Chapter 6 Thermochemistry Thermodynamics The science of the























- Slides: 55

Chapter 6 Thermochemistry

Thermodynamics The science of the relationship between heat and other forms of energy. Thermochemistry An area of thermodynamics that concerns the study of the heat absorbed or evolved by a chemical reaction 6|2

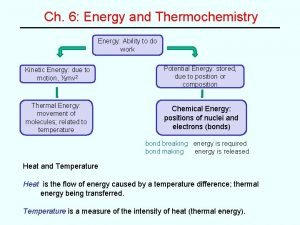
Energy The potential or capacity to move matter. One form of energy can be converted to another form of energy: electromagnetic, mechanical, electrical, or chemical. Next, we’ll study kinetic energy, potential energy, and internal energy. 6|3

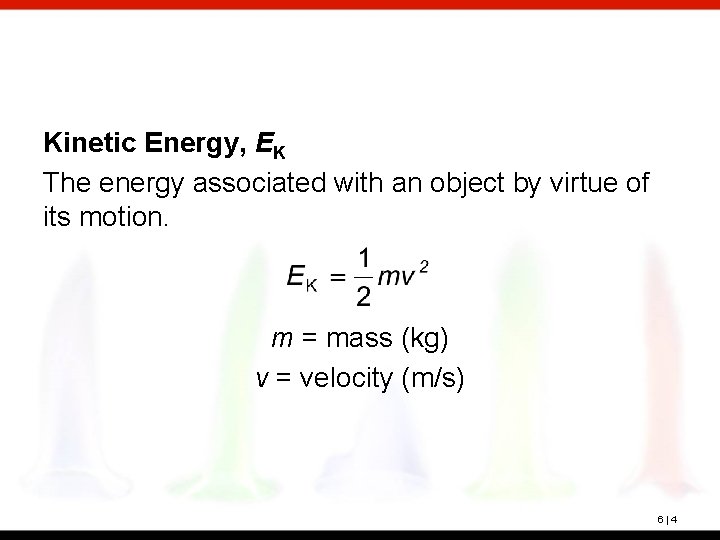
Kinetic Energy, EK The energy associated with an object by virtue of its motion. m = mass (kg) v = velocity (m/s) 6|4

The SI unit of energy is the joule, J, pronounced “jewel. ” The calorie is a non-SI unit of energy commonly used by chemists. It was originally defined as the amount of energy required to raise the temperature of one gram of water by one degree Celsius. The exact definition is given by the equation: 6|5

? A person weighing 75. 0 kg (165 lbs) runs a course at 1. 78 m/s (4. 00 mph). What is the person’s kinetic energy? 6|6

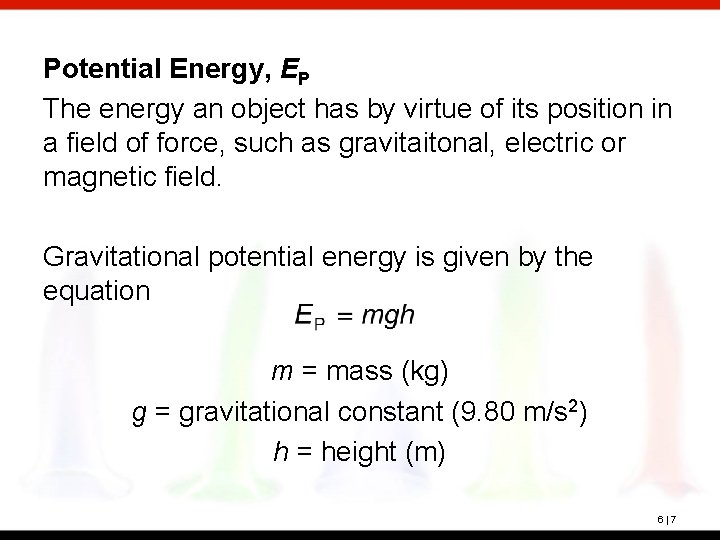
Potential Energy, EP The energy an object has by virtue of its position in a field of force, such as gravitaitonal, electric or magnetic field. Gravitational potential energy is given by the equation m = mass (kg) g = gravitational constant (9. 80 m/s 2) h = height (m) 6|7

Internal Energy, U The sum of the kinetic and potential energies of the particles making up a substance. Total Energy Etot = EK + EP + U 6|8

Law of Conservation of Energy may be converted from one form to another, but the total quantity of energy remains constant. 6|9

Thermodynamic System The substance under study in which a change occurs is called thermodynamic system (or just system). Thermodynamic Surroundings Everything else in the vicinity is called thermodynamic surroundings (or just the surroundings). 6 | 10

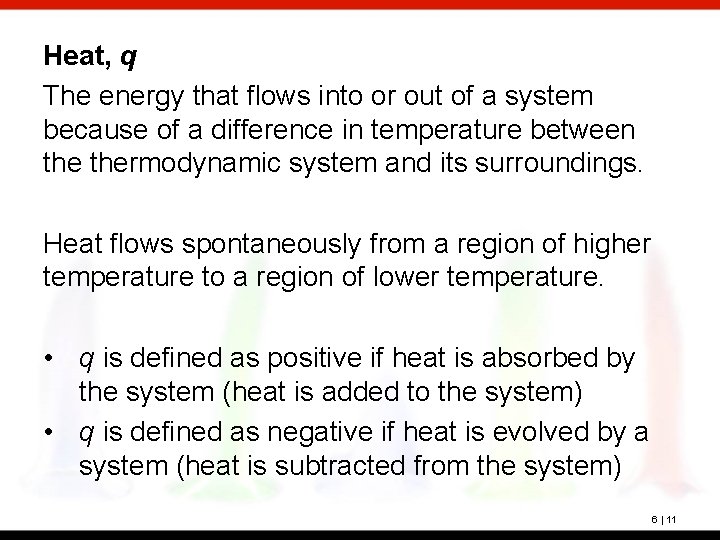
Heat, q The energy that flows into or out of a system because of a difference in temperature between thermodynamic system and its surroundings. Heat flows spontaneously from a region of higher temperature to a region of lower temperature. • q is defined as positive if heat is absorbed by the system (heat is added to the system) • q is defined as negative if heat is evolved by a system (heat is subtracted from the system) 6 | 11

Heat of Reaction The value of q required to return a system to the given temperature at the completion of the reaction (at a given temperature) 6 | 12

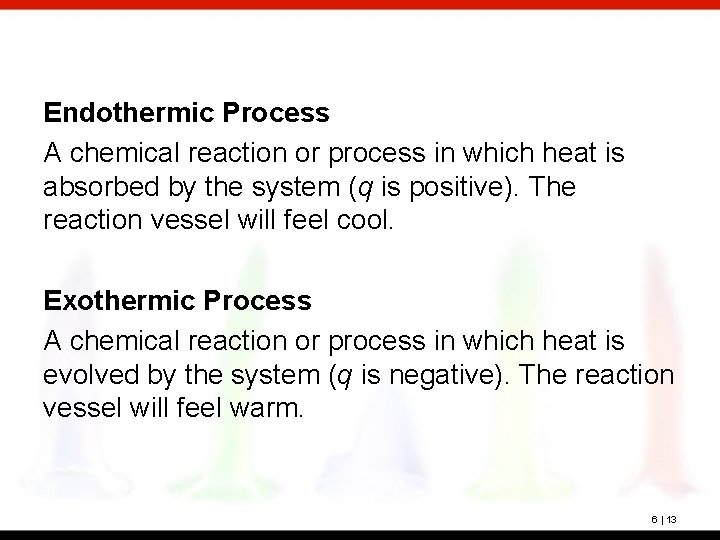
Endothermic Process A chemical reaction or process in which heat is absorbed by the system (q is positive). The reaction vessel will feel cool. Exothermic Process A chemical reaction or process in which heat is evolved by the system (q is negative). The reaction vessel will feel warm. 6 | 13

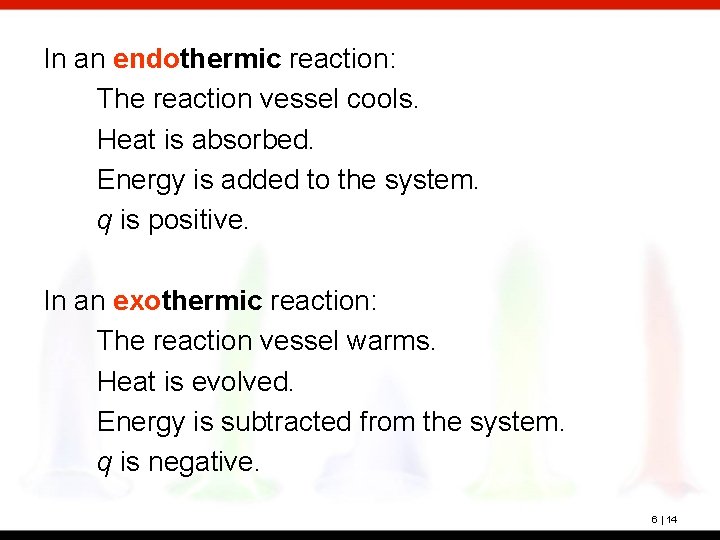
In an endothermic reaction: The reaction vessel cools. Heat is absorbed. Energy is added to the system. q is positive. In an exothermic reaction: The reaction vessel warms. Heat is evolved. Energy is subtracted from the system. q is negative. 6 | 14

Enthalpy, H An extensive property of a substance that can be used to obtain the heat absorbed or evolved in a chemical reaction. Extensive Property A property that depends on the amount of substance. Mass and volume are extensive properties. 6 | 15

A state function is a property of a system that depends only on its present state, which is determined by variables such as temperature and pressure, and is independent of any previous history of the system. 6 | 16

The altitude of a campsite is a state function. It is independent of the path taken to reach it. 6 | 17

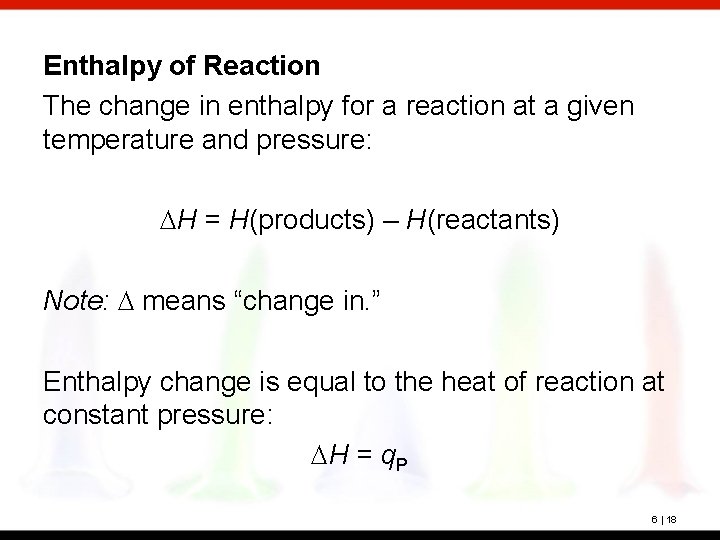
Enthalpy of Reaction The change in enthalpy for a reaction at a given temperature and pressure: DH = H(products) – H(reactants) Note: D means “change in. ” Enthalpy change is equal to the heat of reaction at constant pressure: DH = q. P 6 | 18

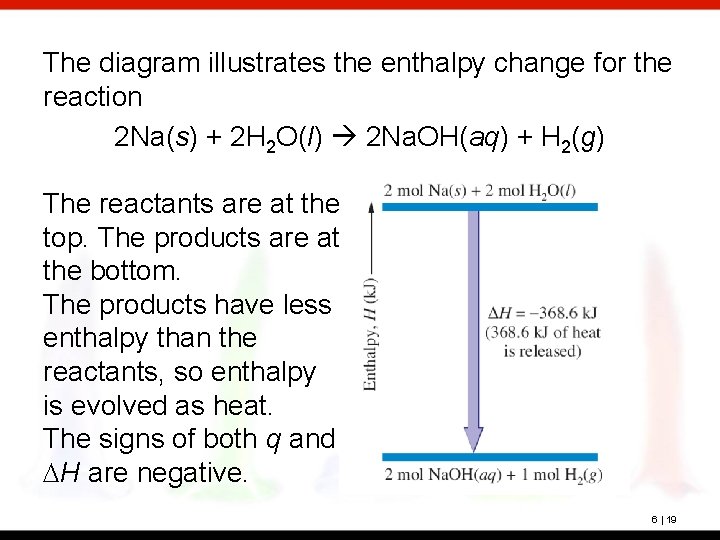
The diagram illustrates the enthalpy change for the reaction 2 Na(s) + 2 H 2 O(l) 2 Na. OH(aq) + H 2(g) The reactants are at the top. The products are at the bottom. The products have less enthalpy than the reactants, so enthalpy is evolved as heat. The signs of both q and DH are negative. 6 | 19

Enthalpy and Internal Energy The precise definition of enthalpy, H, is H = U + PV Many reactions take place at constant pressure, so the change in enthalpy can be given by DH = DU + PDV Rearranging: DU = DH – PDV The term (–PDV) is the energy needed to change volume against the atmospheric pressure, P. It is called pressure-volume work. 6 | 20

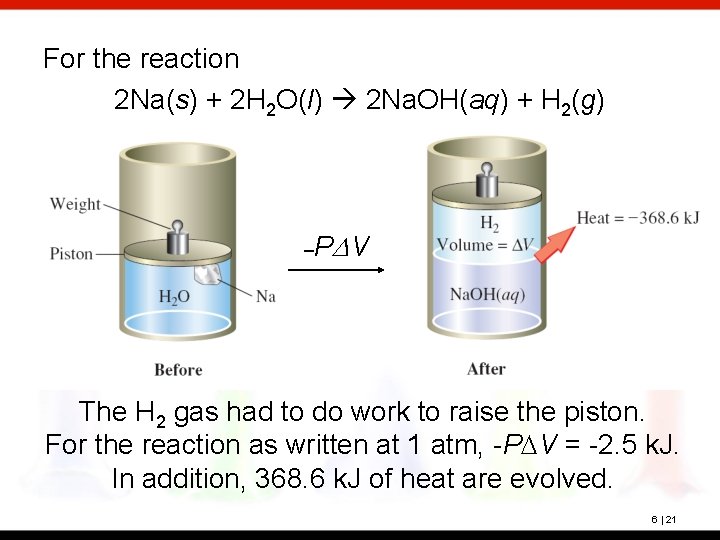
For the reaction 2 Na(s) + 2 H 2 O(l) 2 Na. OH(aq) + H 2(g) –P DV The H 2 gas had to do work to raise the piston. For the reaction as written at 1 atm, -PDV = -2. 5 k. J. In addition, 368. 6 k. J of heat are evolved. 6 | 21

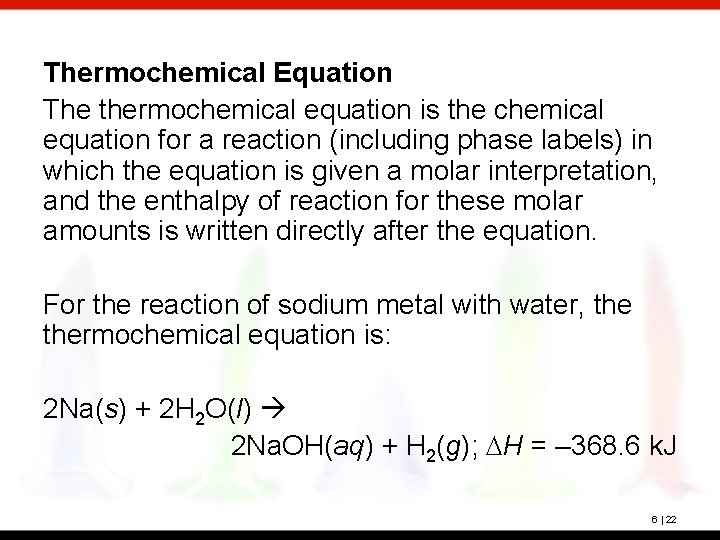
Thermochemical Equation The thermochemical equation is the chemical equation for a reaction (including phase labels) in which the equation is given a molar interpretation, and the enthalpy of reaction for these molar amounts is written directly after the equation. For the reaction of sodium metal with water, thermochemical equation is: 2 Na(s) + 2 H 2 O(l) 2 Na. OH(aq) + H 2(g); DH = – 368. 6 k. J 6 | 22

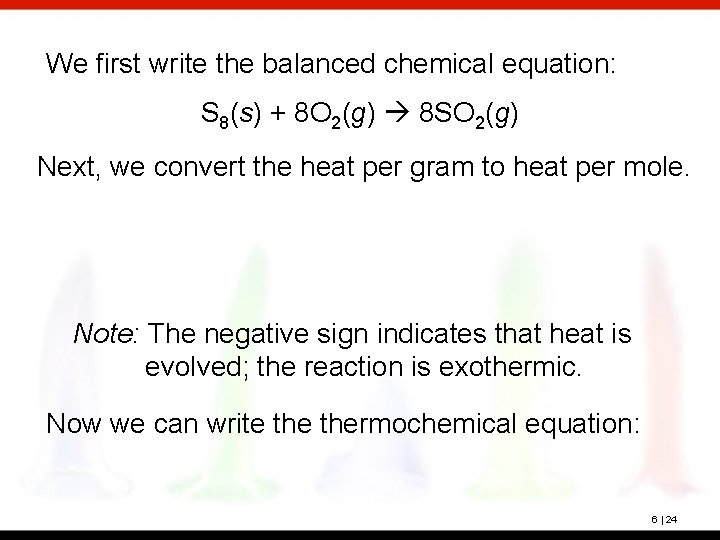
? Sulfur, S 8, burns in air to produce sulfur dioxide. The reaction evolves 9. 31 k. J of heat per gram of sulfur at constant pressure. Write thermochemical equation for this reaction. 6 | 23

We first write the balanced chemical equation: S 8(s) + 8 O 2(g) 8 SO 2(g) Next, we convert the heat per gram to heat per mole. Note: The negative sign indicates that heat is evolved; the reaction is exothermic. Now we can write thermochemical equation: 6 | 24

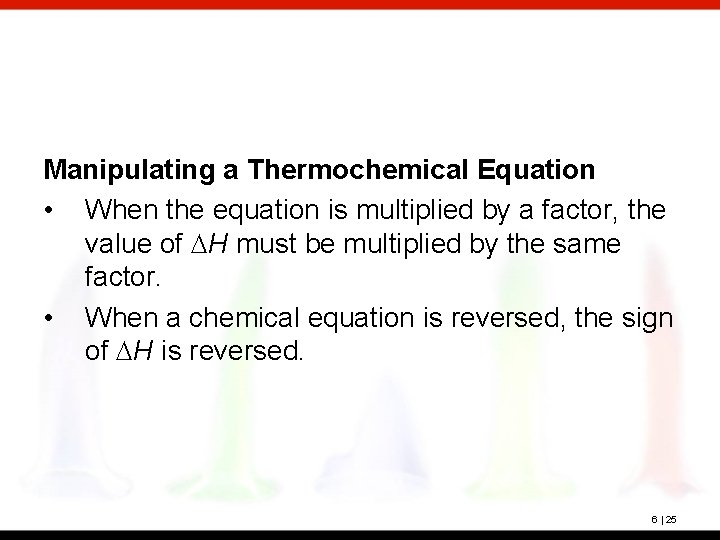
Manipulating a Thermochemical Equation • When the equation is multiplied by a factor, the value of DH must be multiplied by the same factor. • When a chemical equation is reversed, the sign of DH is reversed. 6 | 25

6 | 26

? When sulfur burns in air, the following reaction occurs: S 8(s) + 8 O 2(g) 8 SO 2(g); DH = – 2. 39 x 103 k. J Write thermochemical equation for the dissociation of one mole of sulfur dioxide into its elements. 6 | 27

6 | 28

Applying Stoichiometry to Heats of Reaction 6 | 29

? You burn 15. 0 g sulfur in air. How much heat evolves from this amount of sulfur? The thermochemical equation is S 8(s) + 8 O 2(g) 8 SO 2(g); DH = -2. 39 x 103 k. J 6 | 30

6 | 31

The daily energy requirement for a 20 year-old man weighing 67 kg is 1. 3 x 104 k. J. For a 20 -year-old woman weighing 58 kg, the daily requirement is 8. 8 x 103 k. J. If all this energy were to be provided by the combustion of glucose, C 6 H 12 O 6, how many grams of glucose would have to be consumed by the man and the woman per day? C 6 H 12 O 6(s) + 6 O 2(g) 6 CO 2(g) + 6 H 2 O(l); DH = -2. 82 x 103 k. J ? 6 | 32

6 | 33

Measuring Heats of Reaction We will first look at the heat needed to raise the temperature of a substance because this is the basis of our measurements of heats of reaction. 6 | 34

Heat Capacity, C, of a Sample of Substance The quantity of heat needed to raise the temperature of the sample of substance by one degree Celsius (or one Kelvin). Molar Heat Capacity The heat capacity for one mole of substance. Specific Heat Capacity, s (or specific heat) The quantity of heat needed to raise the temperature of one gram of substance by one degree Celsius (or one Kelvin) at constant pressure. 6 | 35

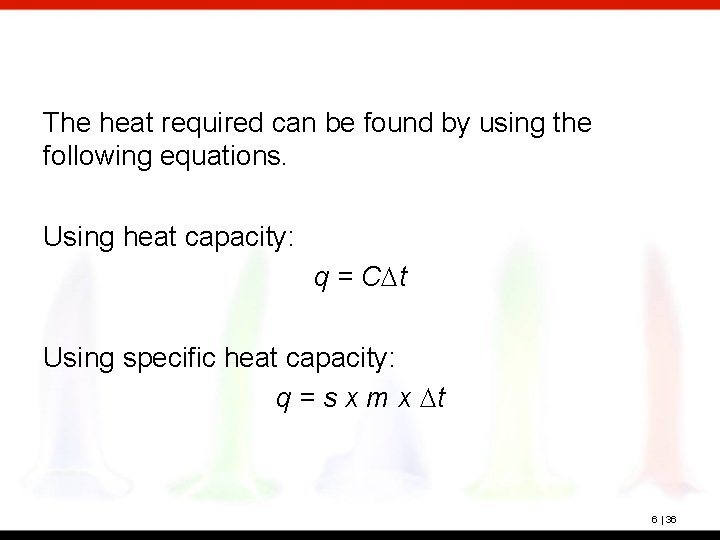
The heat required can be found by using the following equations. Using heat capacity: q = CDt Using specific heat capacity: q = s x m x Dt 6 | 36

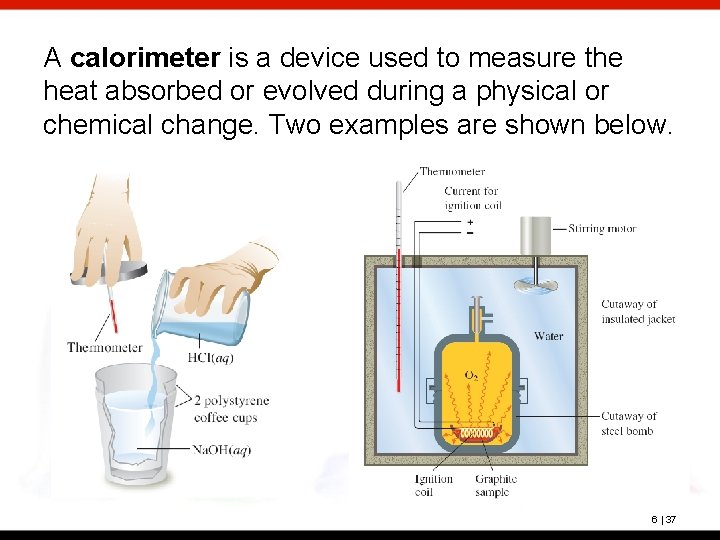
A calorimeter is a device used to measure the heat absorbed or evolved during a physical or chemical change. Two examples are shown below. 6 | 37

? A piece of zinc weighing 35. 8 g was heated from 20. 00°C to 28. 00°C. How much heat was required? The specific heat of zinc is 0. 388 J/(g°C). 6 | 38

Hess’s Law of Heat Summation For a chemical equation that can be written as the sum of two or more steps, the enthalpy change for the overall equation equals the sum of the enthalpy changes for the individual steps. 6 | 39

Suppose we want DH for the reaction 2 C(graphite) + O 2(g) 2 CO(g) It is difficult to measure directly. However, two other reactions are known: C(graphite) + O 2(g) CO 2(g); DH = -393. 5 k. J 2 CO 2(g) 2 CO(g) + O 2(g); DH = – 566. 0 k. J In order for these to add to give the reaction we want, we must multiply the first reaction by 2. Note that we also multiply DH by 2. 6 | 40

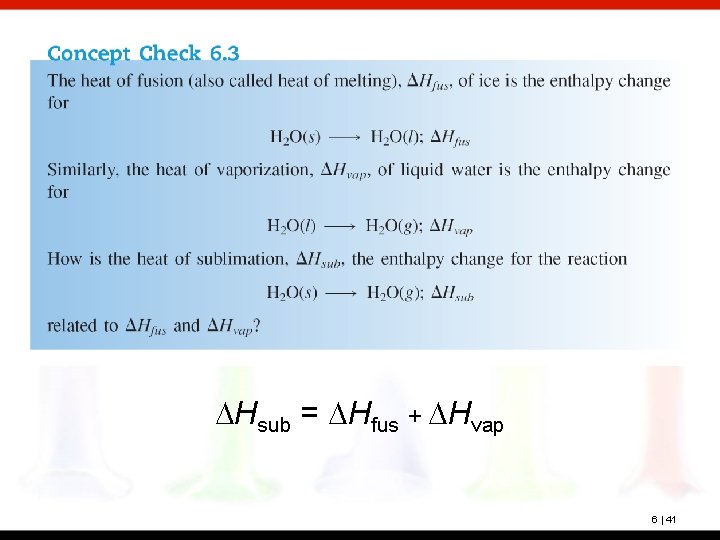
DHsub = DHfus + DHvap 6 | 41

Standard Enthalpies of Formation The term standard state refers to the standard thermodynamic conditions chosen for substances when listing or comparing thermodynamic data: 1 atm pressure and the specified temperature (usually 25°C). These standard conditions are indicated with a degree sign (°). When reactants in their standard states yield products in their standard states, the enthalpy of reaction is called the standard enthalpy of reaction, DH°. (DH° is read “delta H zero. ”) 6 | 42

Elements can exist in more than one physical state, and some elements exist in more than one distinct form in the same physical state. For example, carbon can exist as graphite or as diamond; oxygen can exist as O 2 or as O 3 (ozone). These different forms of an element in the same physical state are called allotropes. The reference form is the most stable form of the element (both physical state and allotrope). 6 | 43

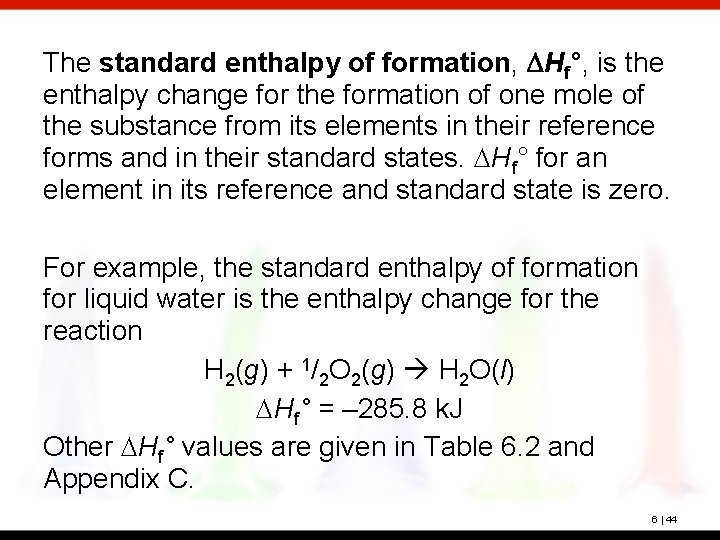
The standard enthalpy of formation, DHf°, is the enthalpy change for the formation of one mole of the substance from its elements in their reference forms and in their standard states. DHf° for an element in its reference and standard state is zero. For example, the standard enthalpy of formation for liquid water is the enthalpy change for the reaction H 2(g) + 1/2 O 2(g) H 2 O(l) DHf° = – 285. 8 k. J Other DHf° values are given in Table 6. 2 and Appendix C. 6 | 44

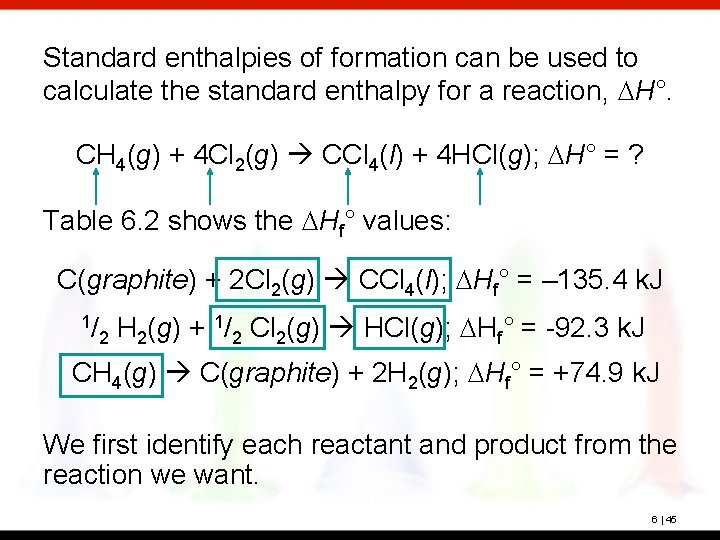
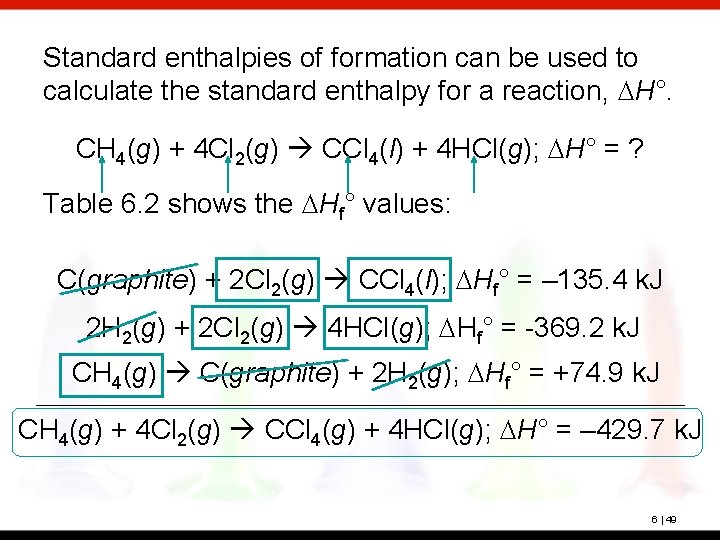
Standard enthalpies of formation can be used to calculate the standard enthalpy for a reaction, DH°. CH 4(g) + 4 Cl 2(g) CCl 4(l) + 4 HCl(g); DH° = ? Table 6. 2 shows the DHf° values: C(graphite) + 2 Cl 2(g) CCl 4(l); DHf° = – 135. 4 k. J 1/ 2 H 2(g) + 1/2 Cl 2(g) HCl(g); DHf° = -92. 3 k. J CH 4(g) C(graphite) + 2 H 2(g); DHf° = +74. 9 k. J We first identify each reactant and product from the reaction we want. 6 | 45

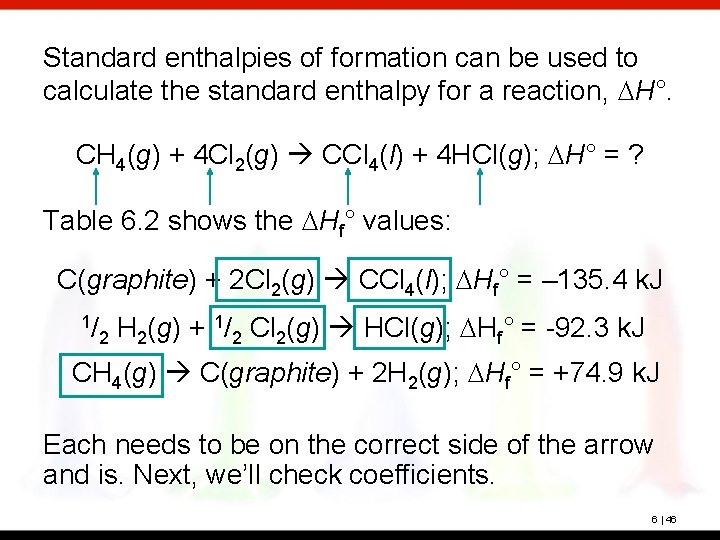
Standard enthalpies of formation can be used to calculate the standard enthalpy for a reaction, DH°. CH 4(g) + 4 Cl 2(g) CCl 4(l) + 4 HCl(g); DH° = ? Table 6. 2 shows the DHf° values: C(graphite) + 2 Cl 2(g) CCl 4(l); DHf° = – 135. 4 k. J 1/ 2 H 2(g) + 1/2 Cl 2(g) HCl(g); DHf° = -92. 3 k. J CH 4(g) C(graphite) + 2 H 2(g); DHf° = +74. 9 k. J Each needs to be on the correct side of the arrow and is. Next, we’ll check coefficients. 6 | 46

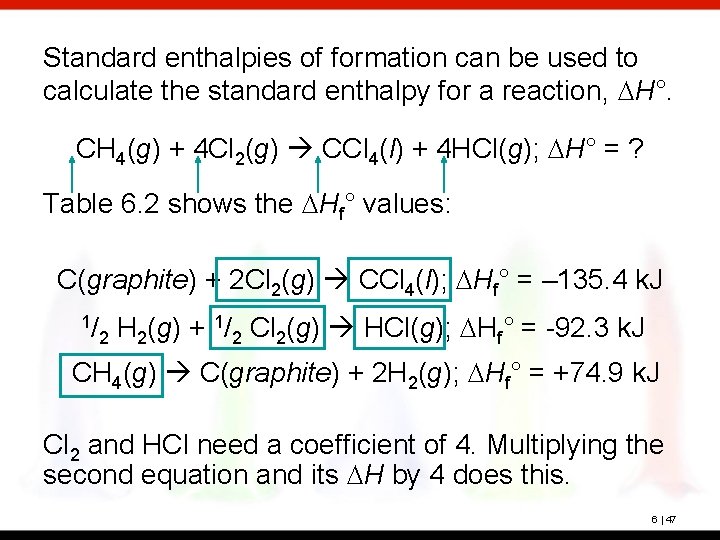
Standard enthalpies of formation can be used to calculate the standard enthalpy for a reaction, DH°. CH 4(g) + 4 Cl 2(g) CCl 4(l) + 4 HCl(g); DH° = ? Table 6. 2 shows the DHf° values: C(graphite) + 2 Cl 2(g) CCl 4(l); DHf° = – 135. 4 k. J 1/ 2 H 2(g) + 1/2 Cl 2(g) HCl(g); DHf° = -92. 3 k. J CH 4(g) C(graphite) + 2 H 2(g); DHf° = +74. 9 k. J Cl 2 and HCl need a coefficient of 4. Multiplying the second equation and its DH by 4 does this. 6 | 47

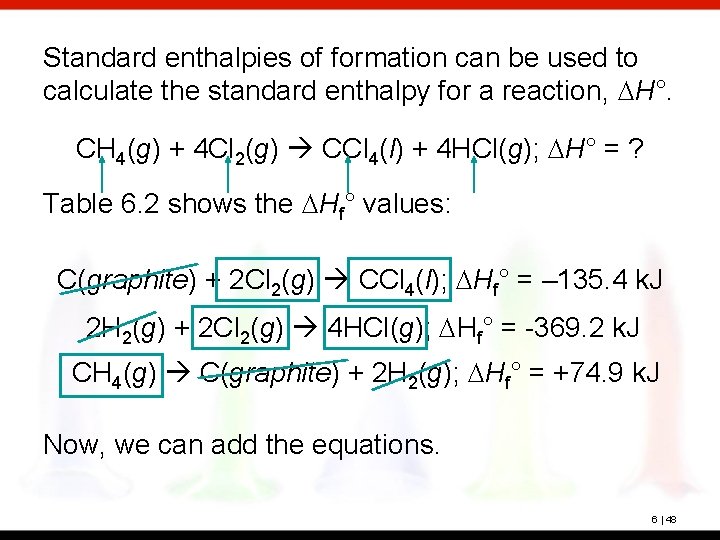
Standard enthalpies of formation can be used to calculate the standard enthalpy for a reaction, DH°. CH 4(g) + 4 Cl 2(g) CCl 4(l) + 4 HCl(g); DH° = ? Table 6. 2 shows the DHf° values: C(graphite) + 2 Cl 2(g) CCl 4(l); DHf° = – 135. 4 k. J 2 H 2(g) + 2 Cl 2(g) 4 HCl(g); DHf° = -369. 2 k. J CH 4(g) C(graphite) + 2 H 2(g); DHf° = +74. 9 k. J Now, we can add the equations. 6 | 48

Standard enthalpies of formation can be used to calculate the standard enthalpy for a reaction, DH°. CH 4(g) + 4 Cl 2(g) CCl 4(l) + 4 HCl(g); DH° = ? Table 6. 2 shows the DHf° values: C(graphite) + 2 Cl 2(g) CCl 4(l); DHf° = – 135. 4 k. J 2 H 2(g) + 2 Cl 2(g) 4 HCl(g); DHf° = -369. 2 k. J CH 4(g) C(graphite) + 2 H 2(g); DHf° = +74. 9 k. J CH 4(g) + 4 Cl 2(g) CCl 4(g) + 4 HCl(g); DH° = – 429. 7 k. J 6 | 49

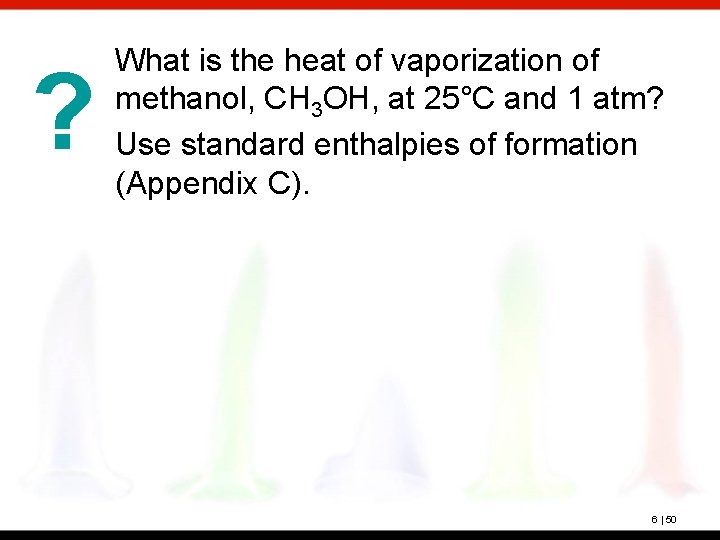
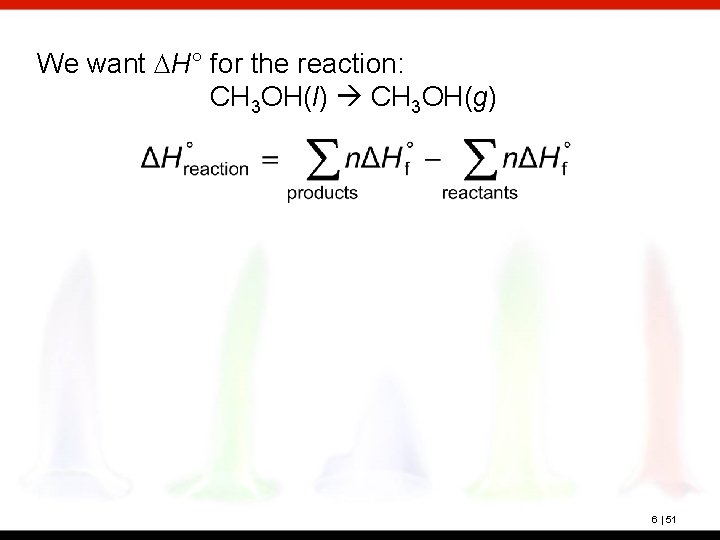
? What is the heat of vaporization of methanol, CH 3 OH, at 25°C and 1 atm? Use standard enthalpies of formation (Appendix C). 6 | 50

We want DH° for the reaction: CH 3 OH(l) CH 3 OH(g) 6 | 51

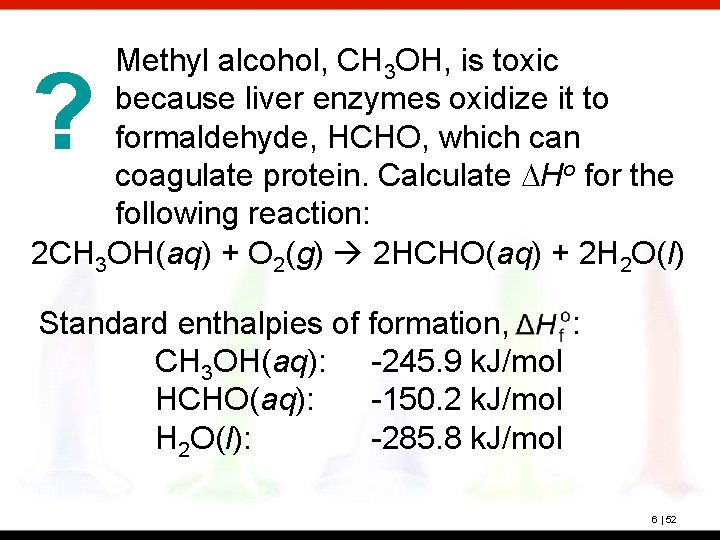
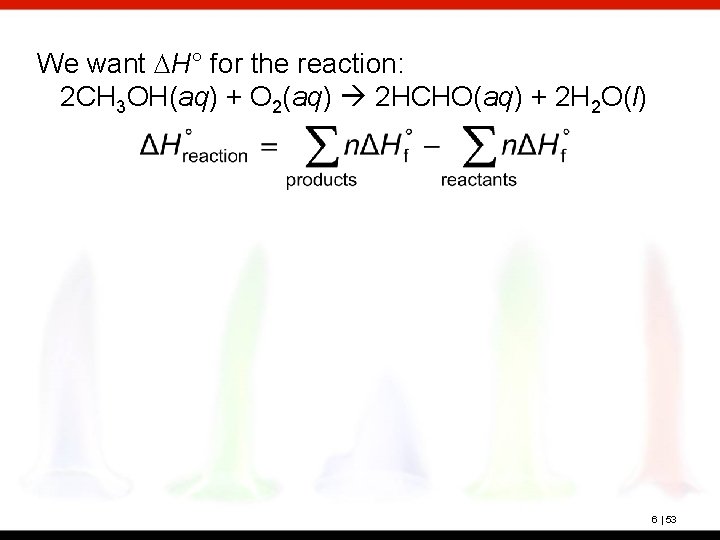
Methyl alcohol, CH 3 OH, is toxic because liver enzymes oxidize it to formaldehyde, HCHO, which can coagulate protein. Calculate DHo for the following reaction: 2 CH 3 OH(aq) + O 2(g) 2 HCHO(aq) + 2 H 2 O(l) ? Standard enthalpies of formation, : CH 3 OH(aq): -245. 9 k. J/mol HCHO(aq): -150. 2 k. J/mol H 2 O(l): -285. 8 k. J/mol 6 | 52

We want DH° for the reaction: 2 CH 3 OH(aq) + O 2(aq) 2 HCHO(aq) + 2 H 2 O(l) 6 | 53

Foods fuels three needs of the body: • They supply substances for the growth and repair of tissue. • They supply substances for the synthesis of compounds used in the regulation processes. • They supply energy. Foods do this by a combustion process. 6 | 54

For glucose, a carbohydrate: C 6 H 12 O 6(s) + 6 O 2(g) 6 CO 2(g) + 6 H 2 O(l); DHf° = – 2803 k. J For glycerol trimyristate, a fat: C 45 H 86 O 6(s) + 127/2 O 2(g) 45 CO 2(g) + 43 H 2 O(l); DHf°= – 27, 820 k. J The average value for carbohydrates is 4. 0 kcal/g and for fats is 9. 0 kcal/g. 6 | 55
 Science textbook form 3
Science textbook form 3 Chapter 17 thermochemistry practice problems
Chapter 17 thermochemistry practice problems Chapter 17 thermochemistry
Chapter 17 thermochemistry Chapter 17 thermochemistry answer key
Chapter 17 thermochemistry answer key Chapter 17 thermochemistry
Chapter 17 thermochemistry Chapter 6 thermochemistry
Chapter 6 thermochemistry Chapter 17 thermochemistry
Chapter 17 thermochemistry What are your favorite subjects?
What are your favorite subjects? Taxonomy is the branch of science that deals with –
Taxonomy is the branch of science that deals with – Reschual
Reschual Chemical engineering thermodynamics 8th solution chapter 3
Chemical engineering thermodynamics 8th solution chapter 3 Thermodynamics for chemical engineering
Thermodynamics for chemical engineering Chemical engineering thermodynamics 8th solution chapter 6
Chemical engineering thermodynamics 8th solution chapter 6 Which of the following system is a control mass system
Which of the following system is a control mass system Chemical engineering thermodynamics 8th solution chapter 10
Chemical engineering thermodynamics 8th solution chapter 10 Thermodynamics chapter 4
Thermodynamics chapter 4 Duhem theorem
Duhem theorem Heat capacity
Heat capacity Q=w/j
Q=w/j Thermodynamics chapter 2
Thermodynamics chapter 2 Thermochemistry
Thermochemistry Thermochemistry is the study of *
Thermochemistry is the study of * Thermochemistry is the study of *
Thermochemistry is the study of * Balanced thermochemical equation
Balanced thermochemical equation Thermochemistry
Thermochemistry Zero point energy correction gaussian
Zero point energy correction gaussian Thermochemical equation
Thermochemical equation Thermochemistry clipart
Thermochemistry clipart Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Thermochemistry is study of
Thermochemistry is study of Thermochemistry equations
Thermochemistry equations Thermochemistry one pager
Thermochemistry one pager Thermochemistry equations
Thermochemistry equations Kirchhoff's law enthalpy example
Kirchhoff's law enthalpy example Thermochemistry equations
Thermochemistry equations Thermochemistry cartoon
Thermochemistry cartoon Ap chemistry thermodynamics
Ap chemistry thermodynamics Ap chem unit 7
Ap chem unit 7 Thermochemistry is the study of
Thermochemistry is the study of General chemistry thermochemistry
General chemistry thermochemistry Study of energy transformations
Study of energy transformations Entalpi pengatoman standar
Entalpi pengatoman standar Energy changes
Energy changes What is thermochemistry
What is thermochemistry Thermochemistry concepts
Thermochemistry concepts Unit 9 thermochemistry
Unit 9 thermochemistry Thermochemistry is study of
Thermochemistry is study of Energy diagram thermochemistry
Energy diagram thermochemistry Thermochemistry is concerned with the
Thermochemistry is concerned with the Kinetic energy thermochemistry
Kinetic energy thermochemistry Thermochemistry
Thermochemistry A piece of metal is heated then submerged in cool water
A piece of metal is heated then submerged in cool water Bomb calorimetry equation
Bomb calorimetry equation Thermochemistry video
Thermochemistry video Thermochemistry
Thermochemistry Thermochemistry
Thermochemistry