Chapter 6 The property of Water and Vapor






























- Slides: 53

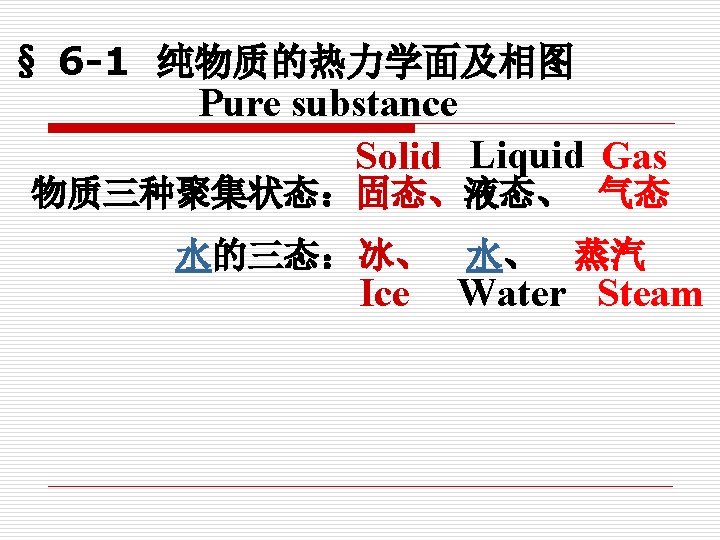
Chapter 6. The property of Water and Vapor (第 6章. 水和水蒸气的性质) o o o 6. 1 Basic conceptions (基本概念) 6. 2 Phase change Process of Pure Substance (纯质的相变过程) 6. 3 Property Tables (水蒸气性质表) 6. 4 h-s diagrams for Water Vapor (水蒸气的h-s图) 6. 5 Thermodynamic Processes of Water Vapor (水蒸气的热力过程)



Phase changes (相变): 任何一种物质都可以经历下列所述的相变. Any kind of substance may undergo various types of phase changes as following : 溶解(Fusion/melting) = solid to liquid 凝固(Freezing) = liquid to solid 气化(Vaporization)= liquid to gas 凝结(Condensation)= gas to liquid 升华(Sublimation) = solid to gas

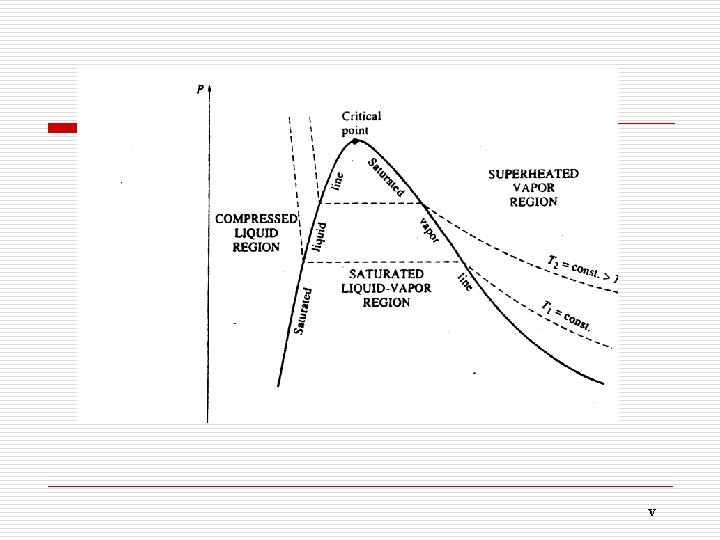
The sublimation line separates the solid and vapor regions; The vaporization line separates the liquid and vapor it ends at the critical point. The melting line regions; separates the solid and liquid regions; The three lines meet at the triple point. Triple point(三相点):all three phases coexist in equilibrium. (三种相态以平衡共存) Critical point (临界点): The point at which the saturated liquid and saturated vapor states are identical. No distinction can be made between liquid and vapor phases above the critical point. (临界点就是气相与液相一致的状态点, 温度高于临界点 之上, 气相与液相没有明显的区别)




§ 6 -2 汽化与饱和 6. 2. 1 汽化与凝结 气化(Vaporization): 由液态变成气态的物理过程 (不涉及化学变化) The Process of Changing from liquid into gas/vapor is called vaporization. 凝结(Condensation): 由气态变成液态的物理过程 The process of changing from gas/vapor into liquid is called Condensation

o. The rate of condensation depends upon the density of vapor in the space above the liquid. o(凝结的速率取决于液面上方气相空间气体分子的密度, 即蒸气压力) o. Liquid molecule has to overcome the surface tension to become vapor, therefore vaporization consumes energy. ( 液相分子必须克服表面张力才能进入气相空间而气化, 因此气化是要耗能的) o. The rate of Vaporization depends on the liquid temperature. (气化的速率取决于液相的温度)

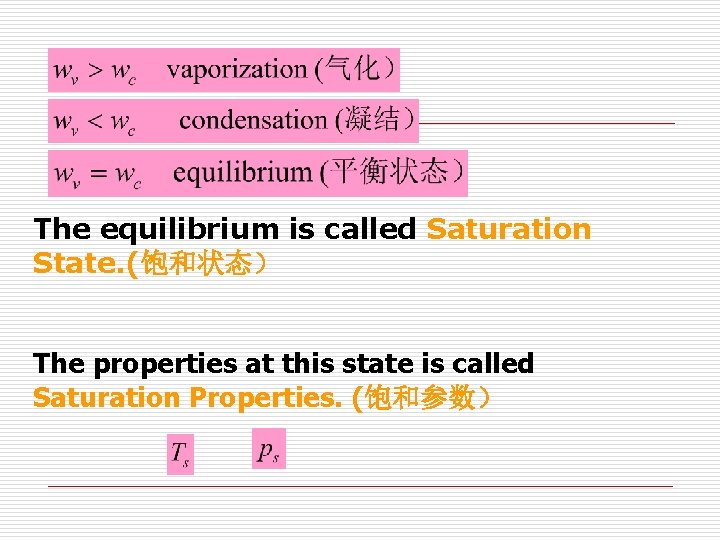
The equilibrium is called Saturation State. (饱和状态) The properties at this state is called Saturation Properties. (饱和参数)

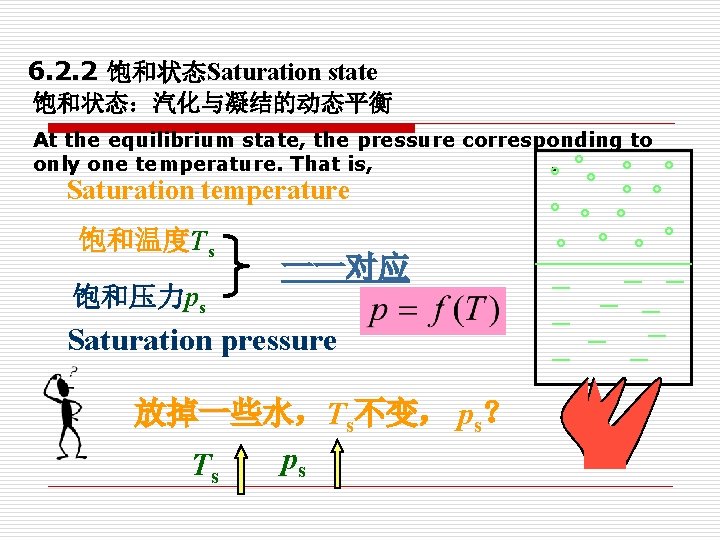
6. 2. 2 饱和状态Saturation state 饱和状态:汽化与凝结的动态平衡 At the equilibrium state, the pressure corresponding to only one temperature. That is, . Saturation temperature 饱和温度Ts 饱和压力ps 一一对应 Saturation pressure 放掉一些水,Ts不变, ps? ps Ts

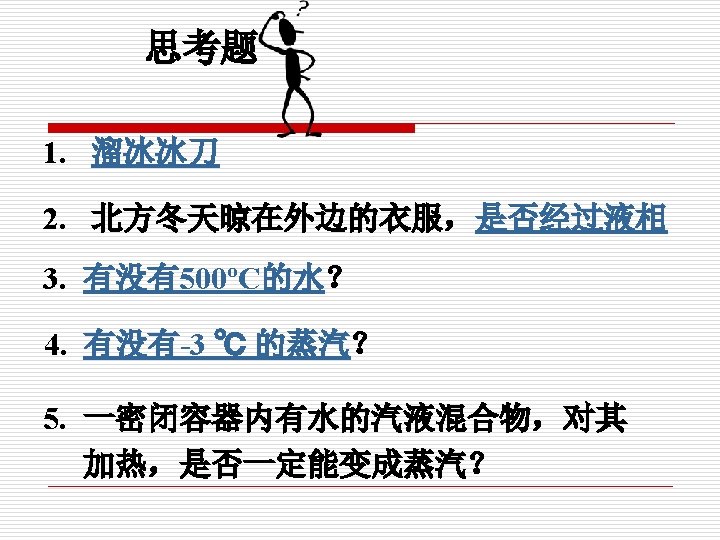
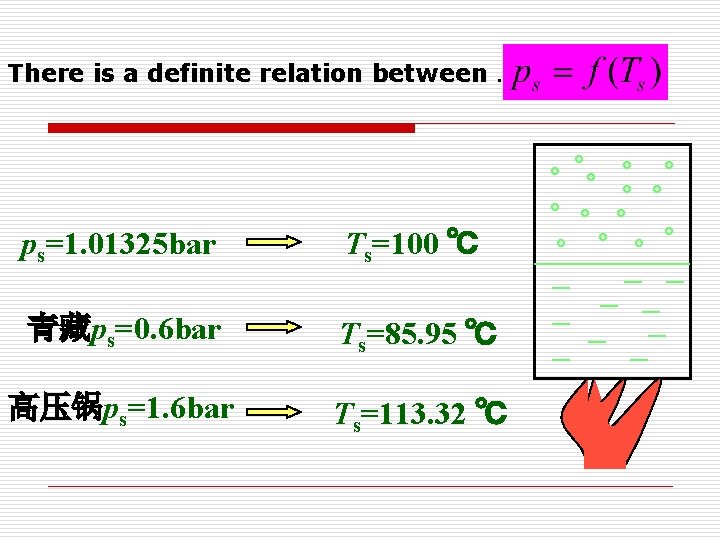
There is a definite relation between . ps=1. 01325 bar Ts=100 ℃ 青藏ps=0. 6 bar Ts=85. 95 ℃ 高压锅ps=1. 6 bar Ts=113. 32 ℃

6. 2. 3 汽化 Vaporization 汽化: 由液态变成气态的物理过程 (不涉及化学变化) Evaporation 蒸发:汽液表面上的汽化 It occurs above the free surface of liquid 沸腾:表面和液体内部同时发生的汽化 It is an intensive vaporization phenomenon occurs in the liquid. Boil (气体和液体均处在饱和状态下)

Evaporation occurs at any temperature and pressure. (蒸发可在任何温度和压力下发生.) Evaporation rate depends on the free surface area, the temperature, the flow rate above, etc. (蒸发的速度与自由液面表面积,液体温度,液面风速等有关. Boiling can only occur as temperature reaches the saturation temperature corresponding to the specified pressure or the pressure drops below the saturation pressure corresponding to the specified temperature. (沸腾只能当温度达到给定压力所对应的饱和温度或压力降到给定 温度所对应的饱和压力时, 才能发生)


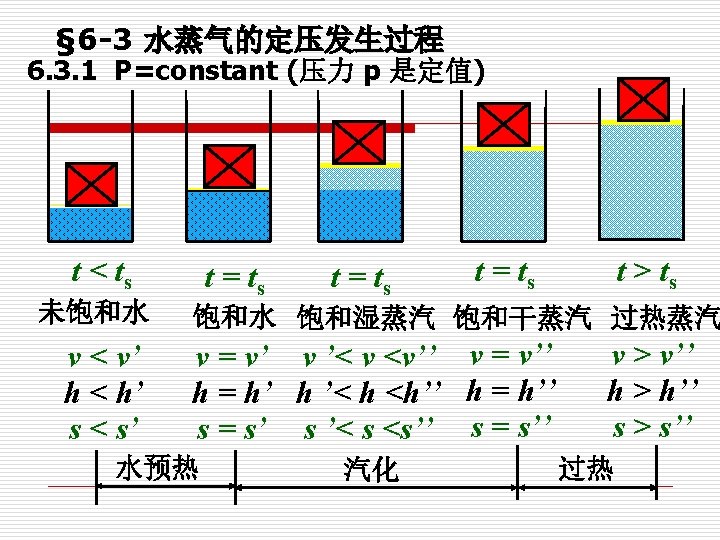
Preheat Stage (预热阶段) Compressed liquid to Saturated liquid. (未饱和液体) Compressed liquid/subcooled liquid is not about to vaporization. As heat added, t slightly. When It becomes Saturated liquid. The liquid is about to vaporize. It is the state at which it is still a liquid, but any heat addition will cause some of the liquid to vaporize.

Vaporization Stage (气化阶段) Saturated liquid to Saturated vapor (饱和液体 饱和蒸气) Saturated vapor is a vapor about to condense. A substance at state between Saturated liquid and Saturated Vapor is called Saturated liquid-vapor mixture/wet vapor. Latent heat of Vaporization (气化潜热): The amount of energy absorbed during vaporization

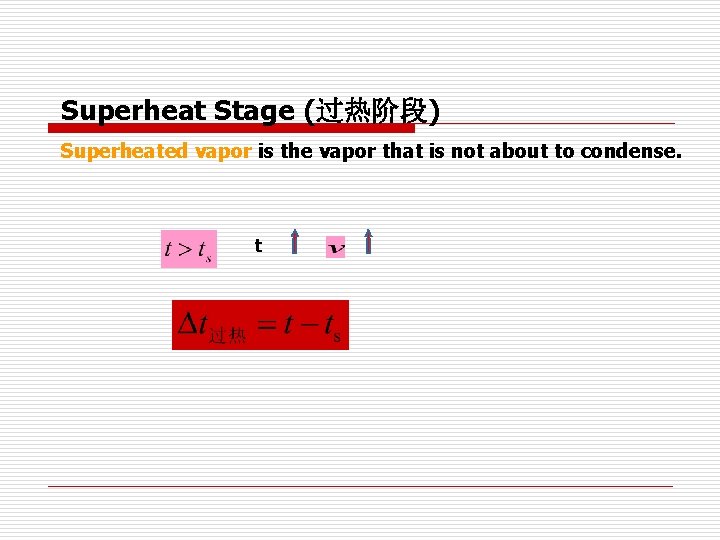
Superheat Stage (过热阶段) Superheated vapor is the vapor that is not about to condense. t


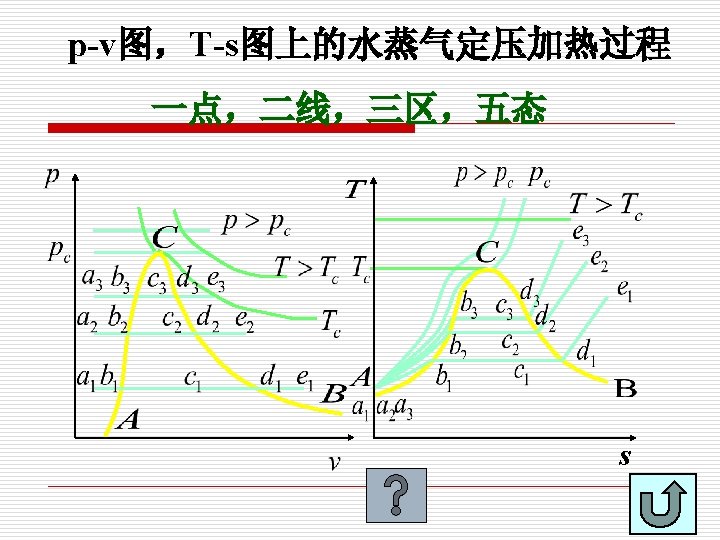
6. 3. 2 定压气化过程的P-v 图和T-s 图 P-v and T-s diagrams State of Liquid and vapor 未饱和液,过冷液 压缩液 Subcooled liquid Compressed liquid 饱和液 Saturated liquid 饱和湿蒸气 Saturated liquid-vapor mixture 饱和蒸气 Saturated vapor 过热蒸气 Superheated vapor 汽化潜热 Latent heat of Vaporization


Saturated liquid line, SLL is formed by connecting a series of boiling points. saturated vapor line, SVL:Connecting a series of points at dry saturated vapor builds a line, known as saturated vapor line, SVL. Vaporization continues by further heat supply to the system until no liquid is left. This state is known as dry saturated vapor, e. g. point d. If the system is slightly cooled at this state, then droplets of liquid will begin to form. The state of substance between saturated liquid and dry vapor is called wet vapor. Further heating of a dry saturated vapor at constant pressure causes a rise of vapor temperature and it becomes superheated vapor. The state of substance is completely defined by its pressure and temperature if it is in liquid or superheated vapor phase.

v

One point (一点): critical point (临界点) Two lines (两线): SLL and SVL (饱和液体线和饱和蒸气线) Three regions(三区): Subcooled liquid region (未饱和液体区) Saturated liquid-vapor mixture region(湿蒸气区) Superheated vapor region (过热蒸气区) Five states(五态): Subcooled liquid(未饱和液体) Saturated liquid (饱和液体) Saturated liquid-vapor mixture (湿蒸气) Saturated vapor (干饱和蒸气) Superheated vapor (过热蒸气)

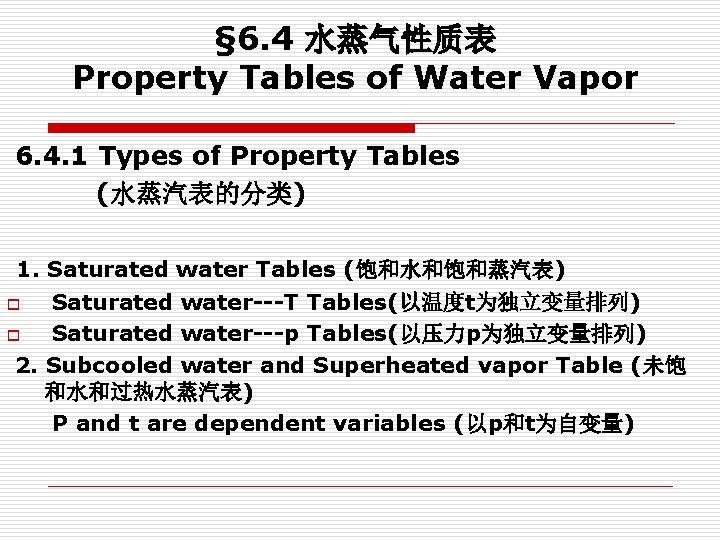
§ 6. 4 水蒸气性质表 Property Tables of Water Vapor 6. 4. 1 Types of Property Tables (水蒸汽表的分类) 1. Saturated water Tables (饱和水和饱和蒸汽表) Saturated water---T Tables(以温度t为独立变量排列) o Saturated water---p Tables(以压力p为独立变量排列) 2. Subcooled water and Superheated vapor Table (未饱 和水和过热水蒸汽表) P and t are dependent variables (以p和t为自变量) o

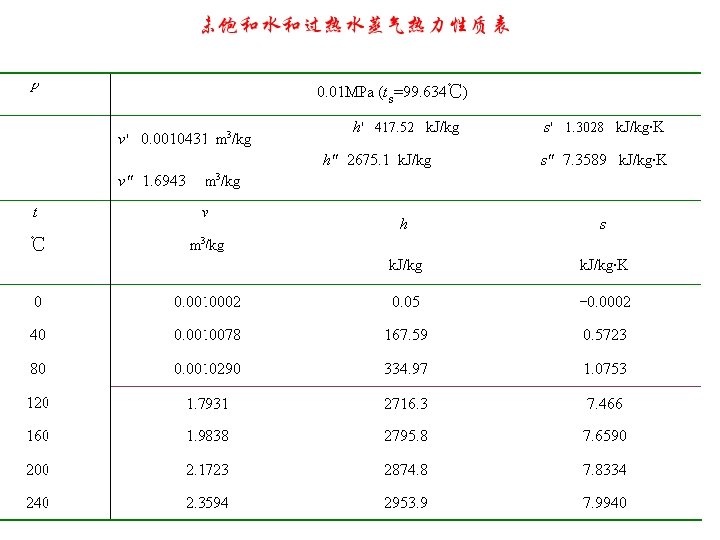
2、Reference State and Reference Values (基准点的规定) For water, the saturated liquid at 0. 01℃ is taken as reference state. (取水的三相点,即 0. 01 o. C为基准点,规定在 此温度下液态水的热力学能和熵为零。) The internal energy and entropy are assigned zero at this state.

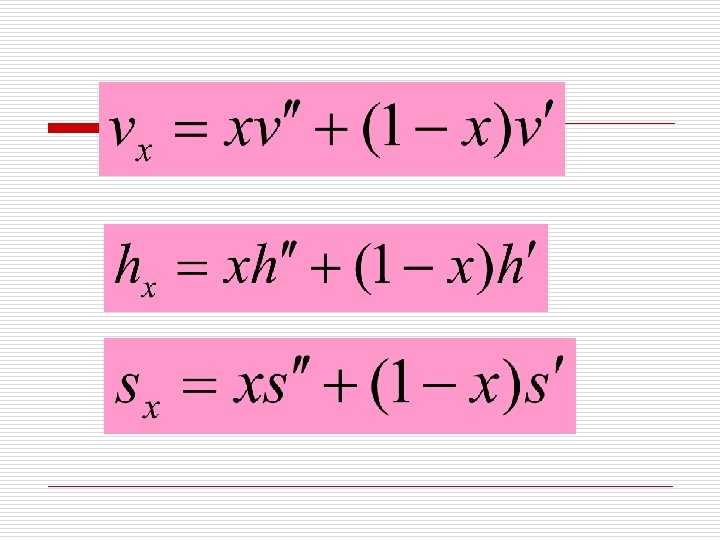
3. Properties of Saturated liquid-vapor mixture at Pressure p (压力为p的湿饱和蒸汽) The state of wet vapor can not be defined by just pressure and temperature until one other property is given. The condition or quality of wet vapor is often defined by its dryness or wetness fraction. 定义干度x才可以确定湿蒸汽的状态。 We define dryness as dryness fraction, x = mixture, the mass of dry vapor in 1 kg of the wetness fraction, 1 - x = mixture. the mass of liquid in 1 kg of the




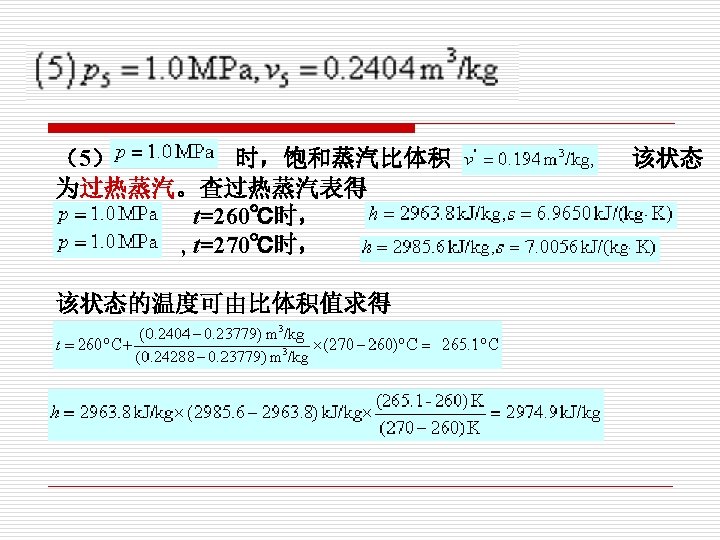
4. Exercise. Consulting property tables of water vapor to determine the state of each point and their h,s,x. (利用水蒸气表判断下列各点的状态,并确 定其h,s,x的值。)









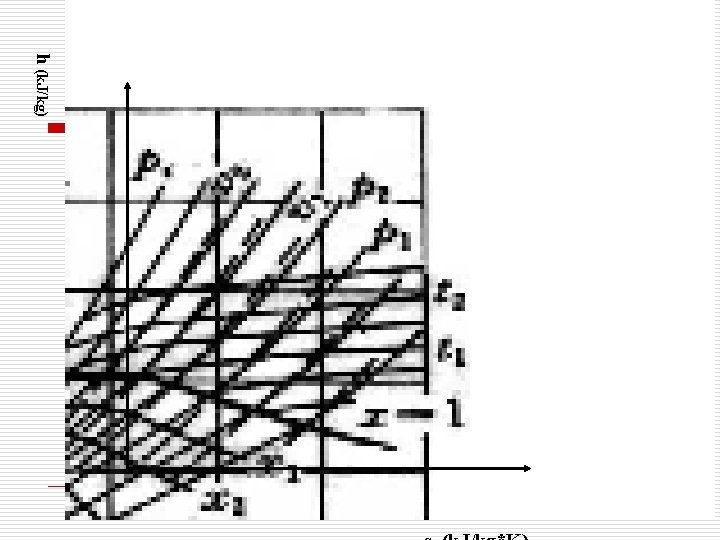
§ 6. 5 h-s diagram of water vapor (水蒸汽的h-s图) 。 1. SLL and SVL 2. (界限曲线,x=0、x=1两条曲线) 2. Constant Pressure Line and Constant Temperature Line (定压线和定温线) In Saturated liquid-vapor region, constant pressure line and constant temperature line are identical. ( 在湿蒸汽区定压线与定温线重合。) 3. Constant Volume Line(定容线。) 4. Constant Dryness Line (定干度线。) Given Two Properties, all the other state properties can be determined from the Diagram. (给定两个参数,即可查出其余全部参数)

。 Priorities (优点): Fast and convenient. 方便、快速。 Drawbacks (缺点): Not so accurate as Property tables 精确性稍差


h (k. J/kg)

§ 6. 6 Thermodynamic Processes of Water Vapor (水蒸气的热力过程) 6. 6. 1 Characteristics of water vapor (水蒸汽的特点) 1. Water vapor can not be treated as ideal gas (水蒸汽不 是理想气体) pv=RT is not suitable. (理想气体状态方程不适用。) 2. Analysis and Calculation on Water vapor can be solved by using Tables and Diagrams ( 水蒸汽的分析和 计算采用水蒸汽图和表。) 3. The First and the Second Laws must be obeyed during processes of water vapor (热力学第一定律和第二 定律成立)

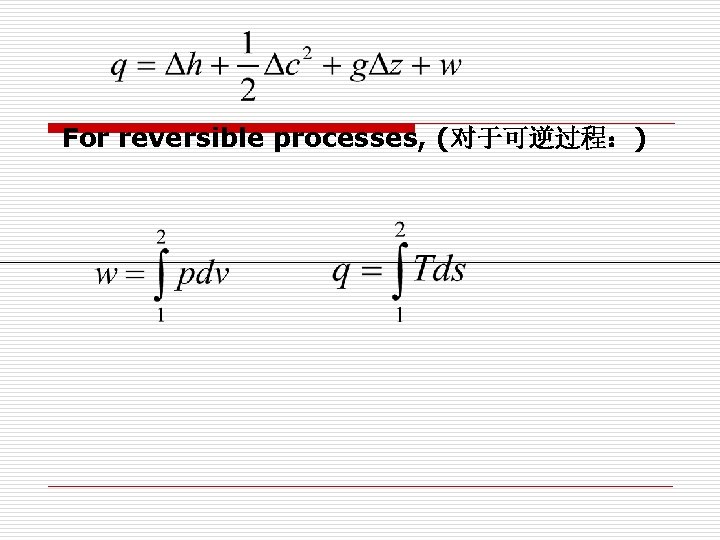
For reversible processes, (对于可逆过程:)

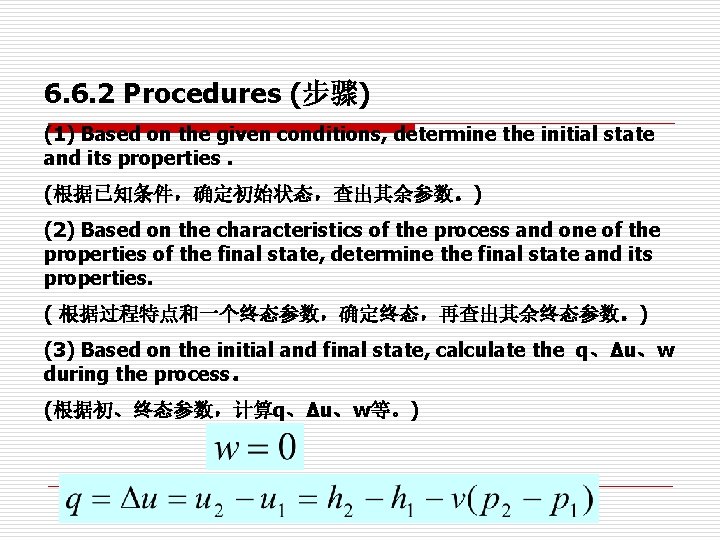
6. 6. 2 Procedures (步骤) (1) Based on the given conditions, determine the initial state and its properties. (根据已知条件,确定初始状态,查出其余参数。) (2) Based on the characteristics of the process and one of the properties of the final state, determine the final state and its properties. ( 根据过程特点和一个终态参数,确定终态,再查出其余终态参数。) (3) Based on the initial and final state, calculate the q、Δu、w during the process。 (根据初、终态参数,计算q、Δu、w等。)

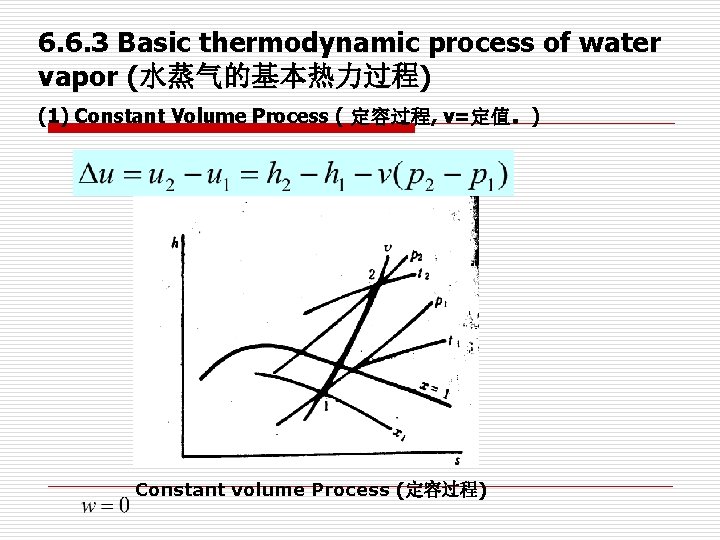
6. 6. 3 Basic thermodynamic process of water vapor (水蒸气的基本热力过程) (1) Constant Volume Process ( 定容过程, v=定值。) Constant volume Process (定容过程)

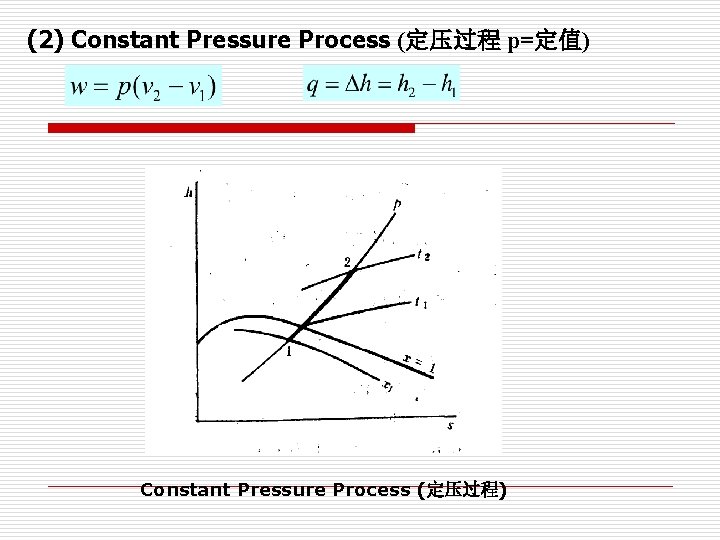
(2) Constant Pressure Process (定压过程 p=定值) Constant Pressure Process (定压过程)

(3) Constant Temperature Process (定温过程 T=定值) Isothermal Process (定温过程)

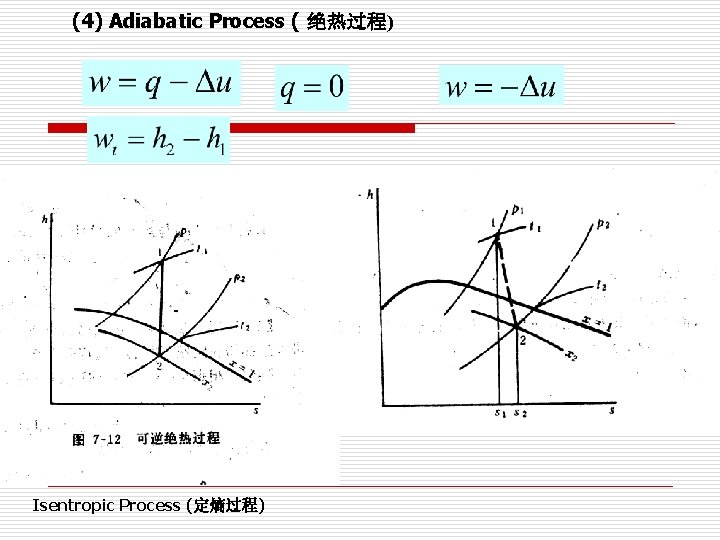
(4) Adiabatic Process ( 绝热过程) Isentropic Process (定熵过程)

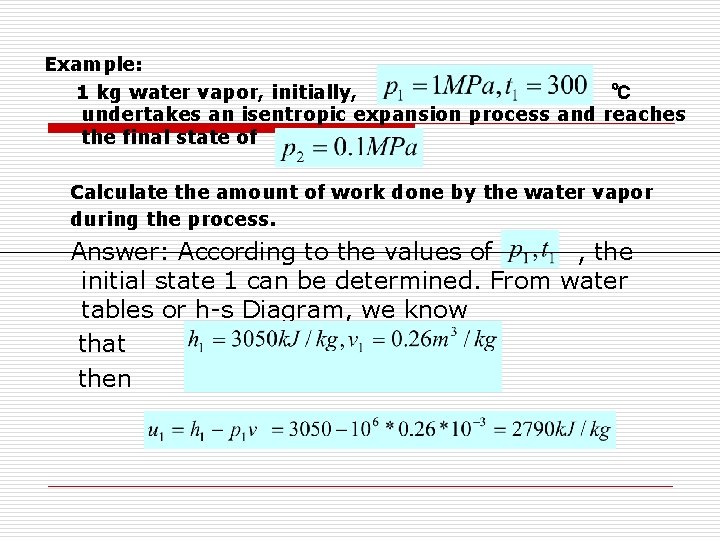
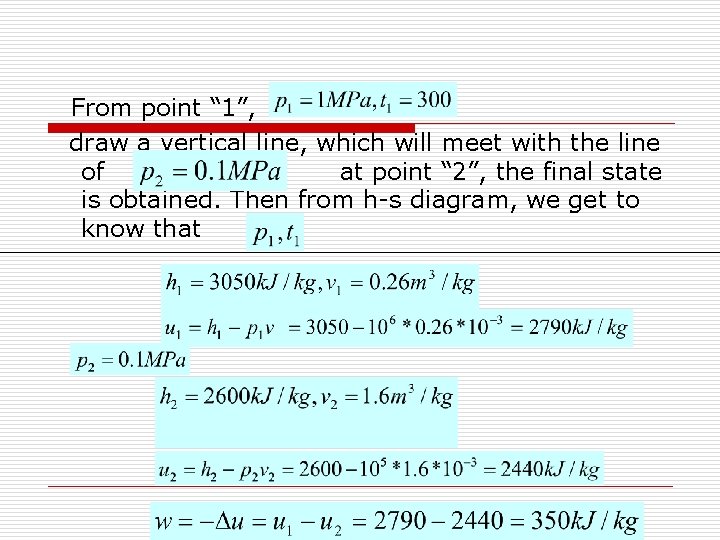
Example: 1 kg water vapor, initially, ℃ undertakes an isentropic expansion process and reaches the final state of Calculate the amount of work done by the water vapor during the process. Answer: According to the values of , the initial state 1 can be determined. From water tables or h-s Diagram, we know that then

From point “ 1”, draw a vertical line, which will meet with the line of at point “ 2”, the final state is obtained. Then from h-s diagram, we get to know that
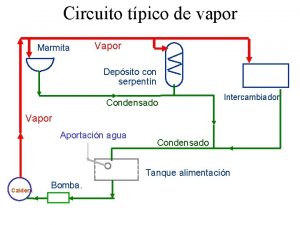
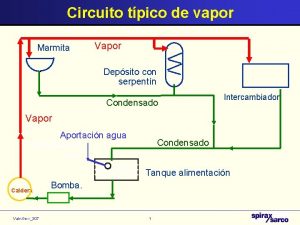
 Caldeira flamotubular
Caldeira flamotubular Water and water and water water
Water and water and water water What is the earth called a blue planet
What is the earth called a blue planet How to calculate vapor pressure of water
How to calculate vapor pressure of water Only water vapor evaporates through stomata.
Only water vapor evaporates through stomata. The capacity of air to hold water vapor
The capacity of air to hold water vapor Vapor pressure of water equation
Vapor pressure of water equation Water vapor in the atmosphere percentage
Water vapor in the atmosphere percentage Water vapor mixing ratio
Water vapor mixing ratio Raoult's law and dalton's law
Raoult's law and dalton's law Highest clouds
Highest clouds Phosphorus equation
Phosphorus equation To cook by the vapor produced from boiling water
To cook by the vapor produced from boiling water Obstructed and unobstructed heritage
Obstructed and unobstructed heritage Physical property and chemical property
Physical property and chemical property Commutative property vs associative property
Commutative property vs associative property A paved blacktop parking lot was built
A paved blacktop parking lot was built Vapor pressure worksheet
Vapor pressure worksheet Vapor pressure and intermolecular forces
Vapor pressure and intermolecular forces High specific heat water property
High specific heat water property How does the property of water support fish in general
How does the property of water support fish in general Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Glasgow thang điểm
Glasgow thang điểm Chúa yêu trần thế
Chúa yêu trần thế Môn thể thao bắt đầu bằng chữ đua
Môn thể thao bắt đầu bằng chữ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thế nào là giọng cùng tên
Thế nào là giọng cùng tên Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phối cảnh
Phối cảnh Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Bảng số nguyên tố lớn hơn 1000
Bảng số nguyên tố lớn hơn 1000