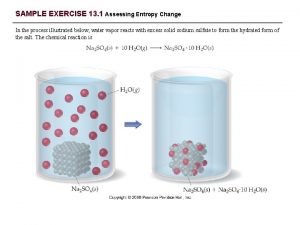
Liquids Vapor Pressure Vapor Gas phase of a


















- Slides: 26

Liquids & Vapor Pressure

Vapor • Gas phase of a substance that is normally a liquid at room temperature. • Some evaporation occurs at all temperatures. • Generally, the easier a substance evaporates, the weaker the intermolecular forces.

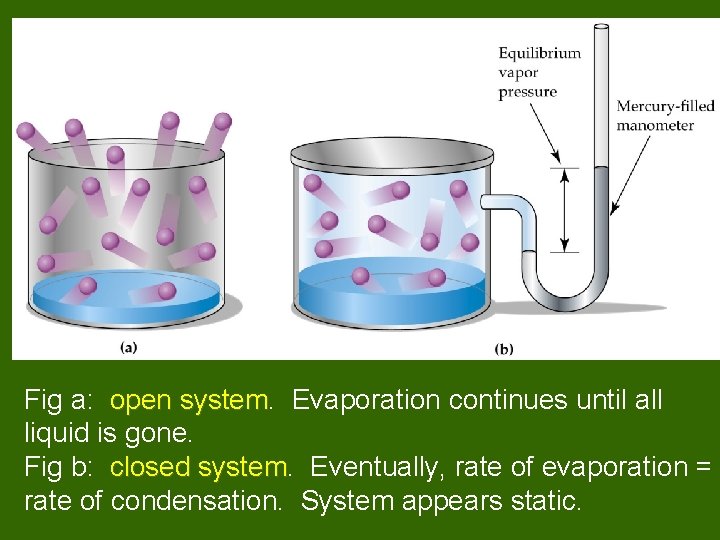
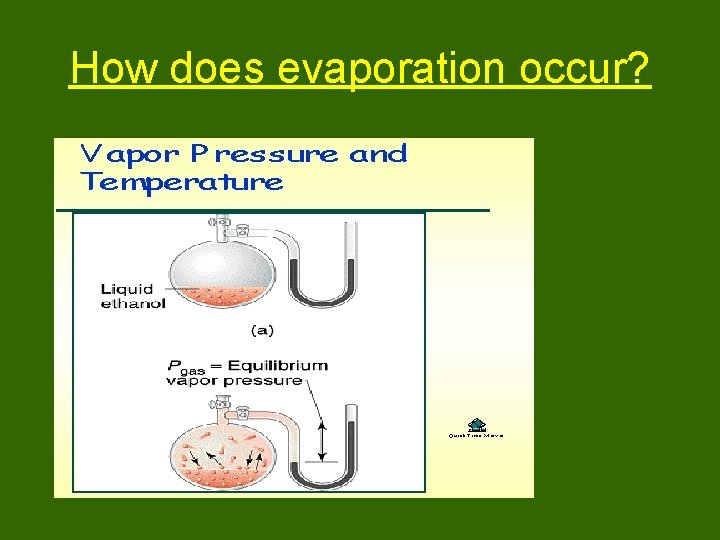
Fig a: open system Evaporation continues until all liquid is gone. Fig b: closed system Eventually, rate of evaporation = rate of condensation. System appears static.

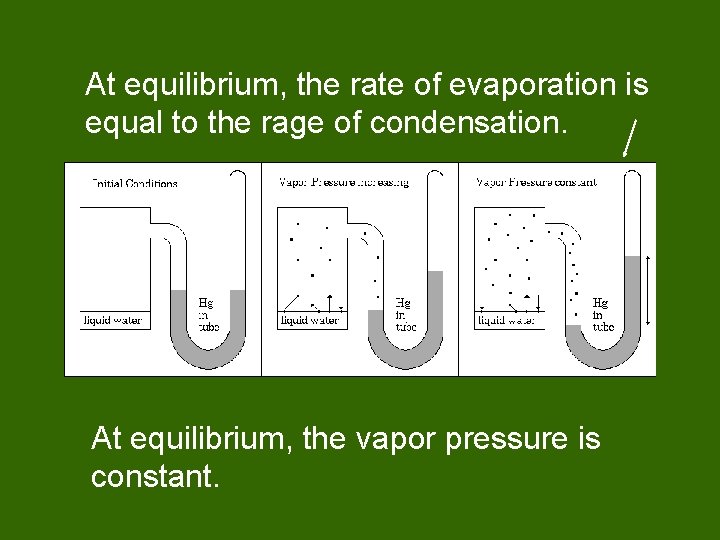
At equilibrium, the rate of evaporation is equal to the rage of condensation. At equilibrium, the vapor pressure is constant.

How does evaporation occur?

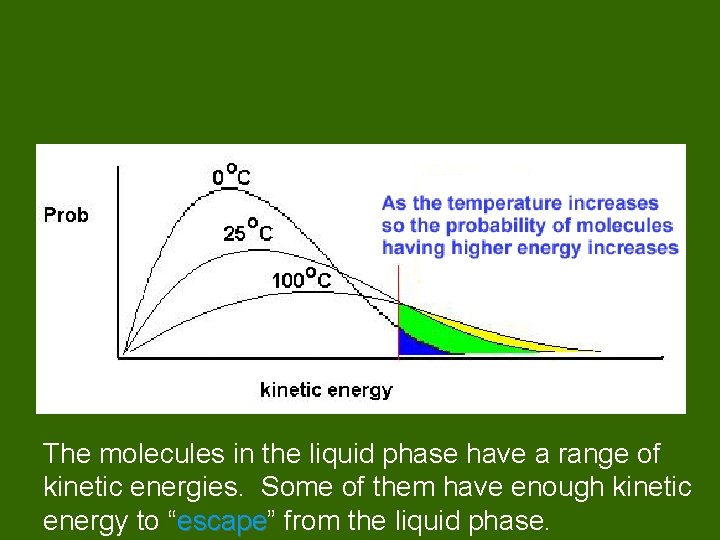
The molecules in the liquid phase have a range of kinetic energies. Some of them have enough kinetic energy to “escape” escape from the liquid phase.

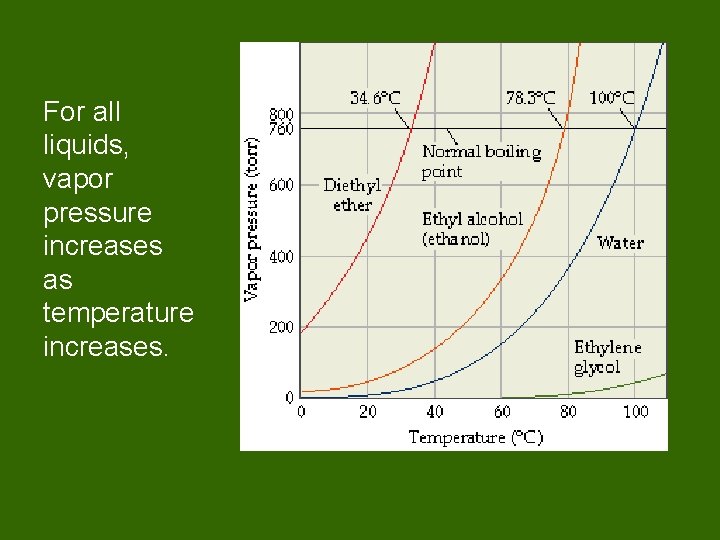
Vapor Pressure • Pressure exerted by a vapor over its liquid. Measuring vapor pressure • How is vapor pressure affected by temperature? • The higher the temperature, the higher the vapor pressure.

For all liquids, vapor pressure increases as temperature increases.

Vapor Pressure • Vapor pressure does NOT depend on how much liquid is present. • As long as some liquid is present, can be a teaspoon or a gallon. • Vapor pressure depends only on temperature.

Which has the highest v. p. ? 90 50 C 20 C 90 C 70 C

Intermolecular Forces in Liquids WEAK FORCES • High vapor pressure • High rate of evaporation • Low boiling point • Small Hv STRONG FORCES • Low vapor pressure • Low rate of evaporation • High boiling point • Large Hv

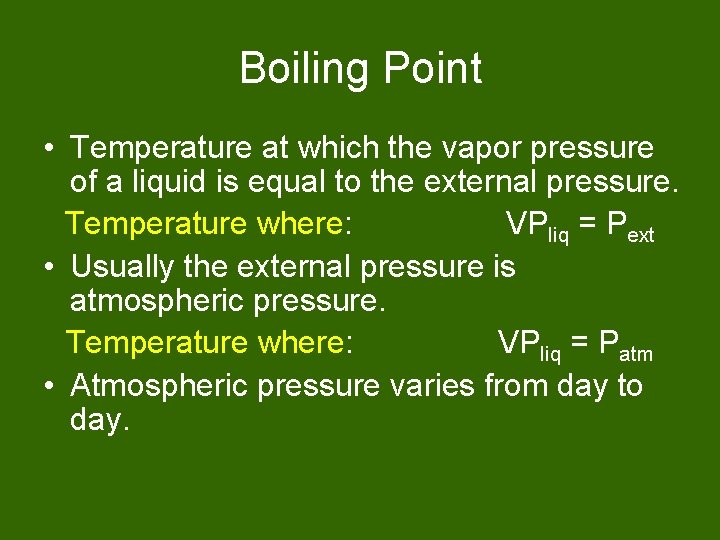
Boiling Point • Temperature at which the vapor pressure of a liquid is equal to the external pressure. Temperature where: VPliq = Pext • Usually the external pressure is atmospheric pressure. Temperature where: VPliq = Patm • Atmospheric pressure varies from day to day.

Boiling Point • Temperature at which the vapor pressure of a liquid = external or atmospheric pressure. • Normal Boiling Point is temperature at which vapor pressure of the liquid = 1 atm. • Substances with high boiling points have strong molecular interactions & low vapor pressures.

Normal Boiling Point • Temperature at which the vapor pressure of a liquid is equal to exactly 1 atmosphere or 760 torr or 101. 3 k. Pa.

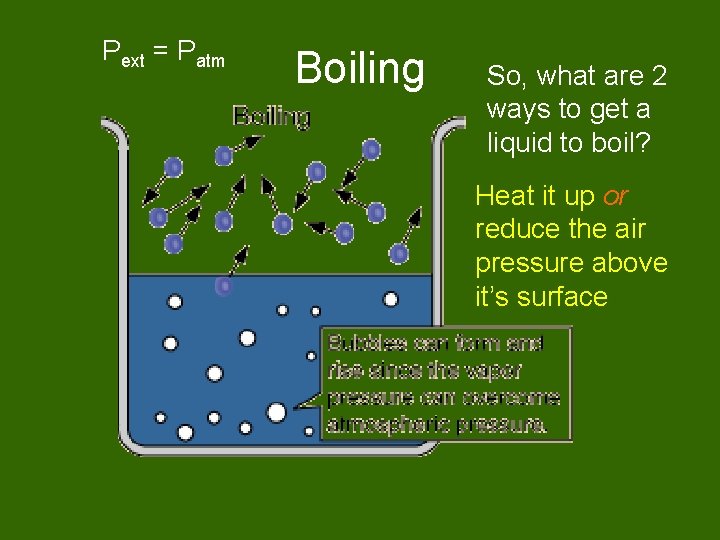
Pext = Patm Boiling So, what are 2 ways to get a liquid to boil? Heat it up or reduce the air pressure above it’s surface

Raise the vapor pressure of the liquid by heating it up.

Reduce the external pressure using a vacuum pump.

Strong or Weak attractive forces? • • High vapor pressure Large Hv High boiling point Low vapor pressure Small Hv Evaporates rapidly Evaporates slowly • • Weak Strong Weak Strong

Boiling and Pressure • If you increase the external pressure (say you are camping in Death Valley), the o. C. boiling point is ____ than 100 > • If you decrease the external pressure (say you are eating Raman noodles at the top of Mt. Whitney), the boiling point is o. C. ____ than 100 <

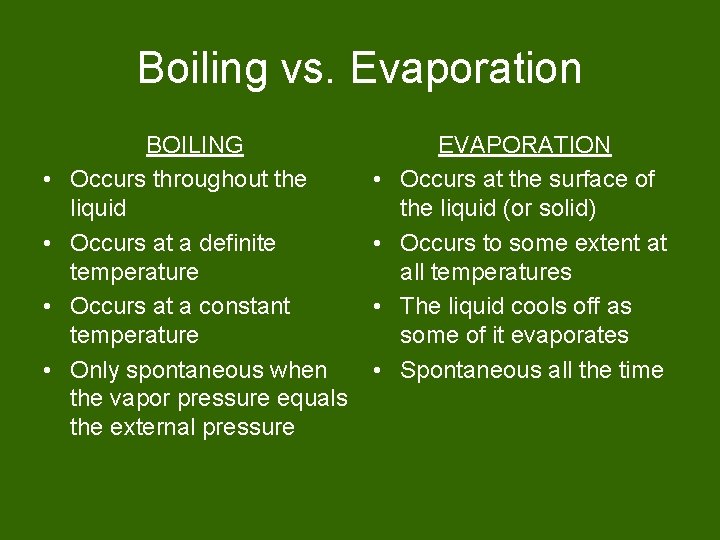
Boiling vs. Evaporation • • BOILING Occurs throughout the liquid Occurs at a definite temperature Occurs at a constant temperature Only spontaneous when the vapor pressure equals the external pressure • • EVAPORATION Occurs at the surface of the liquid (or solid) Occurs to some extent at all temperatures The liquid cools off as some of it evaporates Spontaneous all the time

Can you explain this … • Drinking Bird

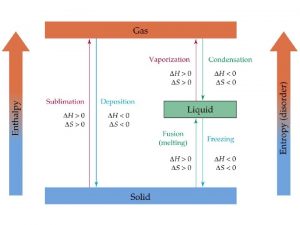
Phase Changes • Melting • Boiling (occurs throughout, constant temperature) – Evaporation (occurs at surface, temperature drops) • • Sublimation Freezing Deposition Condensation

Phase Changes are accompanied by energy changes. • Which phase changes absorb energy? Melting, boiling • Which phase changes release energy? Condensation, Freezing

It’s always from the system’s perspective. • Endothermic Process: Energy is absorbed • Exothermic Process: Energy is released

Melting Point • = temperature at which the liquid phase and solid phase of a substance can coexist at equilibrium. • The higher the melting point, the stronger the molecular interactions.

Freezing Point • Temperature at which a liquid is converted to a crystalline solid. • How does freezing point compare to melting point? They’re the same.
 Superaquecimento
Superaquecimento Regional metamorphism
Regional metamorphism How to calculate vapor pressure of water
How to calculate vapor pressure of water Glycerin vapor pressure
Glycerin vapor pressure Absolute humidity
Absolute humidity Raoult's law and dalton's law
Raoult's law and dalton's law Why freezing point decreases on adding solute
Why freezing point decreases on adding solute Tb=kbm
Tb=kbm Vapor pressure worksheet
Vapor pressure worksheet Calculate the osmotic pressure of a solution
Calculate the osmotic pressure of a solution Which of the following involves a colligative property
Which of the following involves a colligative property Vapor pressure lowering
Vapor pressure lowering Hemolysis and crenation
Hemolysis and crenation Raoult's law for non volatile solute
Raoult's law for non volatile solute Shortcut vapor pressure equation
Shortcut vapor pressure equation Imf
Imf Ion dipole intermolecular forces
Ion dipole intermolecular forces Vapor pressure
Vapor pressure Normal phase vs reverse phase chromatography
Normal phase vs reverse phase chromatography Hplc reverse phase vs normal phase
Hplc reverse phase vs normal phase Mobile phase and stationary phase
Mobile phase and stationary phase Chromatography means
Chromatography means Normal phase vs reverse phase chromatography
Normal phase vs reverse phase chromatography Line vs phase voltage
Line vs phase voltage Hplc detector types
Hplc detector types In a triangle connected source feeding a y connected load
In a triangle connected source feeding a y connected load Csce 441
Csce 441