M k d Meaning x 106 x 103 x 10 -1 centi milli micro c m μ x 10 -2 x 10 -3 x 10 -6 Memorize the information in the table above

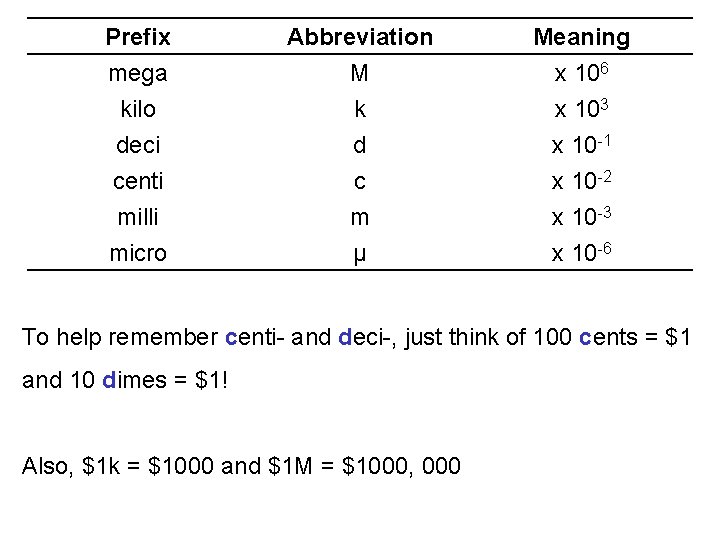
Prefix mega kilo deci Abbreviation M k d Meaning x 106 x 103 x 10 -1 centi milli micro c m μ x 10 -2 x 10 -3 x 10 -6 To help remember centi- and deci-, just think of 100 cents = $1 and 10 dimes = $1! Also, $1 k = $1000 and $1 M = $1000, 000

Errors involving prefix modifiers has been a particular problem with the dispensing of medications and there have been many deaths as a result. When looking at a measurement you should consider: What is being measured ? What are the units ? What prefix modifiers are being used ?

Essential Ideas 1. 4 Measurements Scientists use the SI system of units. To assist in written very large and very small numbers a system of decimal prefixes has been developed.

Essential Ideas 1. 5 Measurement Uncertainty, Accuracy and Precision

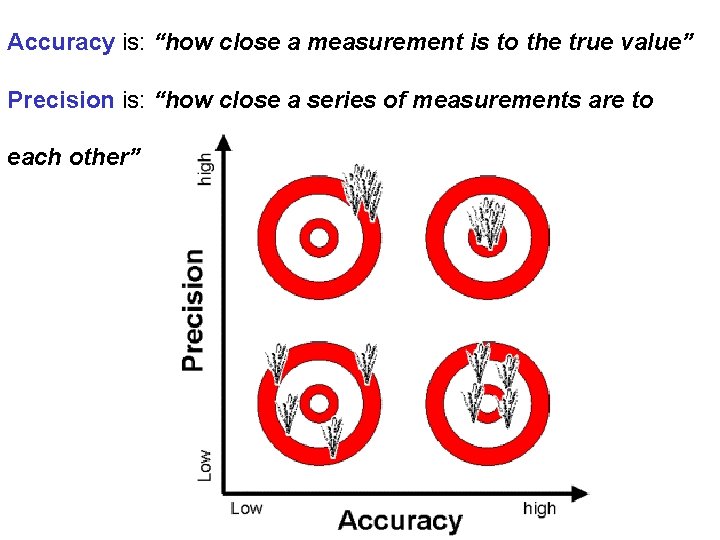
Accuracy is: “how close a measurement is to the true value” Precision is: “how close a series of measurements are to each other”

Accuracy and precision depend upon systematic and random error. Systematic error is when all measurements are either larger or smaller than the actual value in a consistent fashion. e. g. a scale always weighs objects to be 2 pounds heavier than they really are. Systematic error can be corrected for through calibration.

Random error results in measurements that are both larger and smaller than the actual value. Random error results from limitations of the person making the measurement and the device being used. Random error can be reduced by taking a large number of measurements and determining the average.

Exact numbers are numbers that have no uncertainty associated with them. • Whole number of objects • Unit definitions e. g. 12 inches in a foot • Simple Fractions (1/2, 1/3, 5/9 etc) 3 pennies 10 mm in 1 cm

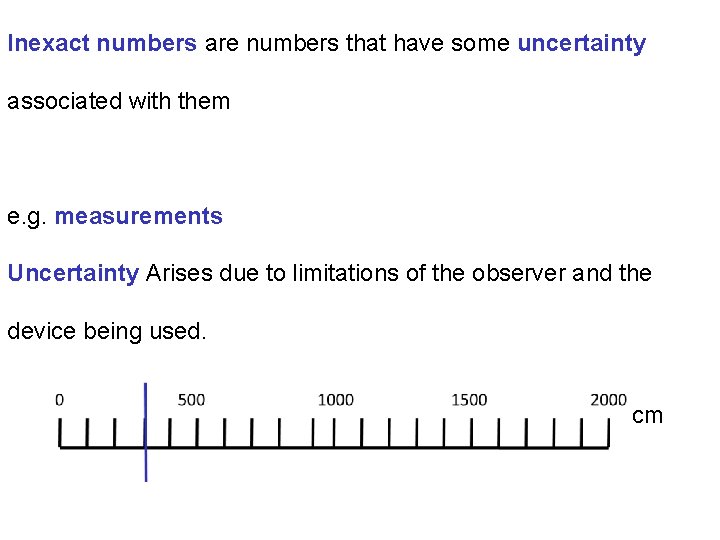
Inexact numbers are numbers that have some uncertainty associated with them e. g. measurements Uncertainty Arises due to limitations of the observer and the device being used. cm

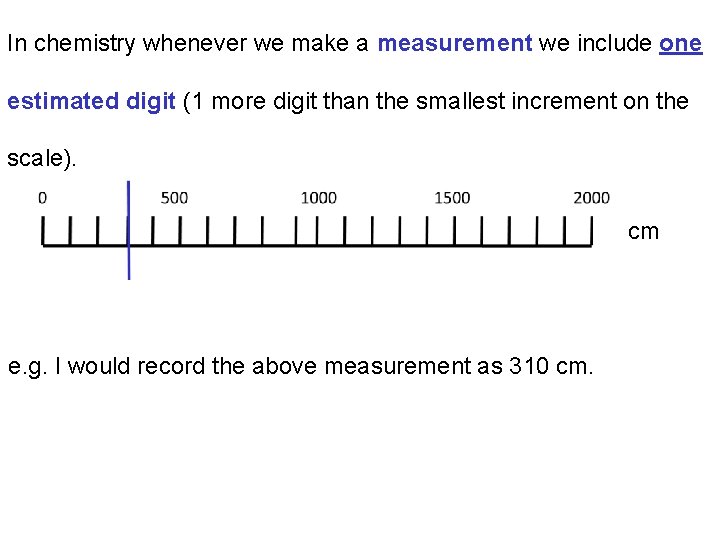
In chemistry whenever we make a measurement we include one estimated digit (1 more digit than the smallest increment on the scale). cm e. g. I would record the above measurement as 310 cm.

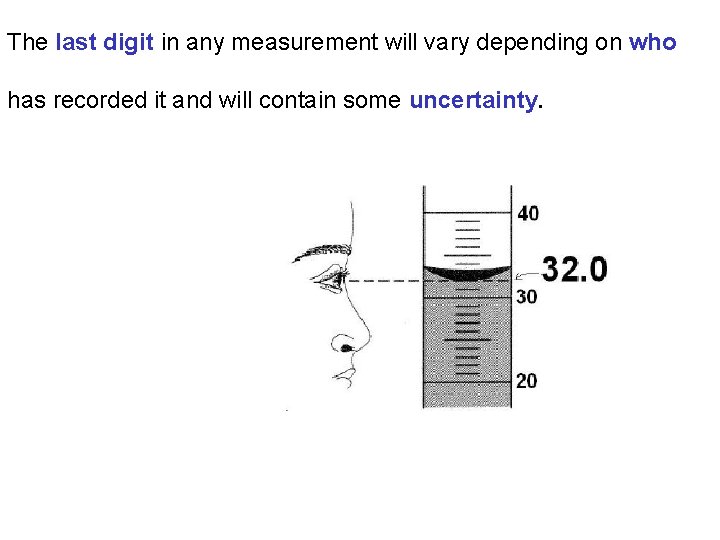
The last digit in any measurement will vary depending on who has recorded it and will contain some uncertainty.

We define the number of significant figures in a measurement as: “the number of digits known with certainty plus one digit that is estimated” Number of significant figures = all certain digits + one estimated

The first rule in determining the number of significant figures in a measurement is that all non-zero numbers are significant. e. g. 15 has 2 significant figures.

When the number contains zeros determining the number of significant figures becomes more complex. § “Leading zeroes” are not significant. 0. 00000012 and 0. 012 both have 2 significant figures. §“Confined zeros” located between non-zero numbers are significant. 0. 05003 has 4 significant figures.

If the number contains a decimal point trailing zeros are significant. 0. 2500 has 4 significant figures If the number does not contain a decimal point trailing zeros are not significant. 2500 has 2 significant figures

1. All non-zero numbers are significant. 2. Confined (Buried) zeroes are significant 3. Leading zeroes are not significant 4. Trailing zeroes are only significant if the number contains a decimal point

Operational rules are used to determine how many significant figures our answer requires. 1. For division and multiplication our answer contains the number of significant figures as the number with the least number of significant figures. e. g. 26. 003/25. 0 = 1. 04

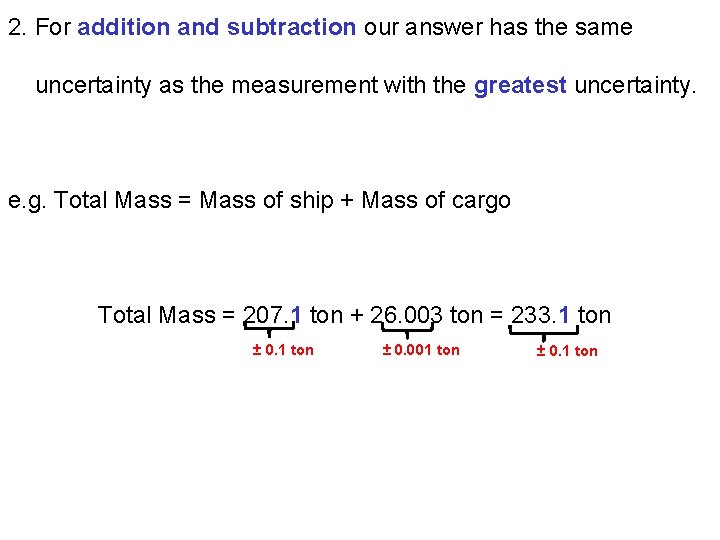
2. For addition and subtraction our answer has the same uncertainty as the measurement with the greatest uncertainty. e. g. Total Mass = Mass of ship + Mass of cargo Total Mass = 207. 1 ton + 26. 003 ton = 233. 1 ton ± 0. 001 ton ± 0. 1 ton

When I enter the following into my calculator: d = 26. 003 g / 25. 0 m. L I obtain: d = 1. 04012 g/m. L What do I do with these numbers? But I know my answer should contain 3 sig. figs.

Rounding rules describe how we deal with numbers we are truncating. 1. If the first number being dropped is ≤ 4 leave the last digit as is. 2. If the first number being dropped is ≥ 5 increase the value of the last digit by one. d = 26. 003 g / 25. 0 m. L = 1. 04012 g/m. L

Exact numbers play no role in determining the number of significant figures in calculations. e. g. What is the total length of 3 sticks each with a length of 30. 0 cm placed end-to-end? Total length = 3 x 30. 0 cm = 90. 0 cm

Essential Ideas 1. 5 Measurement Uncertainty, Accuracy and Precision Scientists always include one estimated digit in their measurements. Exact numbers have no uncertainty. The number of certain digits plus the uncertain digit of a measurement is referred to as the number of significant figures a measurement has. Operational rules describe how the correct number of significant figures is arrived at when measured values are used in calculations. Rounding rules are used to truncate the answer of a calculation to the correct number of significant figures. Accuracy describes how close a measured value is to the true value. Precision describes how close a series of measurements are to each other.

Essential Ideas 1. 6 Mathematical treatment of Measurement Results

Decimal numbers become difficult to use and are prone to error when dealing with very large or very small numbers: e. g. 6023000000 molecules or 0. 000001 meters Very large and very small numbers are common in chemistry

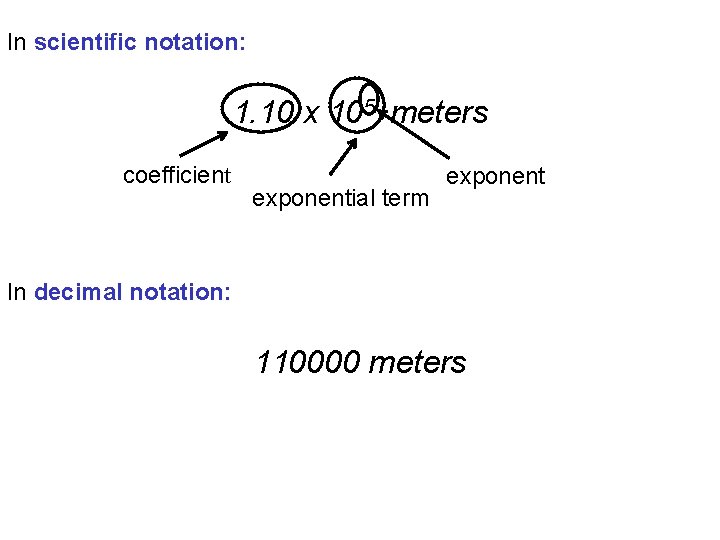
In scientific notation: 1. 10 x 105 meters coefficient exponential term exponent In decimal notation: 110000 meters

Scientific notation is often needed to express the correct number of significant figures. 1. 10 x 105 meters (3 s. f. ) In decimal notation: 110000 meters (2 s. f. )

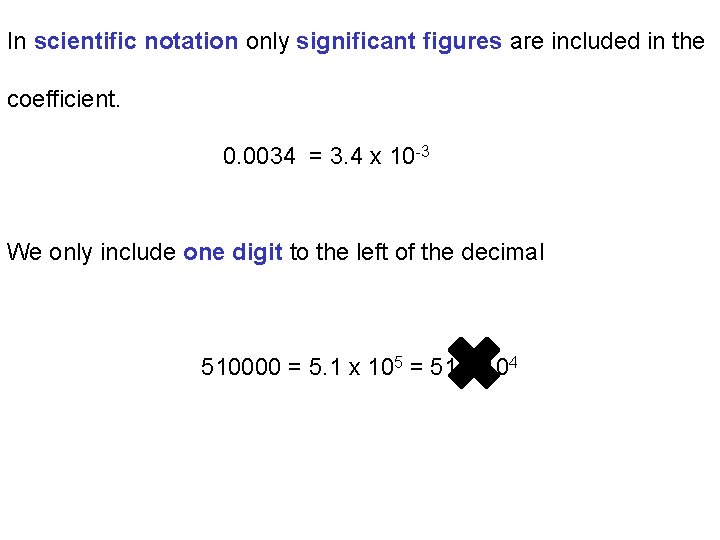
In scientific notation only significant figures are included in the coefficient. 0. 0034 = 3. 4 x 10 -3 We only include one digit to the left of the decimal 510000 = 5. 1 x 105 = 51 x 104

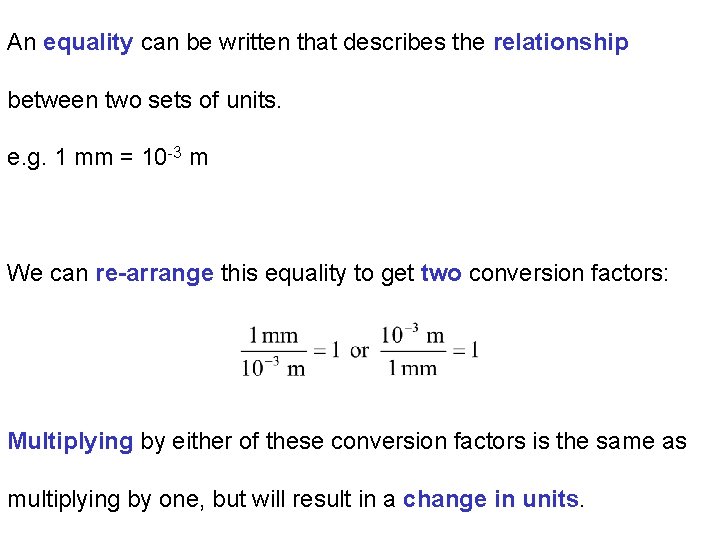
An equality can be written that describes the relationship between two sets of units. e. g. 1 mm = 10 -3 m We can re-arrange this equality to get two conversion factors: Multiplying by either of these conversion factors is the same as multiplying by one, but will result in a change in units.

We use a conversion factor that divides by the units we wish to cancel and multiplies by the units we want in our answer. e. g. Q. Express 1400 millimeters in meters. A.

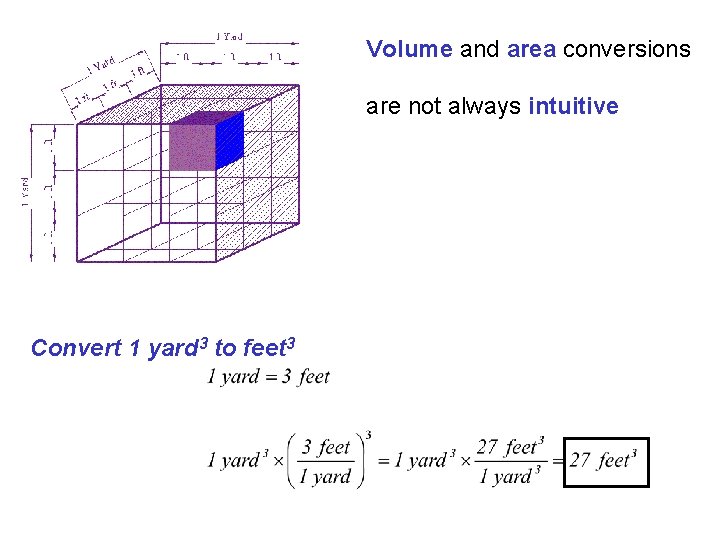
Volume and area conversions are not always intuitive Convert 1 yard 3 to feet 3

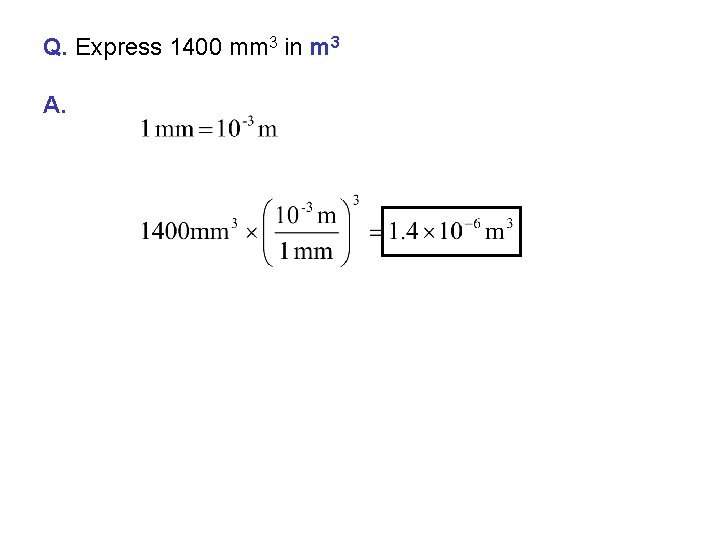
Q. Express 1400 mm 3 in m 3 A.

Unit conversions involving time are common and may require multiple conversion factors. Q. Express 2 weeks in seconds A.

Unit conversions involving ratio’s can often be confusing. Speed is the ratio of distance travelled in a given time. Q. Express 3 mi/hr in mi/s. A.

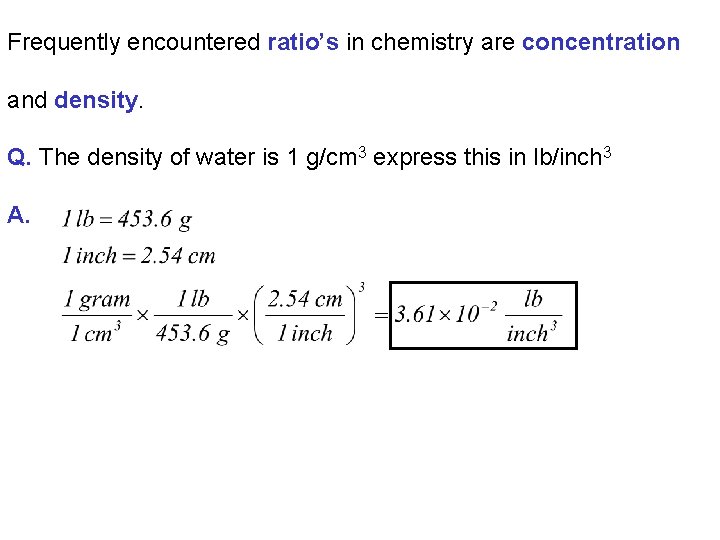
Frequently encountered ratio’s in chemistry are concentration and density. Q. The density of water is 1 g/cm 3 express this in lb/inch 3 A.

The three systems of units for temperature commonly used are the Celsius, Fahrenheit and Kelvin scale. The Fahrenheit scale is the least used in chemistry, although it is the most commonly used in daily life in the USA. For temperature we use formulas to convert from different systems of units.

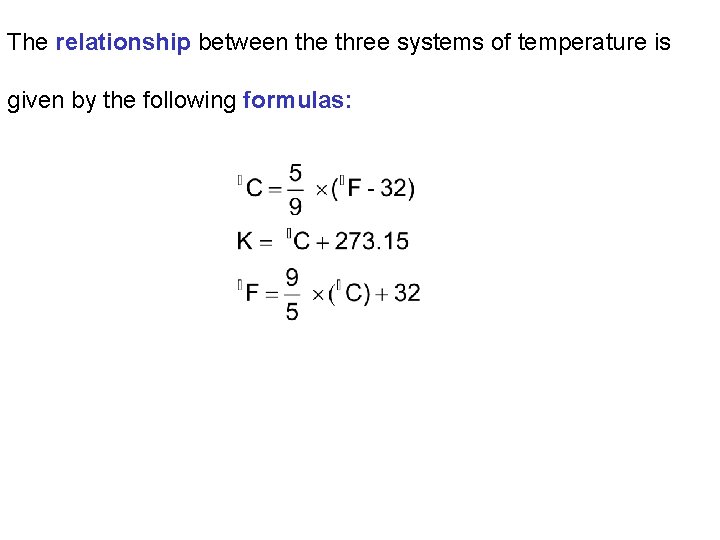
The relationship between the three systems of temperature is given by the following formulas:

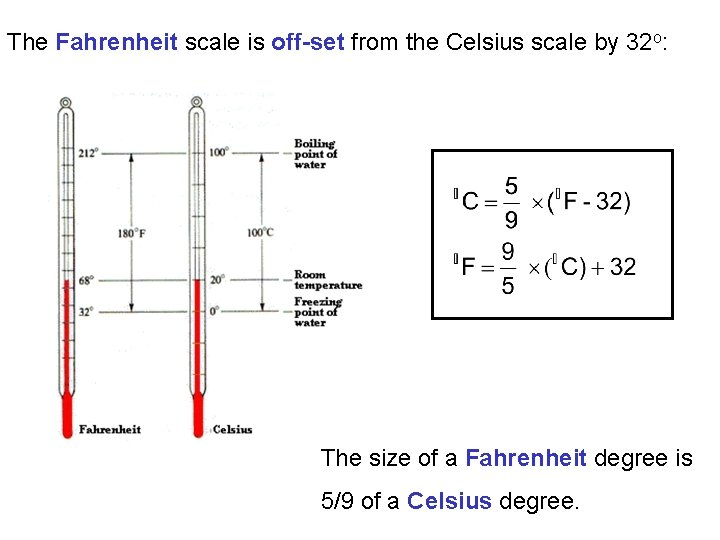
The Fahrenheit scale is off-set from the Celsius scale by 32 o: The size of a Fahrenheit degree is 5/9 of a Celsius degree.

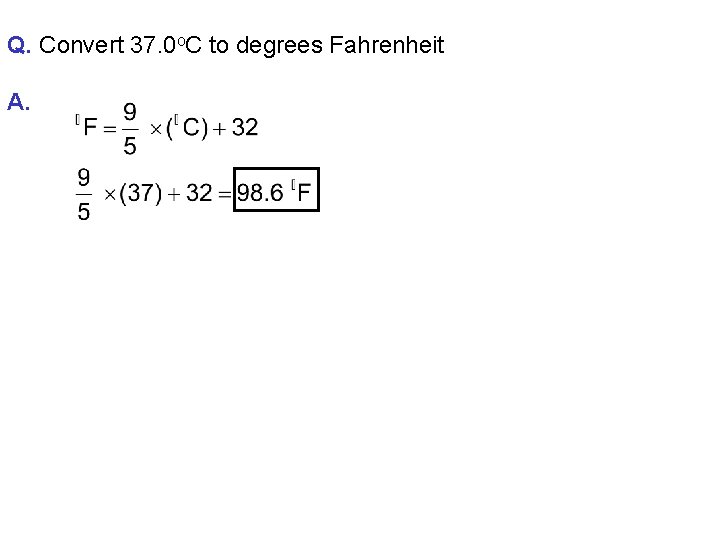
Q. Convert 37. 0 o. C to degrees Fahrenheit A.

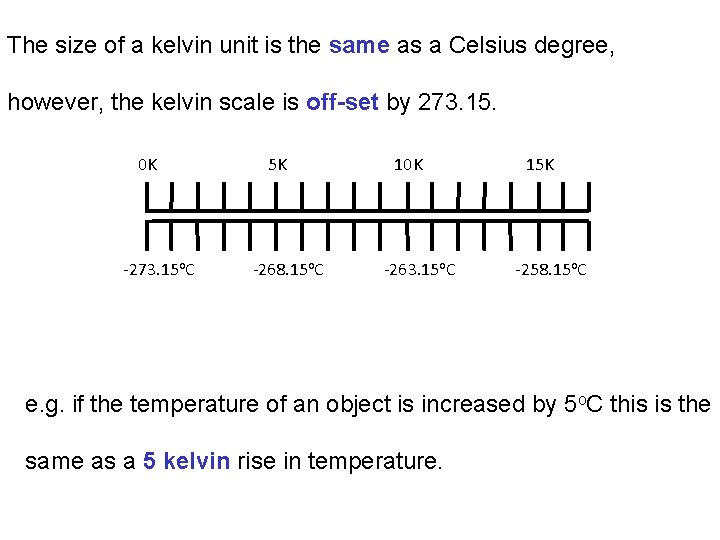
The size of a kelvin unit is the same as a Celsius degree, however, the kelvin scale is off-set by 273. 15. 0 K -273. 15 o. C 5 K -268. 15 o. C 10 K -263. 15 o. C 15 K -258. 15 o. C e. g. if the temperature of an object is increased by 5 o. C this is the same as a 5 kelvin rise in temperature.

Q. Convert 37. 00 o. C to Kelvin A.

Google is a great tool for checking your answers to conversion problems. Of course you still need to practice for when you can’t use a computer or need to show your working !!

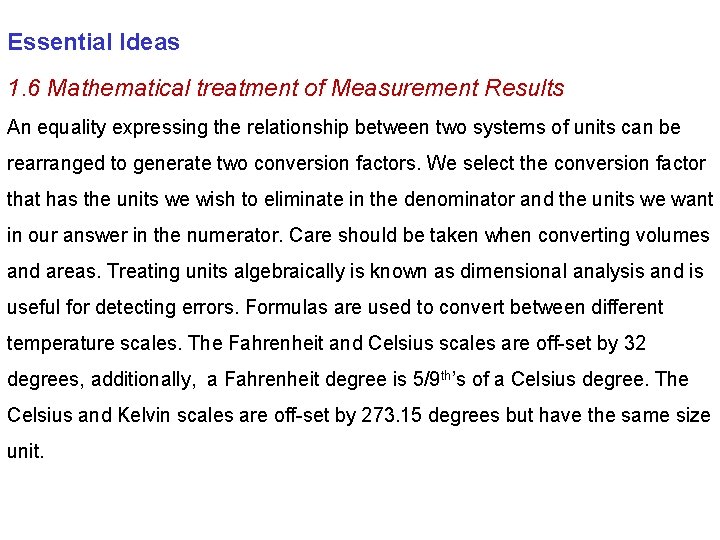
Essential Ideas 1. 6 Mathematical treatment of Measurement Results An equality expressing the relationship between two systems of units can be rearranged to generate two conversion factors. We select the conversion factor that has the units we wish to eliminate in the denominator and the units we want in our answer in the numerator. Care should be taken when converting volumes and areas. Treating units algebraically is known as dimensional analysis and is useful for detecting errors. Formulas are used to convert between different temperature scales. The Fahrenheit and Celsius scales are off-set by 32 degrees, additionally, a Fahrenheit degree is 5/9 th’s of a Celsius degree. The Celsius and Kelvin scales are off-set by 273. 15 degrees but have the same size unit.
 If your instructor were to ask if you cleaned up your room
If your instructor were to ask if you cleaned up your room Gezang 161
Gezang 161 Gezang 161
Gezang 161 Vidas blue
Vidas blue Port udp 161
Port udp 161 Opwekking 86
Opwekking 86 Convenio 161 oit resumen
Convenio 161 oit resumen Ucr cs 161
Ucr cs 161 Computer security 161 cryptocurrency lecture
Computer security 161 cryptocurrency lecture Jelena đorđevic 161
Jelena đorđevic 161 Inls 161
Inls 161 Computer science 161
Computer science 161 Astronomy 161
Astronomy 161 Mcp-161
Mcp-161 Glosf
Glosf Pa 161 program
Pa 161 program Ac 161
Ac 161 Error 161
Error 161 Drba-161
Drba-161 Csc 161 rochester
Csc 161 rochester Xdbx
Xdbx Bvp 161
Bvp 161 Gezang 177
Gezang 177 Gretine
Gretine Astronomy 161
Astronomy 161 160 161
160 161 Nabl 161
Nabl 161 180-161
180-161 Give us your hungry your tired your poor
Give us your hungry your tired your poor Participante pasivo
Participante pasivo Basic instructor course tcole
Basic instructor course tcole Basic instructor course texas
Basic instructor course texas Basic instructor course tcole
Basic instructor course tcole Pepperball hotshot
Pepperball hotshot Everyone selected to serve on this jury
Everyone selected to serve on this jury Instructor vs teacher
Instructor vs teacher Ospfv
Ospfv Mptc firearms
Mptc firearms Tcole advanced instructor course
Tcole advanced instructor course Basic instructor course #1014
Basic instructor course #1014 The virtual instructor
The virtual instructor Nfpa 1403 instructor to student ratio
Nfpa 1403 instructor to student ratio Human factors instructor
Human factors instructor Instructor operating station
Instructor operating station Catia instructor
Catia instructor Instructor
Instructor Instructor responsibilities and professionalism
Instructor responsibilities and professionalism Tcole 1014 basic instructor course
Tcole 1014 basic instructor course Jrotc marksmanship instructor course online
Jrotc marksmanship instructor course online Nrp instructor toolkit
Nrp instructor toolkit Utp cable
Utp cable What is cbrf training
What is cbrf training Nra certified instructor logo
Nra certified instructor logo Naismith was an instructor of
Naismith was an instructor of Tcole advanced instructor course
Tcole advanced instructor course Tcole advanced instructor course
Tcole advanced instructor course Jrotc marksmanship instructor course online
Jrotc marksmanship instructor course online 15 sec illusion
15 sec illusion Acr medical term
Acr medical term Basic instructor course #1014
Basic instructor course #1014 Basic instructor course tcole
Basic instructor course tcole Delmar cengage learning instructor resources
Delmar cengage learning instructor resources Instructor office hours
Instructor office hours Diferencia entre gran plano general y plano general
Diferencia entre gran plano general y plano general Where did general lee surrender to general grant?
Where did general lee surrender to general grant? General chemistry with qualitative analysis
General chemistry with qualitative analysis General chemistry thermochemistry
General chemistry thermochemistry General chemistry nomenclature
General chemistry nomenclature General chemistry 2
General chemistry 2 General chemistry ders notları
General chemistry ders notları General chemistry 11th edition
General chemistry 11th edition General chemistry 1 stoichiometry
General chemistry 1 stoichiometry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry Ib chemistry functional groups
Ib chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Coping with gay loneliness
Coping with gay loneliness Stop blaming your parents for your problems
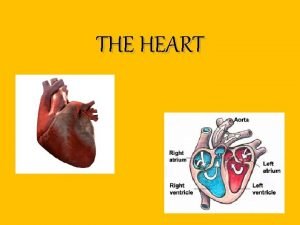
Stop blaming your parents for your problems How does the heart pump work
How does the heart pump work Be glad your nose is on your face
Be glad your nose is on your face Introduce your friend to your teacher
Introduce your friend to your teacher Cara ikatan buku sila
Cara ikatan buku sila In your notebook answer the questions
In your notebook answer the questions In your notebook define the following terms in your own
In your notebook define the following terms in your own Enagic compensation plan
Enagic compensation plan Your conscious awareness of your own name
Your conscious awareness of your own name How to respond to teach me your ways
How to respond to teach me your ways If your vehicle malfunctions turn on your hazard lights
If your vehicle malfunctions turn on your hazard lights It is an idealized image that we have developed over time
It is an idealized image that we have developed over time Article writing format for school magazine
Article writing format for school magazine Where your treasure is there your heart will be also
Where your treasure is there your heart will be also Remember your lord in the days of your youth
Remember your lord in the days of your youth My wish for you is that life becomes
My wish for you is that life becomes Your passenger jammed his finger in the door of your m1114
Your passenger jammed his finger in the door of your m1114 First reconcile with your brother
First reconcile with your brother Solve each proportion write your answer in your notebook
Solve each proportion write your answer in your notebook In your notebook write your cv
In your notebook write your cv Treasure what you have
Treasure what you have How old old are you
How old old are you I throw my warlike shield
I throw my warlike shield