AP CHEMISTRY 1307 General Chemistry I Instructor Mrs


















- Slides: 56

AP CHEMISTRY 1307 General Chemistry I Instructor: Mrs. Anna Mkrtchyan-Antonyan Classroom: 219 Phone: EX. 209 E-Mail: amkrtchyan@agbumds. org Text: Chemistry: Principles and Reactions Masterton • Hurley 5 th Edition

CHEMISTRY: Chemistry is the study of the properties, composition, and structure of matter, the physical and chemical changes it undergoes, and the energy liberated or absorbed during those changes.

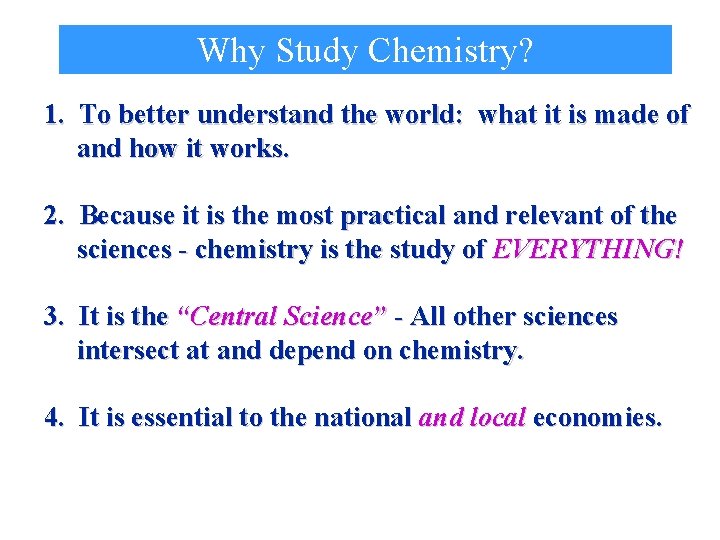
Why Study Chemistry? 1. To better understand the world: what it is made of and how it works. 2. Because it is the most practical and relevant of the sciences - chemistry is the study of EVERYTHING! 3. It is the “Central Science” - All other sciences intersect at and depend on chemistry. 4. It is essential to the national and local economies.

Chapter 1: Matter and Measurements

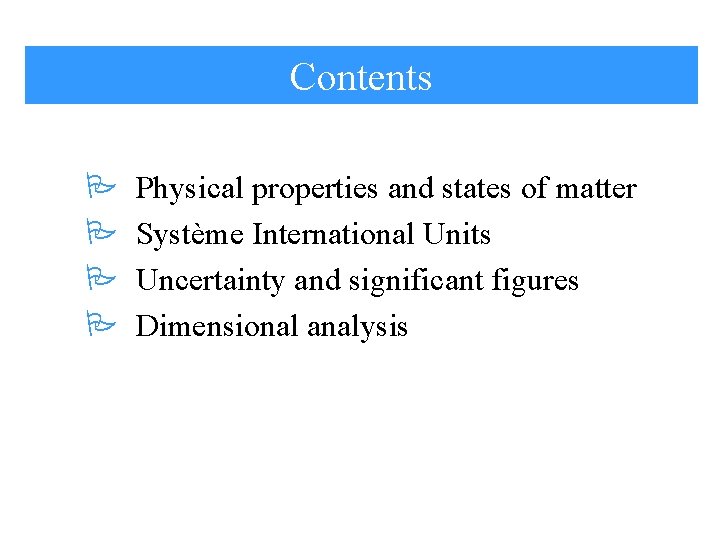
Contents P P Physical properties and states of matter Système International Units Uncertainty and significant figures Dimensional analysis

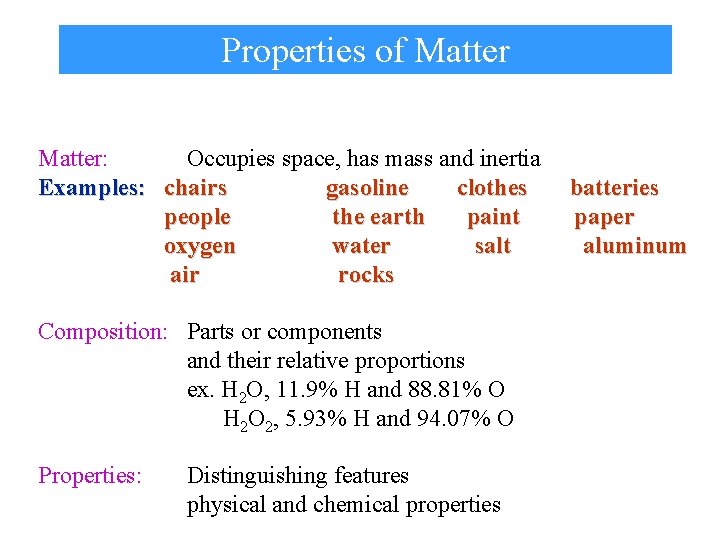
Properties of Matter: Occupies space, has mass and inertia Examples: chairs gasoline clothes people the earth paint oxygen water salt air rocks Composition: Parts or components and their relative proportions ex. H 2 O, 11. 9% H and 88. 81% O H 2 O 2, 5. 93% H and 94. 07% O Properties: Distinguishing features physical and chemical properties batteries paper aluminum

Matter and Change • Physical Property- A property displayed without a change in composition E. g. color. • Chemical Property – Ability or inability of a sample of matter to undergo a change in composition under stated conditions.

Matter and Change • Physical Change - A change in which each substance involved in the change retains its original identity and no new elements or compounds are formed. H 2 O (s) H 2 O (l) “ice” Melting

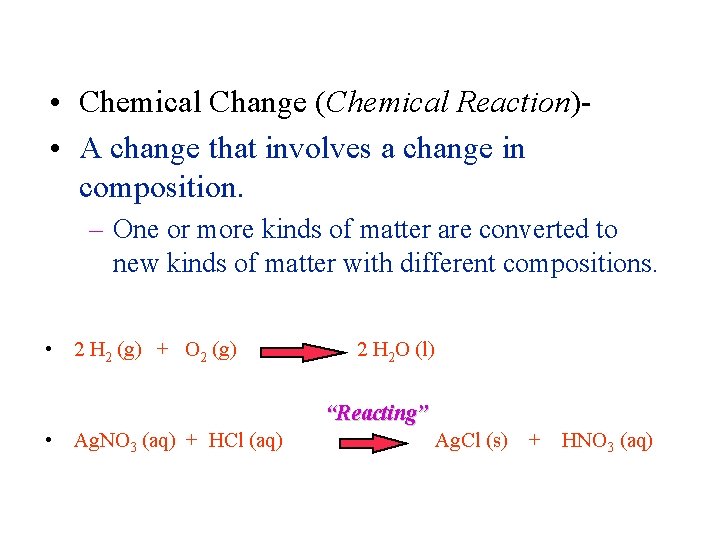
Matter and Change • Chemical Change (Chemical Reaction) • A change that involves a change in composition. – One or more kinds of matter are converted to new kinds of matter with different compositions. • 2 H 2 (g) + O 2 (g) 2 H 2 O (l) “Reacting” • Ag. NO 3 (aq) + HCl (aq) Ag. Cl (s) + HNO 3 (aq)

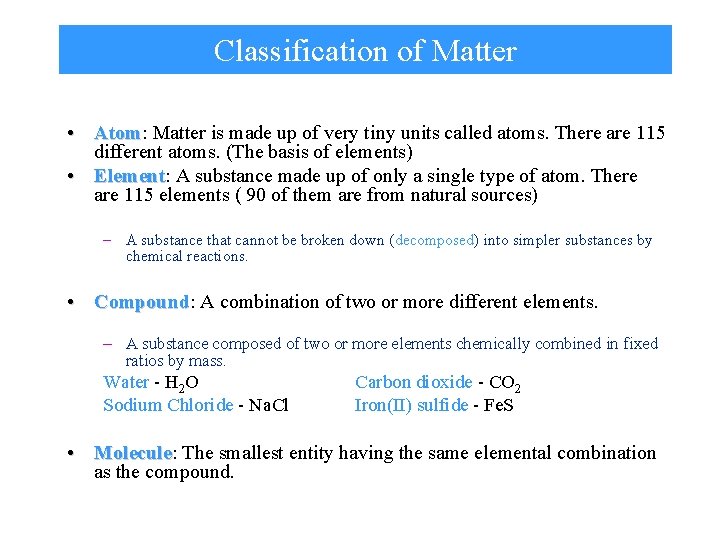
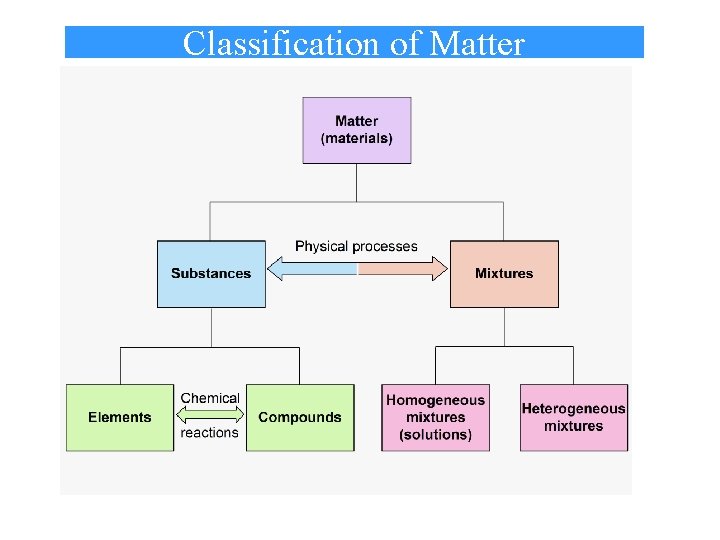
Classification of Matter • Atom: Atom Matter is made up of very tiny units called atoms. There are 115 different atoms. (The basis of elements) • Element: Element A substance made up of only a single type of atom. There are 115 elements ( 90 of them are from natural sources) – A substance that cannot be broken down (decomposed) into simpler substances by chemical reactions. • Compound: Compound A combination of two or more different elements. – A substance composed of two or more elements chemically combined in fixed ratios by mass. Water - H 2 O Sodium Chloride - Na. Cl Carbon dioxide - CO 2 Iron(II) sulfide - Fe. S • Molecule: Molecule The smallest entity having the same elemental combination as the compound.


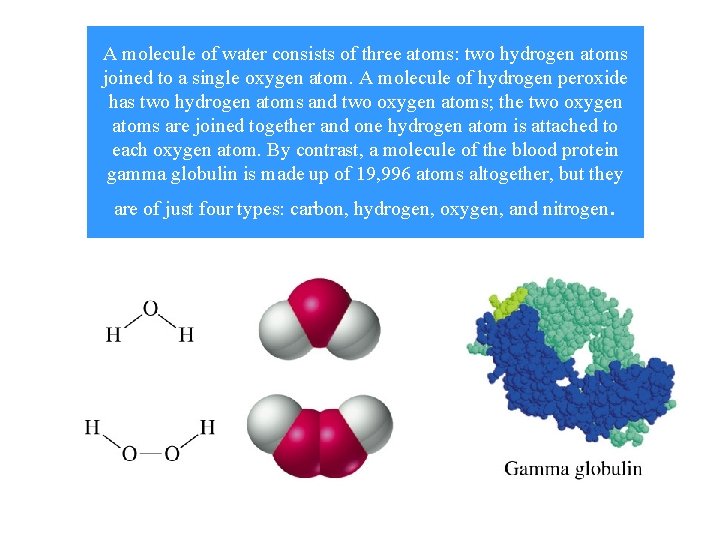
A molecule of water consists of three atoms: two hydrogen atoms joined to a single oxygen atom. A molecule of hydrogen peroxide has two hydrogen atoms and two oxygen atoms; the two oxygen atoms are joined together and one hydrogen atom is attached to each oxygen atom. By contrast, a molecule of the blood protein gamma globulin is made up of 19, 996 atoms altogether, but they are of just four types: carbon, hydrogen, oxygen, and nitrogen .

• Substance: Substance Pure elements and compounds are callled substances • Mixture: Mixture Combination of elements and compounds.

Classification of Matter

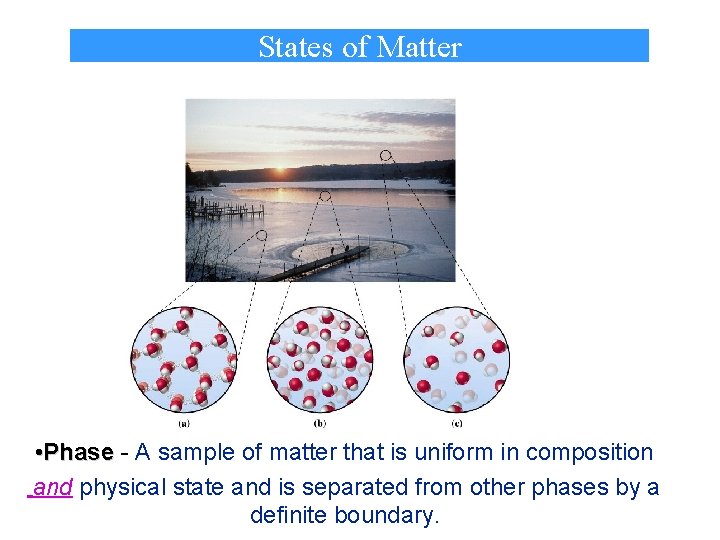
States of Matter • Phase - A sample of matter that is uniform in composition and physical state and is separated from other phases by a definite boundary.

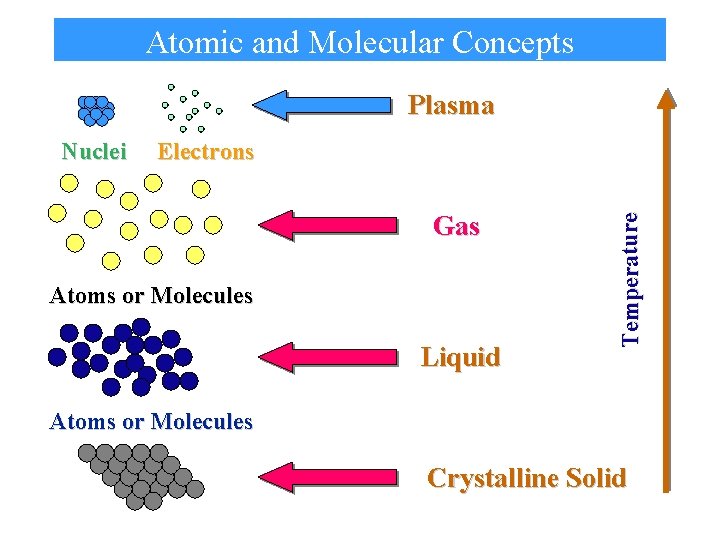
Atomic and Molecular Concepts Plasma Electrons Gas Atoms or Molecules Liquid Temperature Nuclei Atoms or Molecules Crystalline Solid



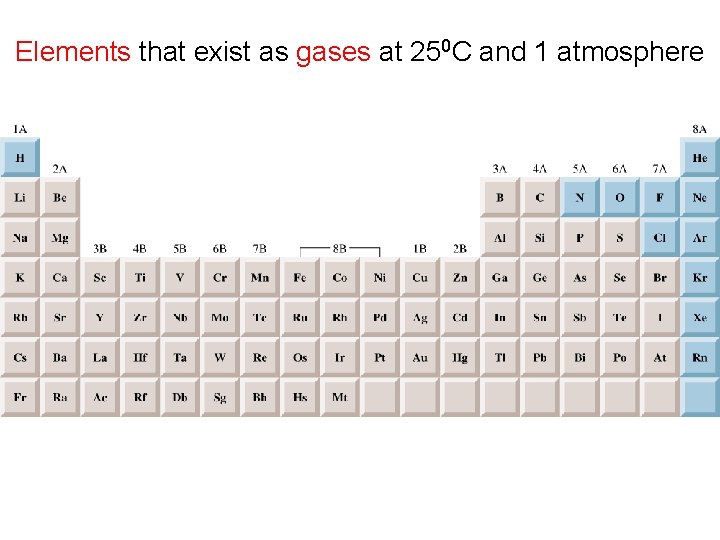
Elements that exist as gases at 250 C and 1 atmosphere

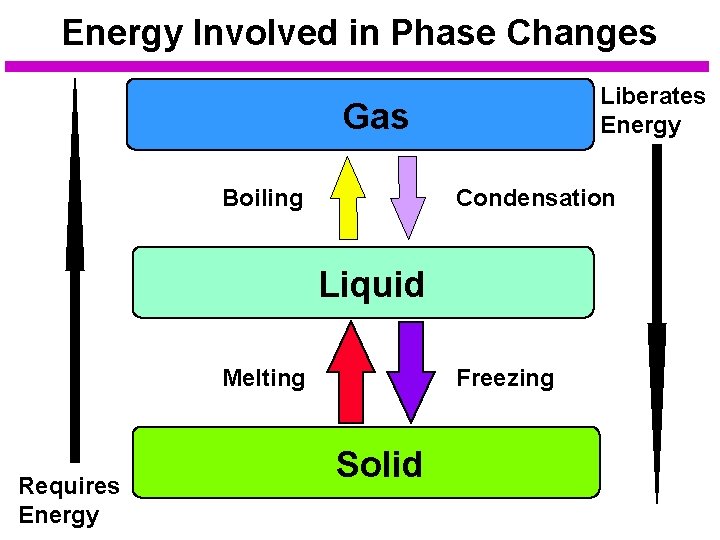
Energy Involved in Phase Changes Liberates Energy Gas Boiling Condensation Liquid Melting Requires Energy Freezing Solid

Measurement • Chemistry is an Observational science. • Chemistry is a Quantitative science. • Measurement - A quantitative observation.

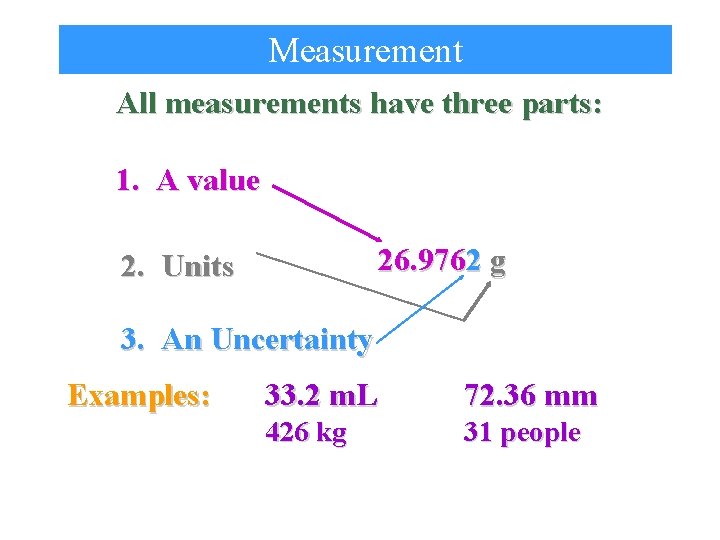
Measurement All measurements have three parts: 1. A value 26. 9762 g 2. Units 3. An Uncertainty Examples: 33. 2 m. L 72. 36 mm 426 kg 31 people

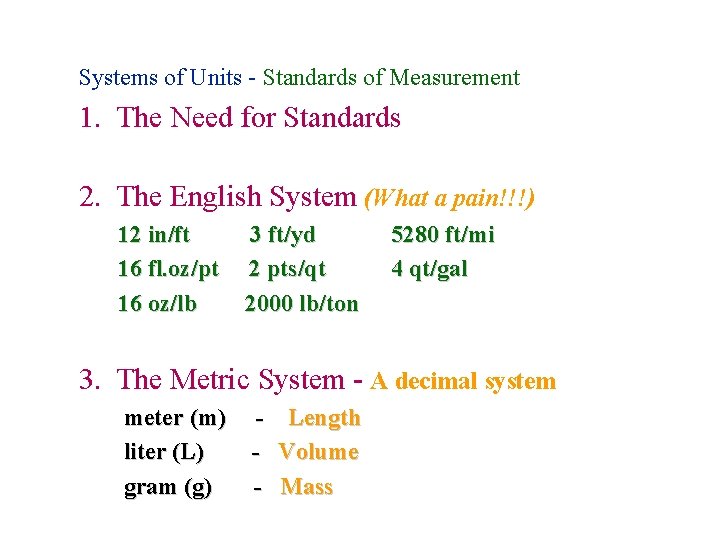
Measurement Systems of Units - Standards of Measurement 1. The Need for Standards 2. The English System (What a pain!!!) 12 in/ft 16 fl. oz/pt 16 oz/lb 3 ft/yd 2 pts/qt 2000 lb/ton 5280 ft/mi 4 qt/gal 3. The Metric System - A decimal system meter (m) liter (L) gram (g) - Length Volume Mass


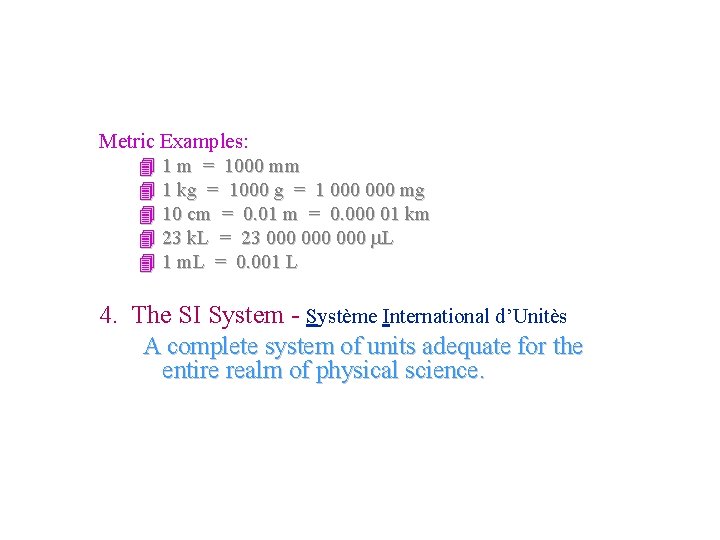
Measurement Metric Examples: 4 1 m = 1000 mm 4 1 kg = 1000 g = 1 000 mg 4 10 cm = 0. 01 m = 0. 000 01 km 4 23 k. L = 23 000 000 L 4 1 m. L = 0. 001 L 4. The SI System - Système International d’Unitès A complete system of units adequate for the entire realm of physical science.

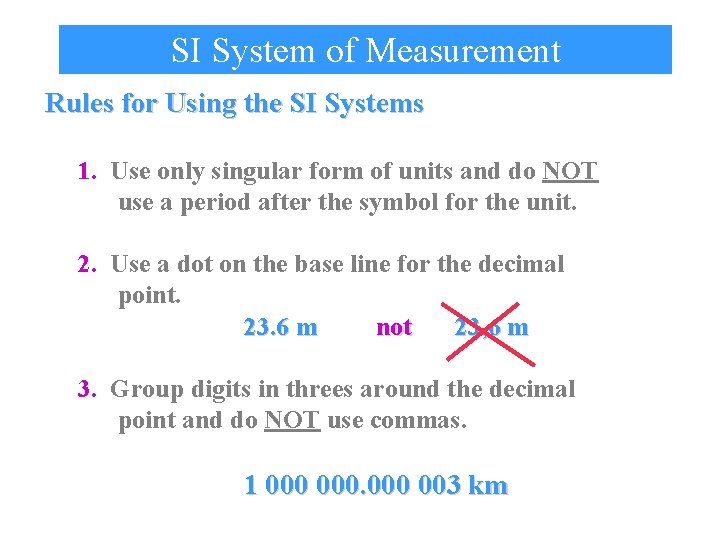
SI System of Measurement Rules for Using the SI Systems 1. Use only singular form of units and do NOT use a period after the symbol for the unit. 2. Use a dot on the base line for the decimal point. 23. 6 m not 23, 6 m 3. Group digits in threes around the decimal point and do NOT use commas. 1 000 003 km

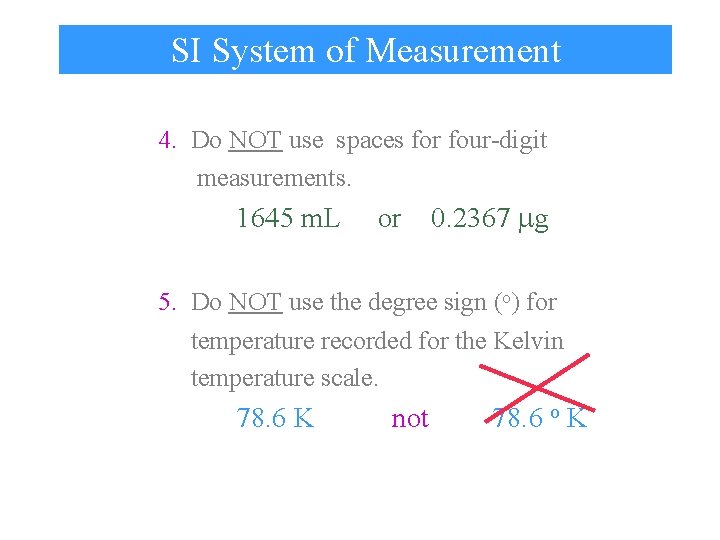
SI System of Measurement 4. Do NOT use spaces for four-digit measurements. 1645 m. L or 0. 2367 g 5. Do NOT use the degree sign (o) for temperature recorded for the Kelvin temperature scale. 78. 6 K not 78. 6 o K

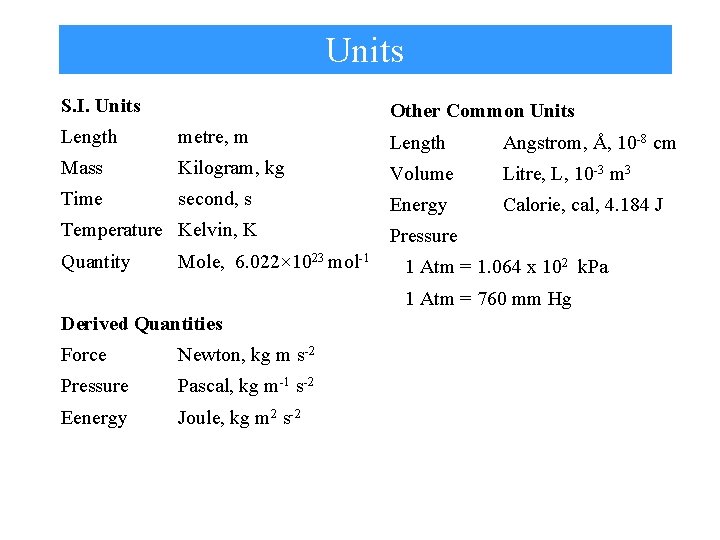
Units S. I. Units Other Common Units Length metre, m Length Angstrom, Å, 10 -8 cm Mass Kilogram, kg Volume Litre, L, 10 -3 m 3 Time second, s Energy Calorie, cal, 4. 184 J Temperature Kelvin, K Quantity Mole, 6. 022× 1023 mol-1 Pressure 1 Atm = 1. 064 x 102 k. Pa 1 Atm = 760 mm Hg Derived Quantities Force Newton, kg m s-2 Pressure Pascal, kg m-1 s-2 Eenergy Joule, kg m 2 s-2

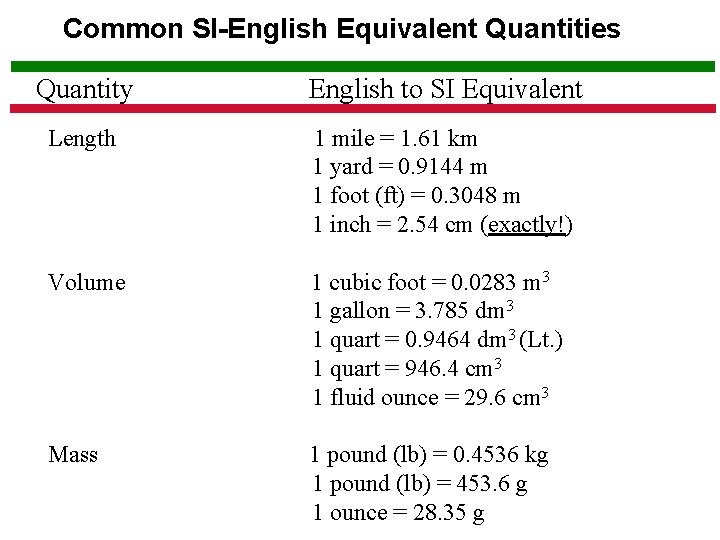
Common SI-English Equivalent Quantities Quantity English to SI Equivalent Length 1 mile = 1. 61 km 1 yard = 0. 9144 m 1 foot (ft) = 0. 3048 m 1 inch = 2. 54 cm (exactly!) Volume 1 cubic foot = 0. 0283 m 3 1 gallon = 3. 785 dm 3 1 quart = 0. 9464 dm 3 (Lt. ) 1 quart = 946. 4 cm 3 1 fluid ounce = 29. 6 cm 3 Mass 1 pound (lb) = 0. 4536 kg 1 pound (lb) = 453. 6 g 1 ounce = 28. 35 g

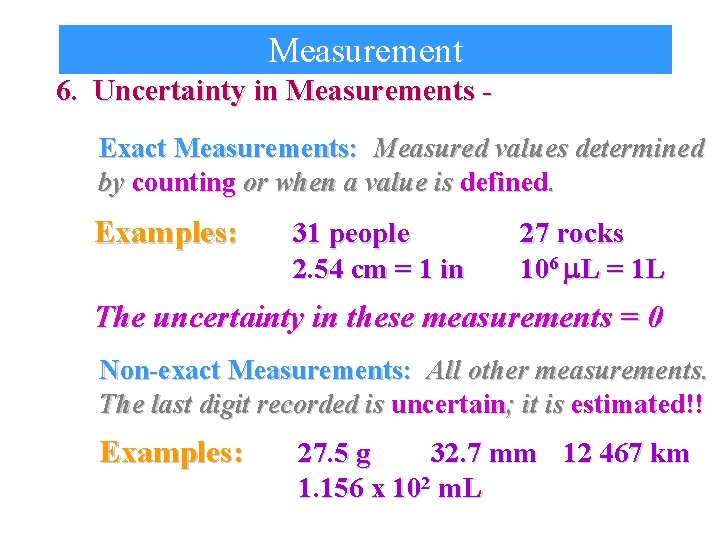
Measurement 6. Uncertainty in Measurements Exact Measurements: Measured values determined by counting or when a value is defined. Examples: 31 people 2. 54 cm = 1 in 27 rocks 106 m. L = 1 L The uncertainty in these measurements = 0 Non-exact Measurements: All other measurements. The last digit recorded is uncertain; it is estimated!! Examples: 27. 5 g 32. 7 mm 12 467 km 1. 156 x 102 m. L

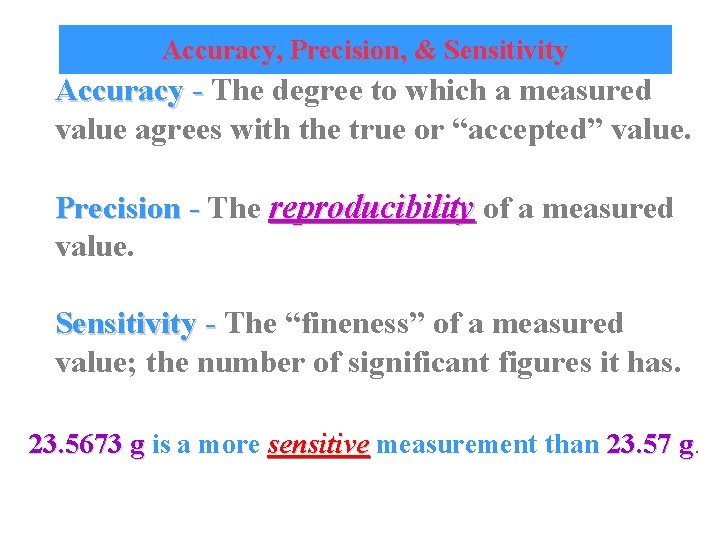
Accuracy, Precision, & Sensitivity Accuracy - The degree to which a measured value agrees with the true or “accepted” value. Precision - The reproducibility of a measured value. Sensitivity - The “fineness” of a measured value; the number of significant figures it has. 23. 5673 g is a more sensitive measurement than 23. 57 g. g

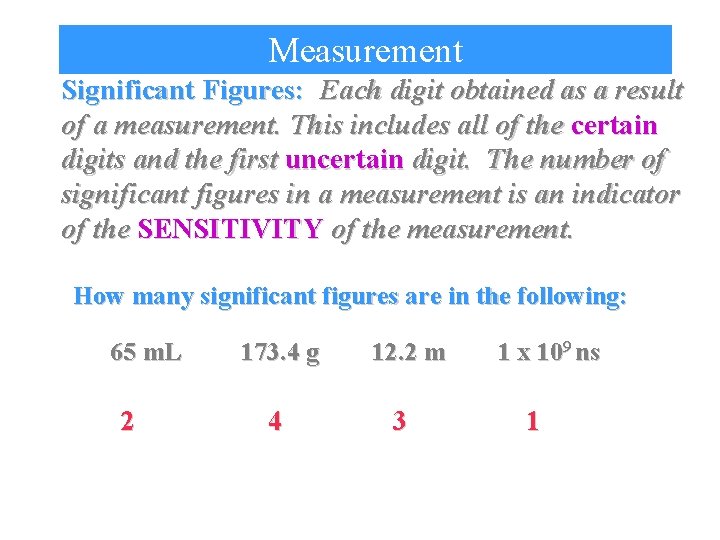
Measurement Significant Figures: Each digit obtained as a result of a measurement. This includes all of the certain digits and the first uncertain digit. The number of significant figures in a measurement is an indicator of the SENSITIVITY of the measurement. How many significant figures are in the following: 65 m. L 2 173. 4 g 4 12. 2 m 3 1 x 109 ns 1

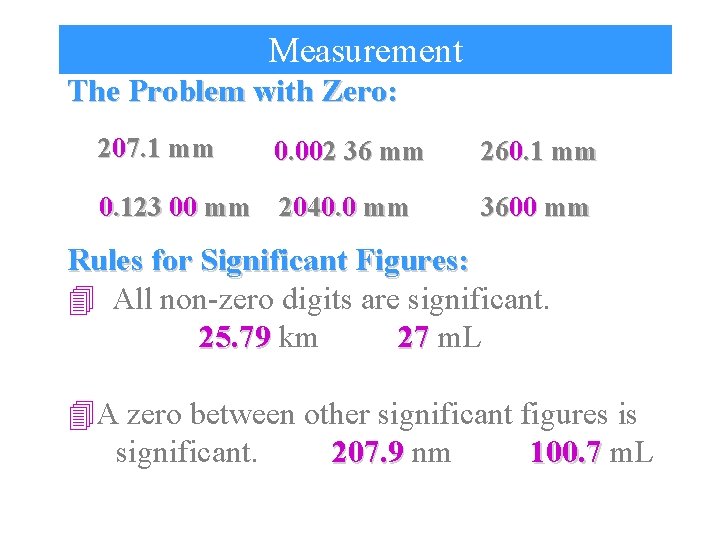
Measurement The Problem with Zero: 207. 1 mm 0. 002 36 mm 0. 123 00 mm 2040. 0 mm 260. 1 mm 3600 mm Rules for Significant Figures: 4 All non-zero digits are significant. 25. 79 km 27 m. L 4 A zero between other significant figures is significant. 207. 9 nm 100. 7 m. L

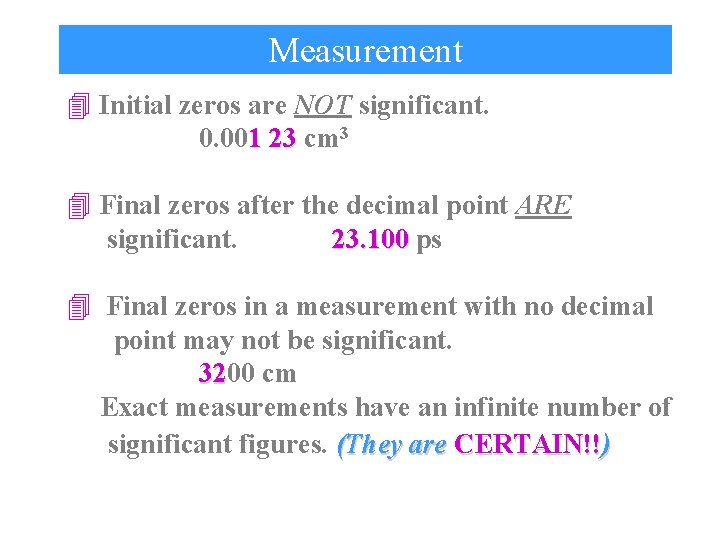
Measurement 4 Initial zeros are NOT significant. 0. 001 23 cm 3 4 Final zeros after the decimal point ARE significant. 23. 100 ps 4 Final zeros in a measurement with no decimal point may not be significant. 3200 32 cm Exact measurements have an infinite number of significant figures. (They are CERTAIN!!)

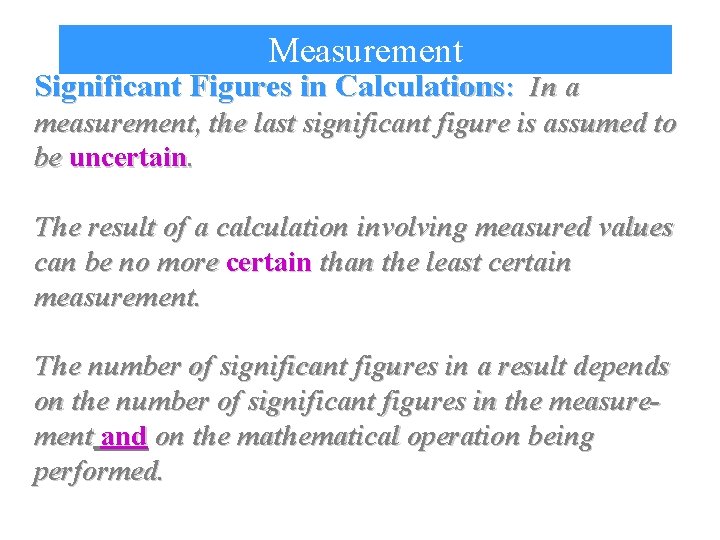
Measurement Significant Figures in Calculations: In a measurement, the last significant figure is assumed to be uncertain. The result of a calculation involving measured values can be no more certain than the least certain measurement. The number of significant figures in a result depends on the number of significant figures in the measurement and on the mathematical operation being performed.

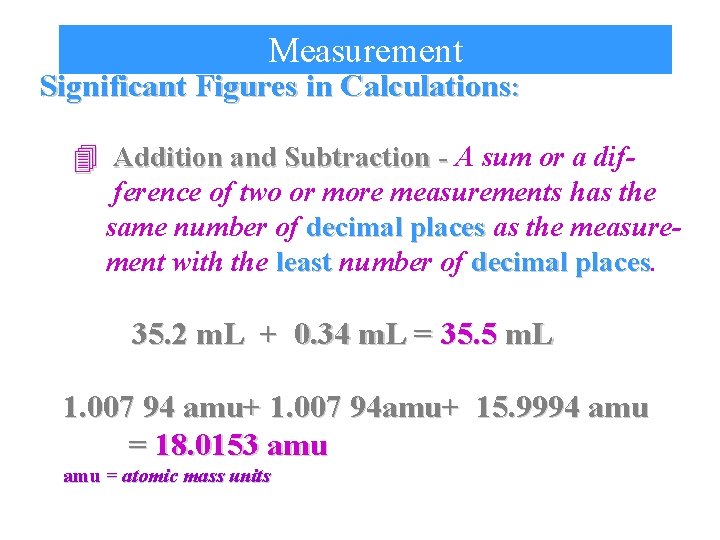
Measurement Significant Figures in Calculations: 4 Addition and Subtraction - A sum or a difference of two or more measurements has the same number of decimal places as the measurement with the least number of decimal places 35. 2 m. L + 0. 34 m. L = 35. 5 m. L 1. 007 94 amu+ 1. 007 94 amu+ 15. 9994 amu = 18. 0153 amu = atomic mass units

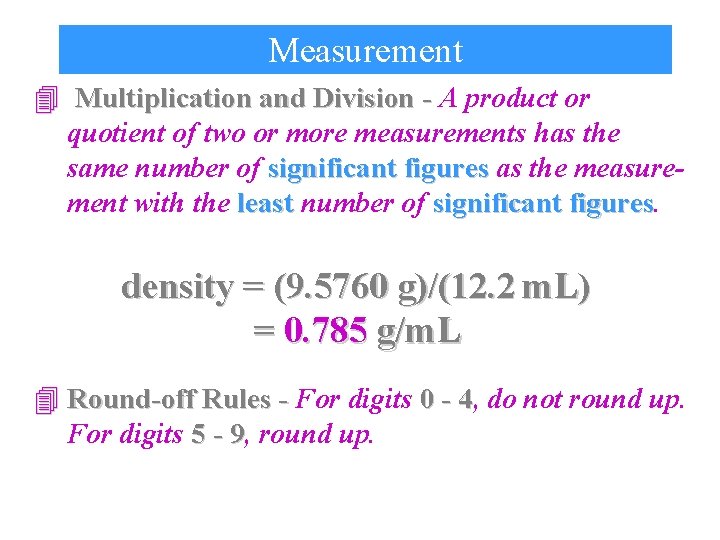
Measurement 4 Multiplication and Division - A product or quotient of two or more measurements has the same number of significant figures as the measurement with the least number of significant figures density = (9. 5760 g)/(12. 2 m. L) = 0. 785 g/m. L 4 Round-off Rules - For digits 0 - 4, 4 do not round up. For digits 5 - 9, 9 round up.

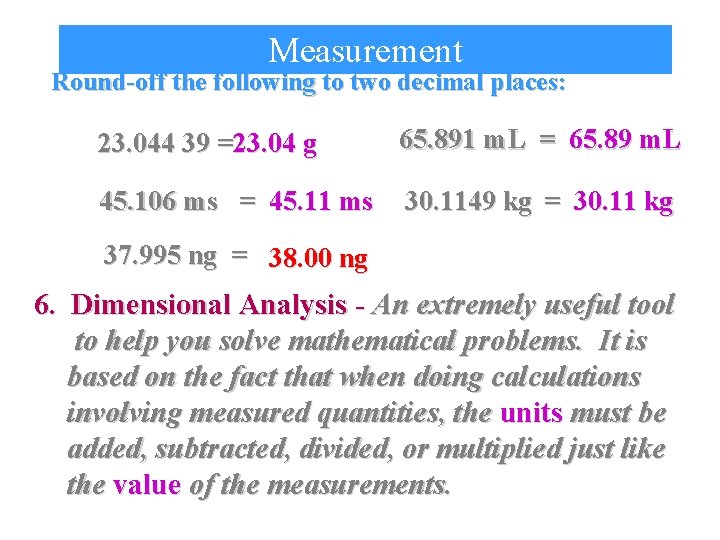
Measurement Round-off the following to two decimal places: 23. 044 39 =23. 04 g 65. 891 m. L = 65. 89 m. L 45. 106 ms = 45. 11 ms 30. 1149 kg = 30. 11 kg 37. 995 ng = 38. 00 ng 6. Dimensional Analysis - An extremely useful tool to help you solve mathematical problems. It is based on the fact that when doing calculations involving measured quantities, the units must be added, subtracted, divided, or multiplied just like the value of the measurements.

Dimensional Analysis How many meters are in each of the following? 21 km 1023 570 mm (21 km)(1 x 103 m) = 21 x 103 m = 2. 1 x 104 m km (1023 570 mm)( 1 m ) = 1. 023 570 m (106 mm)

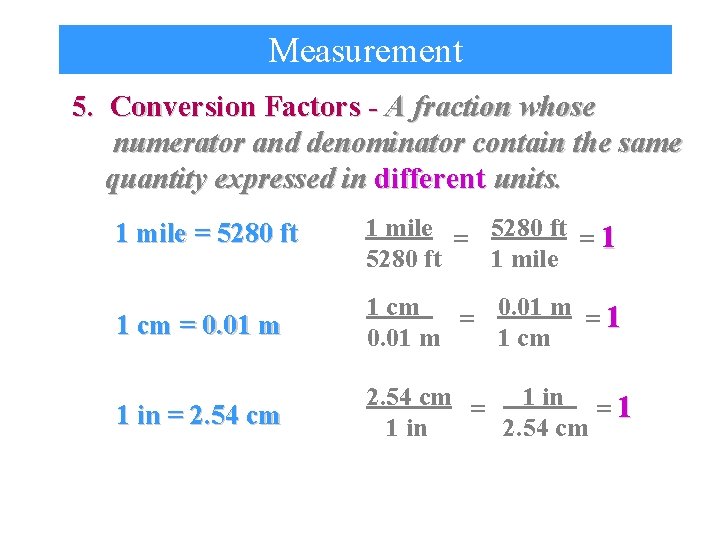
Measurement 5. Conversion Factors - A fraction whose numerator and denominator contain the same quantity expressed in different units. 1 mile = 5280 ft = 1 1 mile 5280 ft 1 cm = 0. 01 m = 1 0. 01 m 1 cm 1 in = 2. 54 cm = 1 in = 1 1 in 2. 54 cm

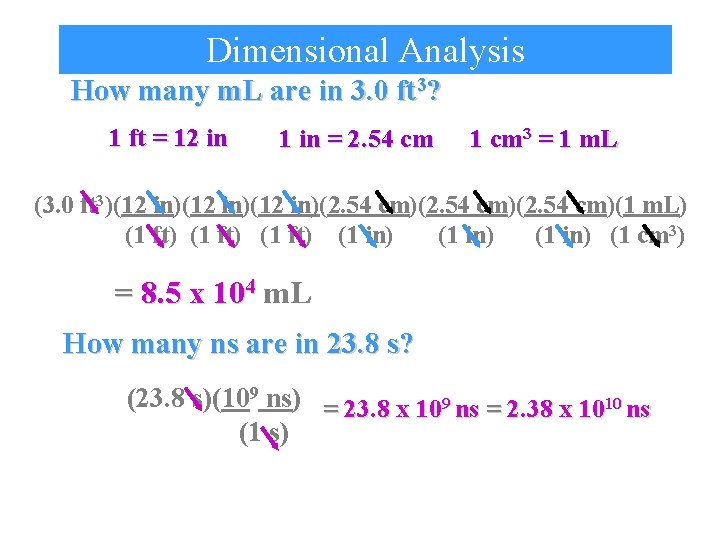
Dimensional Analysis How many m. L are in 3. 0 ft 3? 1 ft = 12 in 1 in = 2. 54 cm 1 cm 3 = 1 m. L (3. 0 ft 3)(12 in)(12 in)(2. 54 cm)(2. 54 cm)(1 m. L) (1 ft) (1 in) (1 cm 3) = 8. 5 x 104 m. L How many ns are in 23. 8 s? (23. 8 s)(109 ns) = 23. 8 x 109 ns = 2. 38 x 1010 ns (1 s)

Classification of Properties of Matter • Properties can be classified as: – Physical or Chemical Properties – Intensive or Extensive Properties

Properties of Matter • Physical Properties - Properties that do NOT involve substances changing into other substances. Melting Point Boiling Point Temperature Density Mass Volume • Chemical Properties - Properties that involve substances changing into other substances. Chemical Reactivity Reduction Potential Flammability Oxidation Potential Prentice-Hall © 2002

Properties of Matter • Extensive Properties - Properties that depend on the amount of matter present in a sample. Mass Volume Heat Capacity • Intensive Properties - Properties that do NOT depend on the amount of matter present in a sample. Color Temperature Density Melting Point Specific Heat Boiling Point Prentice-Hall © 2002

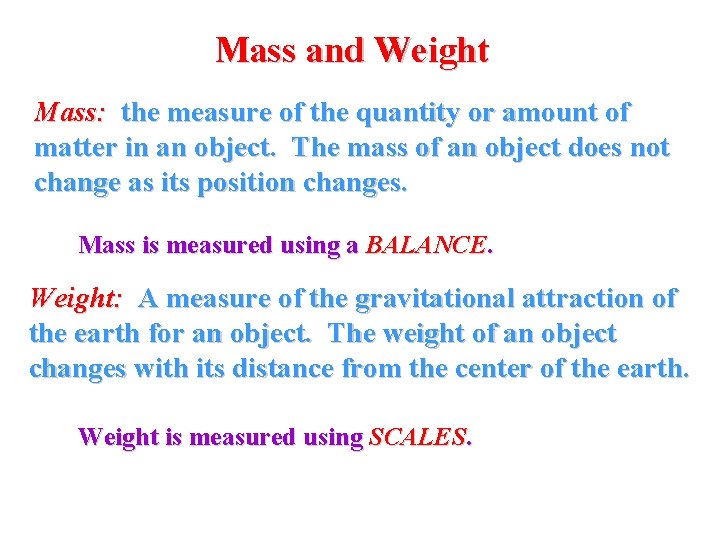
Mass and Weight Mass: the measure of the quantity or amount of matter in an object. The mass of an object does not change as its position changes. Mass is measured using a BALANCE. Weight: A measure of the gravitational attraction of the earth for an object. The weight of an object changes with its distance from the center of the earth. Weight is measured using SCALES.

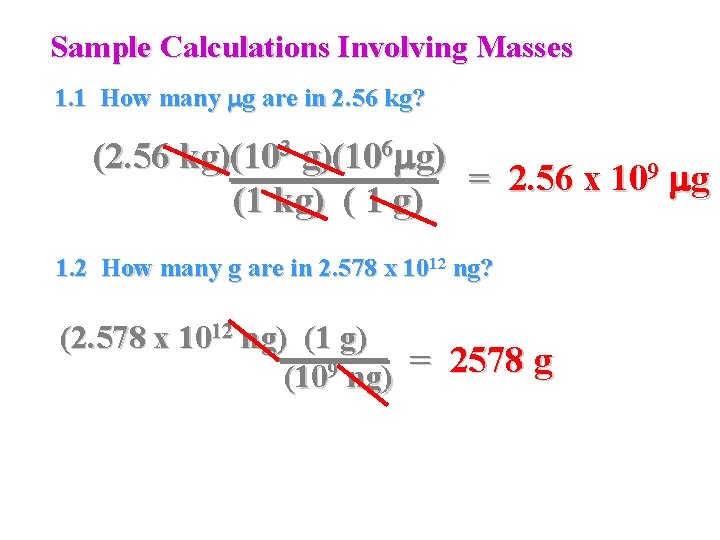
Sample Calculations Involving Masses 1. 1 How many mg are in 2. 56 kg? (2. 56 kg)(103 g)(106 mg) = 2. 56 x 109 mg (1 kg) ( 1 g) 1. 2 How many g are in 2. 578 x 1012 ng? (2. 578 x 1012 ng) (1 g) = 2578 g 9 (10 ng)

Volume

Sample Calculations Involving Volumes 1. 3 How many m. L are in 3. 456 L? (3. 456 L)(1000 m. L)= 3456 m. L L 1. 4 How many m. L are in 23. 7 cm 3? (23. 7 cm 3)( 1 m. L )( 1 L_ _)(106 m. L) (1 cm 3)(1000 m. L)( 1 L ) = 23 700 m. L = 2. 37 x 10 4 m. L

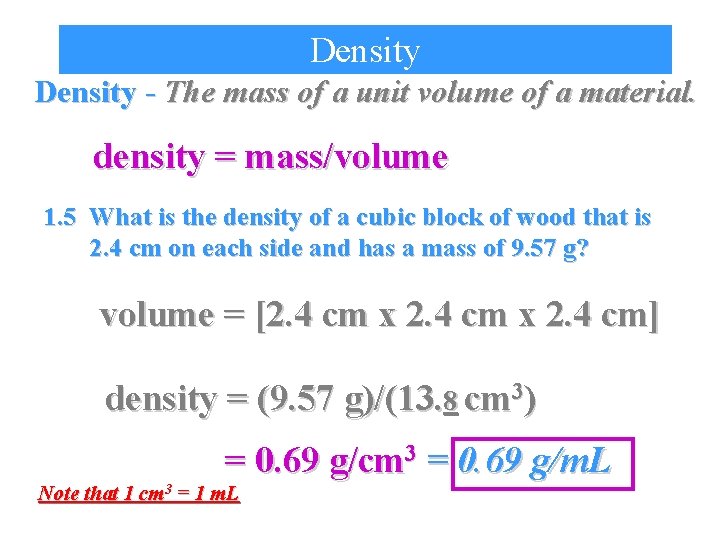
Density - The mass of a unit volume of a material. density = mass/volume 1. 5 What is the density of a cubic block of wood that is 2. 4 cm on each side and has a mass of 9. 57 g? volume = [2. 4 cm x 2. 4 cm] density = (9. 57 g)/(13. 8 cm 3) = 0. 69 g/cm 3 = 0. 69 g/m. L Note that 1 cm 3 = 1 m. L

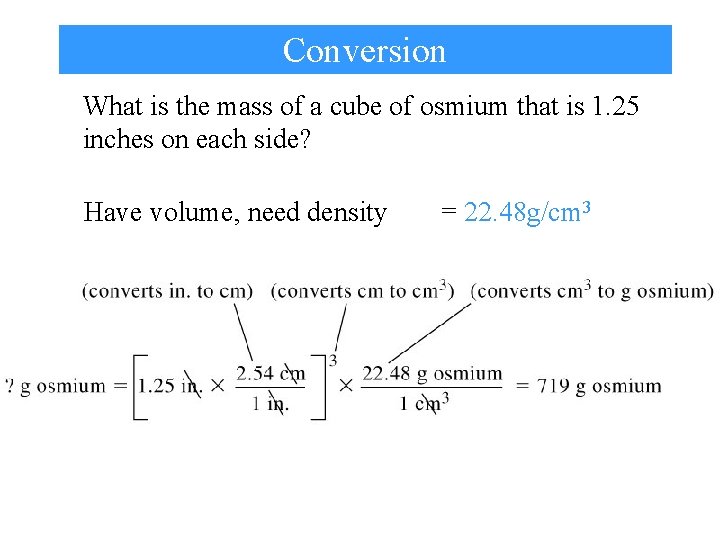
Conversion What is the mass of a cube of osmium that is 1. 25 inches on each side? Have volume, need density = 22. 48 g/cm 3

Temperature and Thermal Energy Temperature: A measure of the “hotness”and “coldness” of an object; a measure of the average kinetic energy of the atoms and molecules of the object. The higher the temperature, the more kinetic energy the atoms and/or molecules have. This is an INTENSIVE property. Thermal Energy: Often called “heat”, it is the form of energy toward which all other forms tend to go.

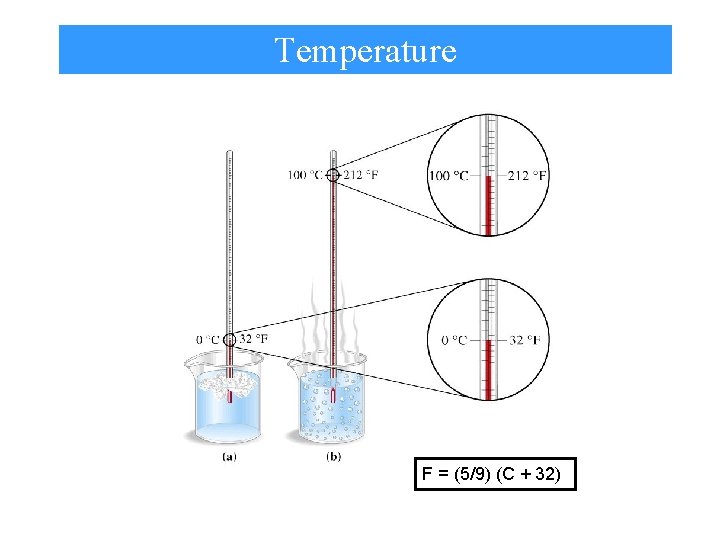
Temperature F = (5/9) (C + 32)

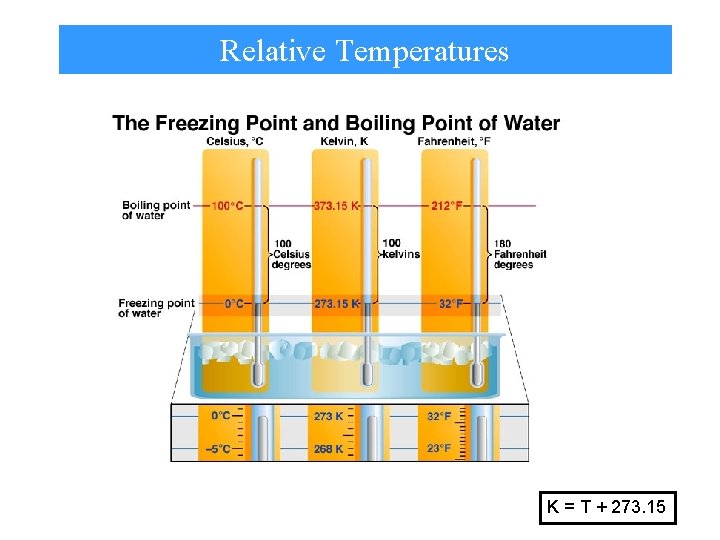
Relative Temperatures K = T + 273. 15


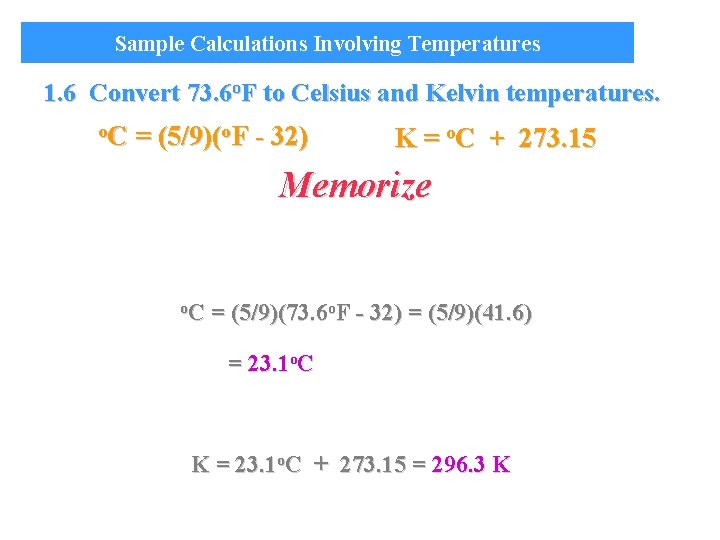
Sample Calculations Involving Temperatures 1. 6 Convert 73. 6 o. F to Celsius and Kelvin temperatures. o. C = (5/9)(o. F - 32) K = o. C + 273. 15 Memorize o. C = (5/9)(73. 6 o. F - 32) = (5/9)(41. 6) = 23. 1 o. C K = 23. 1 o. C + 273. 15 = 296. 3 K

End of Chapter Questions
 Ups flight 1307
Ups flight 1307 Ups airlines flight 1307
Ups airlines flight 1307 They are mrs garcia and mrs castro
They are mrs garcia and mrs castro They are mrs garcia and mrs castro
They are mrs garcia and mrs castro Mrs. darling was ___________ of mrs. s.
Mrs. darling was ___________ of mrs. s. Tipos de participantes en un curso
Tipos de participantes en un curso Basic instructor course texas
Basic instructor course texas Basic instructor course texas
Basic instructor course texas Basic instructor course tcole
Basic instructor course tcole Pepperball training manual
Pepperball training manual Everyone selected to serve on this jury
Everyone selected to serve on this jury Instructor vs teacher
Instructor vs teacher Cisco certified trainer
Cisco certified trainer Mptc firearms
Mptc firearms Basic instructor course texas
Basic instructor course texas Basic instructor course #1014
Basic instructor course #1014 The virtual instructor
The virtual instructor Nfpa 1403
Nfpa 1403 Human factors instructor
Human factors instructor Instructor operating station
Instructor operating station Catia instructor
Catia instructor Instructor
Instructor Ac61-98 plan of action
Ac61-98 plan of action Tcole 1014 basic instructor course
Tcole 1014 basic instructor course Marksmanship instructor
Marksmanship instructor Nrp instructor toolkit
Nrp instructor toolkit Utp cable
Utp cable Cbrf training registry
Cbrf training registry Nra certified instructor logo
Nra certified instructor logo Naismith was an instructor of
Naismith was an instructor of Please clean your own room
Please clean your own room Tcole advanced instructor course
Tcole advanced instructor course Tcole advanced instructor course
Tcole advanced instructor course Jrotc marksmanship instructor course online
Jrotc marksmanship instructor course online Ames room
Ames room Acr medical term
Acr medical term Basic instructor course #1014
Basic instructor course #1014 Basic instructor course #1014
Basic instructor course #1014 Delmar cengage learning instructor resources
Delmar cengage learning instructor resources Instructor office hours
Instructor office hours Planos en cinematografia
Planos en cinematografia Where did general lee surrender to general grant?
Where did general lee surrender to general grant? General chemistry with qualitative analysis
General chemistry with qualitative analysis General chemistry thermochemistry
General chemistry thermochemistry General chemistry nomenclature
General chemistry nomenclature General chemistry 2
General chemistry 2 General chemistry petrucci
General chemistry petrucci General chemistry
General chemistry General chemistry 1 stoichiometry
General chemistry 1 stoichiometry General chemistry
General chemistry General chemistry
General chemistry Exchange energy
Exchange energy General chemistry
General chemistry General chemistry
General chemistry Ib organic chemistry
Ib organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Ttsmp 3
Ttsmp 3