Chapter 11 Intermolecular Forces Liquids and Solids Which












- Slides: 52

Chapter 11 – Intermolecular Forces, Liquids, and Solids Which factor(s) affect the physical properties of a substance? Why does water boil at 100°C and freeze at 0°C? Why does high pressure and low temperature solidify most gasses? Why can’t hydrogen be solidified? In this unit we will look at the factors that affect the physical properties of liquids and solids.

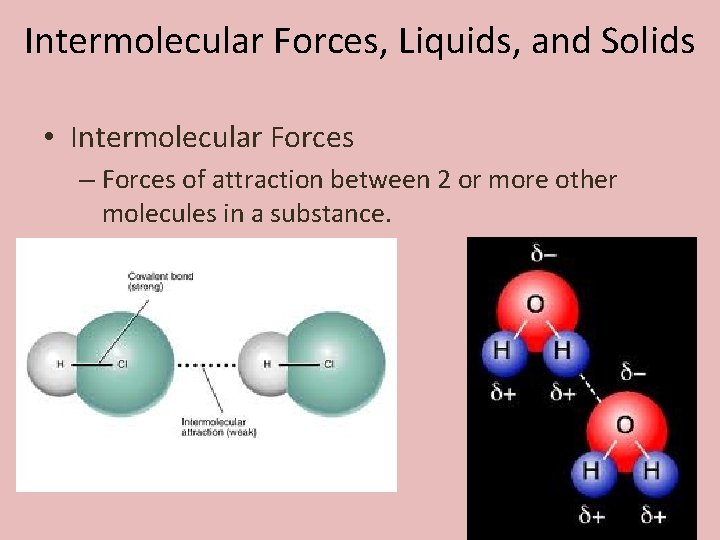
Intermolecular Forces, Liquids, and Solids • Intermolecular Forces – Forces of attraction between 2 or more other molecules in a substance.

Intermolecular Forces, Liquids, and Solids • Intermolecular Forces – 3 types: London Dispersion Forces (weakest), Dipole-Dipole, and Hydrogen Bonding (strongest).

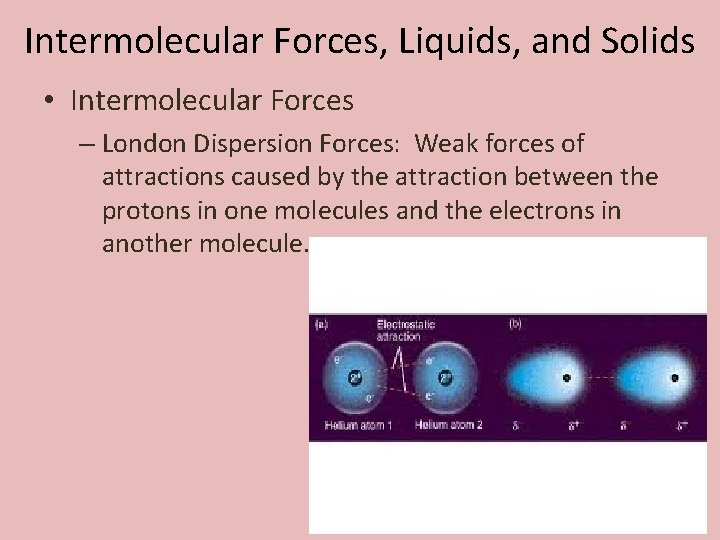
Intermolecular Forces, Liquids, and Solids • Intermolecular Forces – London Dispersion Forces: Weak forces of attractions caused by the attraction between the protons in one molecules and the electrons in another molecule.

Intermolecular Forces, Liquids, and Solids • Intermolecular Forces o. London Dispersion Forces: • Generally, substances that only have LDF’s have low melting and vaporization points. • But substances with higher molecular weights can have so many LDF’s that they will have high melting and vaporization points.

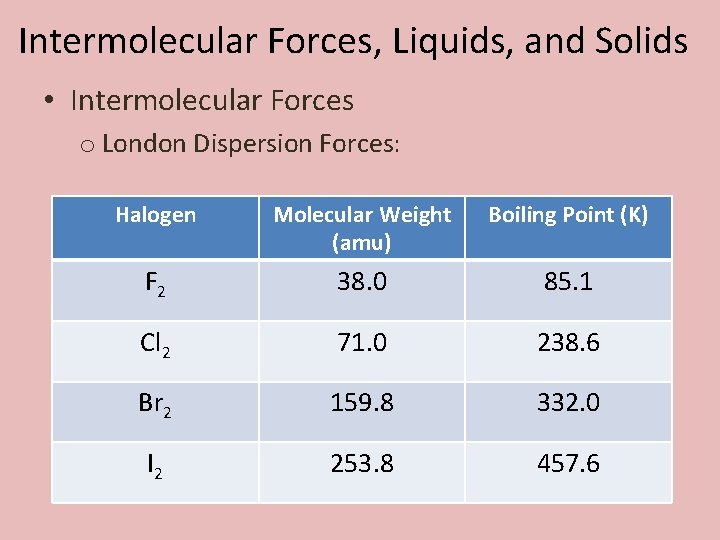
Intermolecular Forces, Liquids, and Solids • Intermolecular Forces o London Dispersion Forces: Halogen Molecular Weight (amu) Boiling Point (K) F 2 38. 0 85. 1 Cl 2 71. 0 238. 6 Br 2 159. 8 332. 0 I 2 253. 8 457. 6

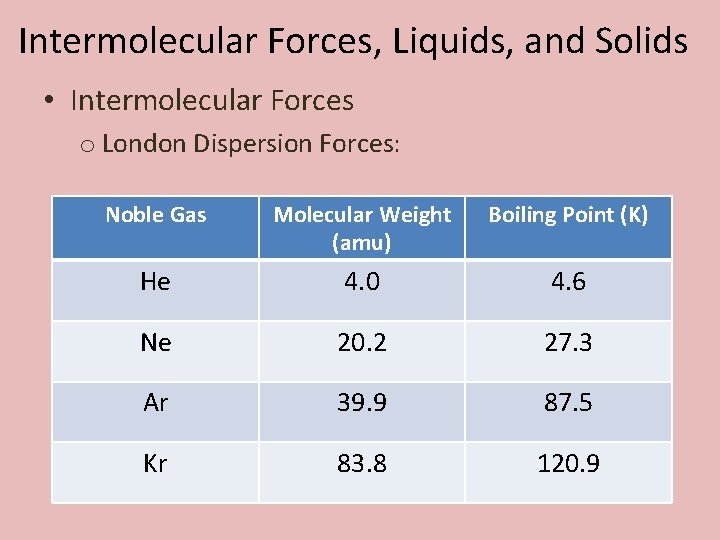
Intermolecular Forces, Liquids, and Solids • Intermolecular Forces o London Dispersion Forces: Noble Gas Molecular Weight (amu) Boiling Point (K) He 4. 0 4. 6 Ne 20. 2 27. 3 Ar 39. 9 87. 5 Kr 83. 8 120. 9

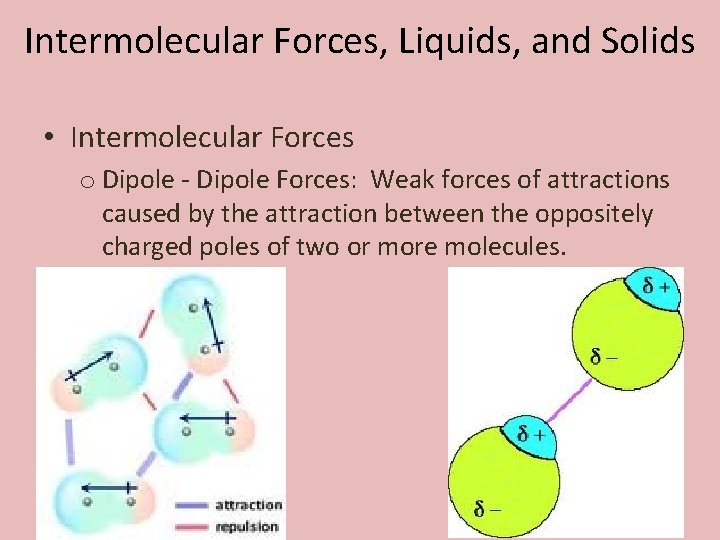
Intermolecular Forces, Liquids, and Solids • Intermolecular Forces o Dipole - Dipole Forces: Weak forces of attractions caused by the attraction between the oppositely charged poles of two or more molecules.

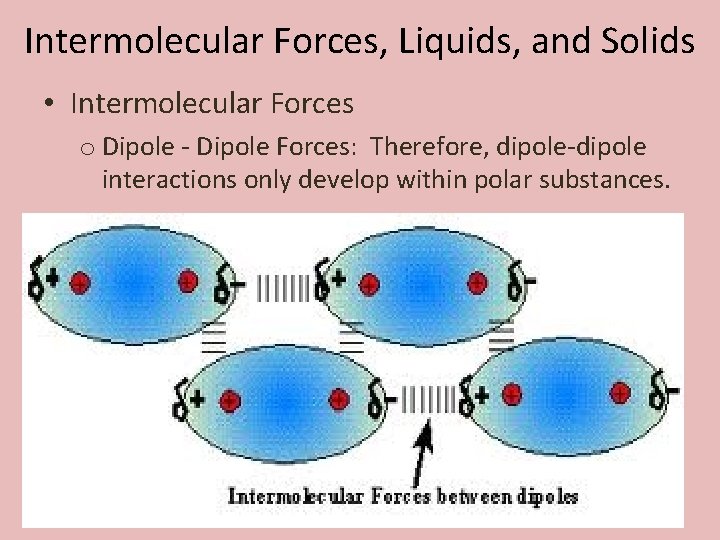
Intermolecular Forces, Liquids, and Solids • Intermolecular Forces o Dipole - Dipole Forces: Therefore, dipole-dipole interactions only develop within polar substances.

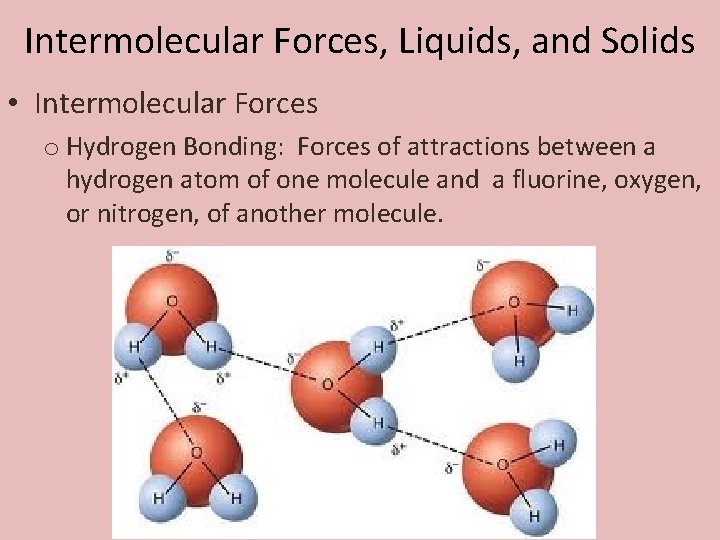
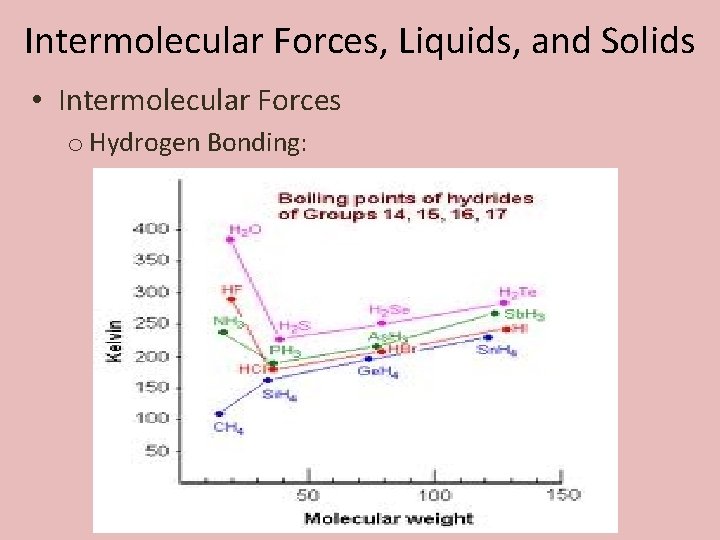
Intermolecular Forces, Liquids, and Solids • Intermolecular Forces o Hydrogen Bonding: Forces of attractions between a hydrogen atom of one molecule and a fluorine, oxygen, or nitrogen, of another molecule.

Intermolecular Forces, Liquids, and Solids • Intermolecular Forces o Hydrogen Bonding: Ammonia’s trigonal pyramidal shape allows the hydrogen atoms of one molecule to be attracted to the nitrogen on another.

Intermolecular Forces, Liquids, and Solids • Intermolecular Forces o Hydrogen Bonding:

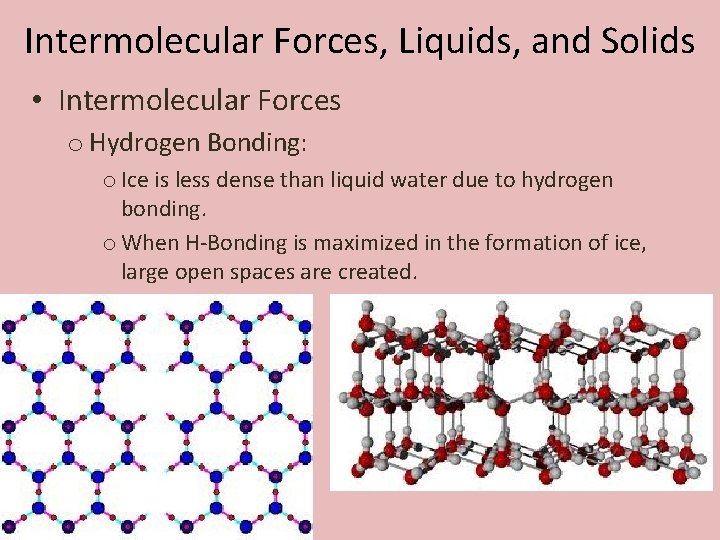
Intermolecular Forces, Liquids, and Solids • Intermolecular Forces o Hydrogen Bonding: o Ice is less dense than liquid water due to hydrogen bonding. o When H-Bonding is maximized in the formation of ice, large open spaces are created.

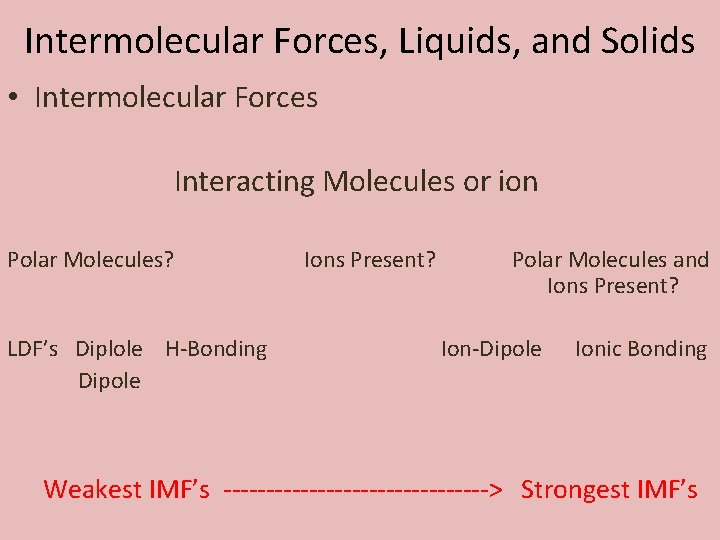
Intermolecular Forces, Liquids, and Solids • Intermolecular Forces Interacting Molecules or ion Polar Molecules? LDF’s Diplole H-Bonding Dipole Ions Present? Polar Molecules and Ions Present? Ion-Dipole Ionic Bonding Weakest IMF’s ----------------> Strongest IMF’s

Intermolecular Forces, Liquids, and Solids • Properties of Liquids o Viscosity – The measure of the resistance of a liquid to flow.

Intermolecular Forces, Liquids, and Solids • Properties of Liquids o Viscosity – Affect by temperature and molecular weight. ØTemperature – IMF’s are overcome with higher temperature. ØMolecular Weight – The larger the molecule, the more ‘tangled’ it becomes, thus resisting flow.

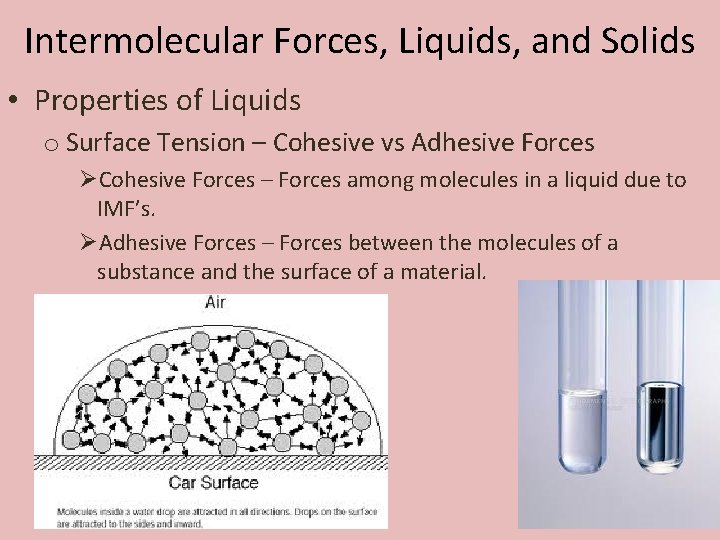
Intermolecular Forces, Liquids, and Solids • Properties of Liquids o Surface Tension – A measure of the inward forces that must be exerted to overcome to expand the surface of a liquid.

Intermolecular Forces, Liquids, and Solids • Properties of Liquids o Surface Tension – Cohesive vs Adhesive Forces ØCohesive Forces – Forces among molecules in a liquid due to IMF’s. ØAdhesive Forces – Forces between the molecules of a substance and the surface of a material.

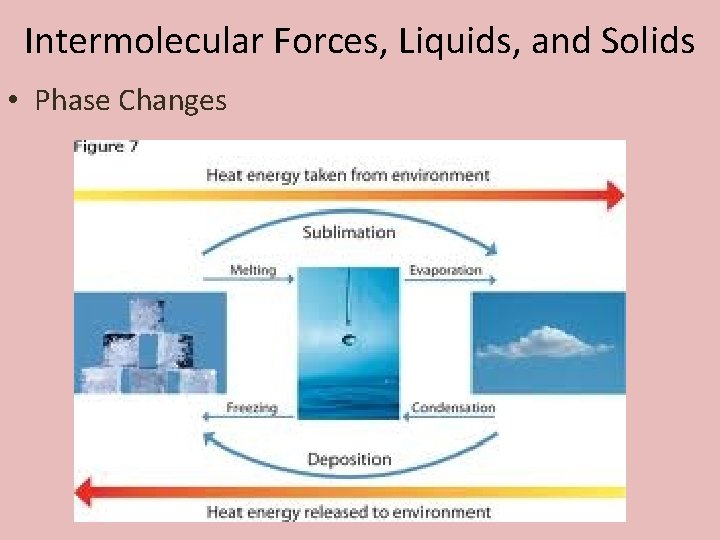
Intermolecular Forces, Liquids, and Solids • Phase Changes

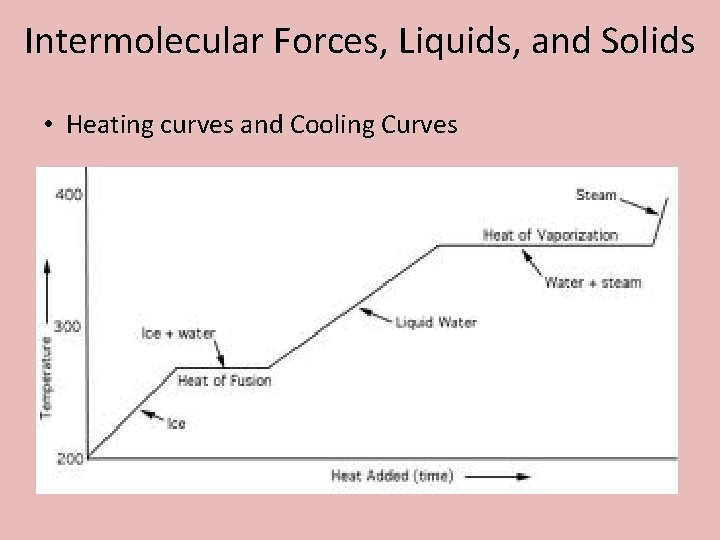
Intermolecular Forces, Liquids, and Solids • Phase Changes o Heat of Fusion (ΔHfus): The amount of energy to melt 1 mole of a substance. o What patter do you see in the heat of fusion of these substances? ΔHfus H 2 O = 6. 01 k. J / mol ΔHfus C 4 H 10 = 5. 00 k. J / mol ΔHfus Hg = 23. 0 k. J / mol

Intermolecular Forces, Liquids, and Solids • Phase Changes o Heat of Vaporization (ΔHvap): The amount of energy to vaporize 1 mole of a substance. o What pattern do you see in the heat of vaporization of these substances? ΔHvap H 2 O = 40. 7 k. J / mol ΔHvap C 4 H 10 = 24. 0 k. J / mol ΔHvap Hg = 58. 0 k. J / mol

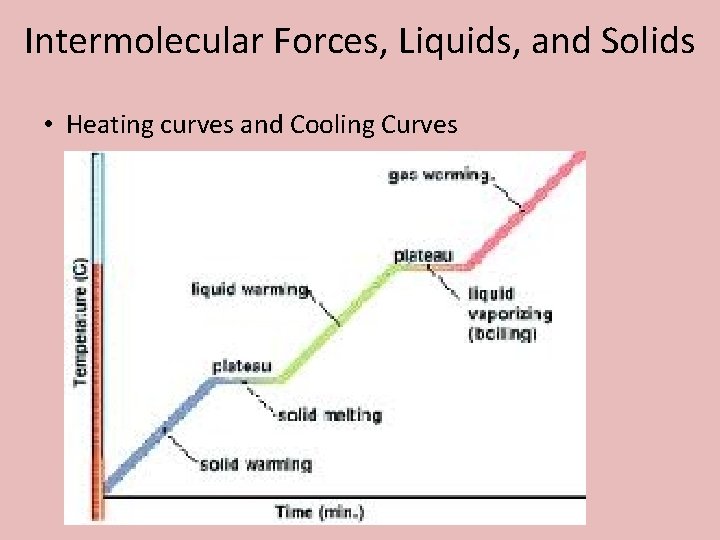
Intermolecular Forces, Liquids, and Solids • Heating curves and Cooling Curves

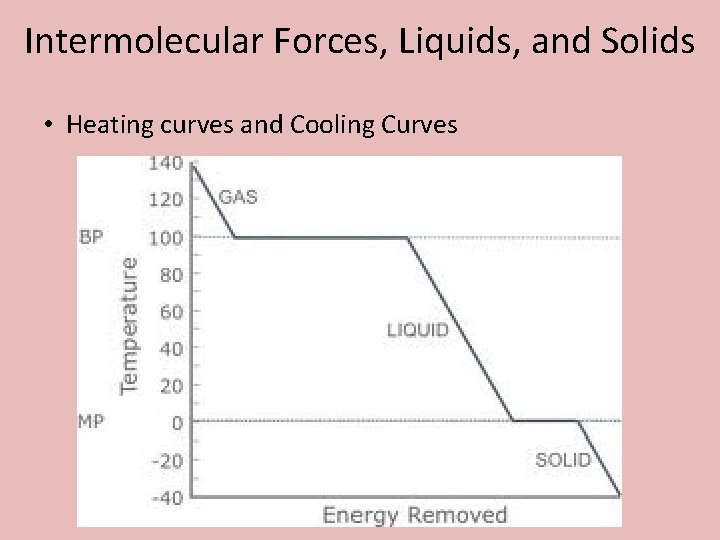
Intermolecular Forces, Liquids, and Solids • Heating curves and Cooling Curves

Intermolecular Forces, Liquids, and Solids • Heating curves and Cooling Curves

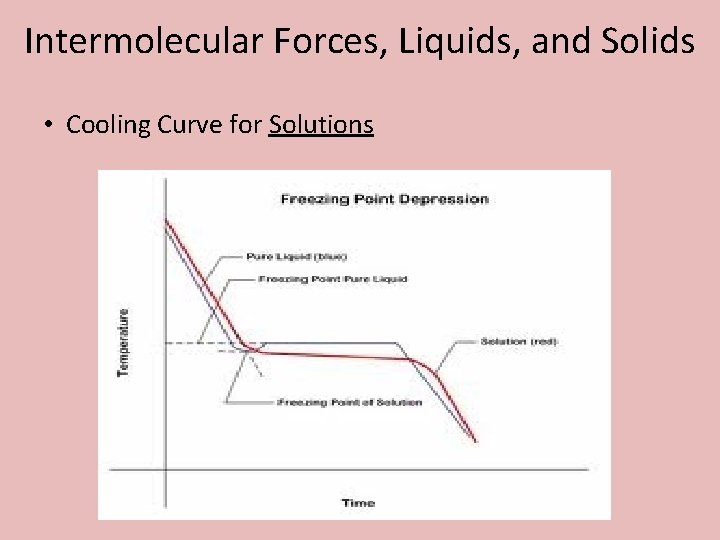
Intermolecular Forces, Liquids, and Solids • Cooling Curve for Solutions

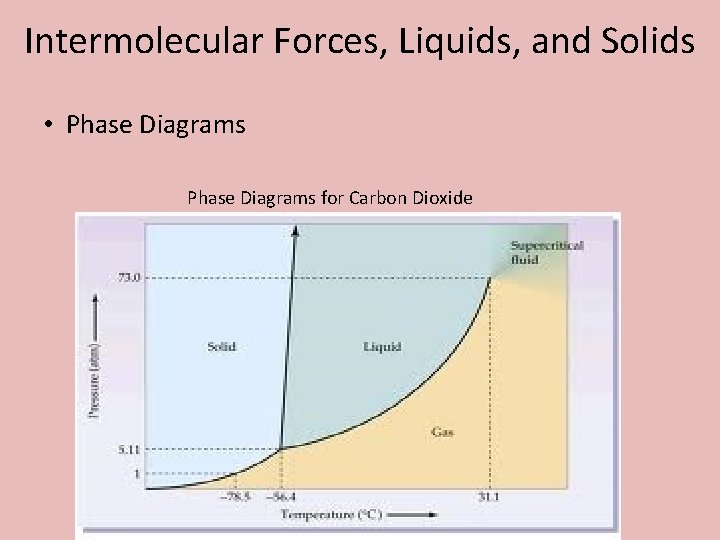
Intermolecular Forces, Liquids, and Solids • Phase Diagrams for Carbon Dioxide

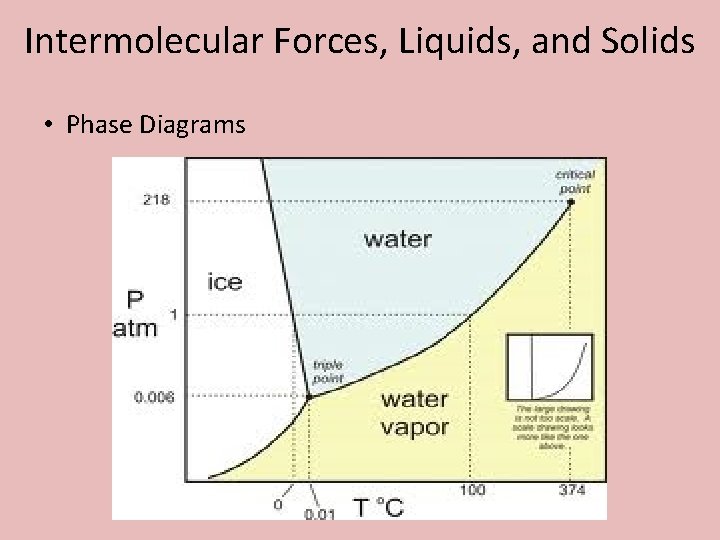
Intermolecular Forces, Liquids, and Solids • Phase Diagrams

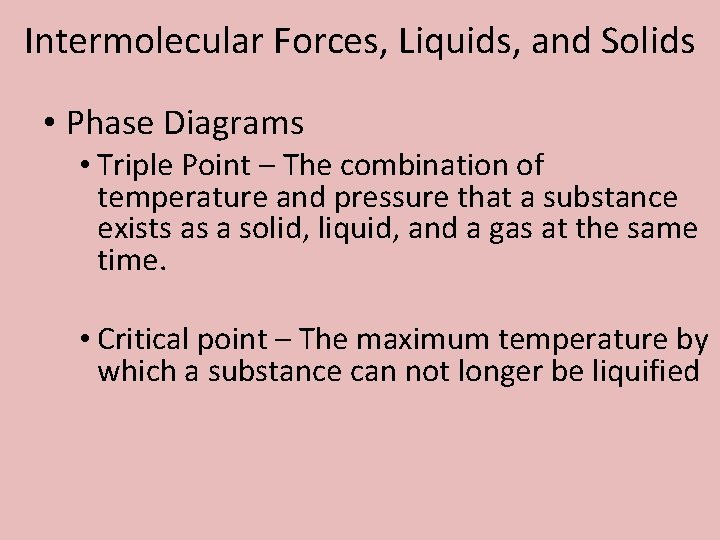
Intermolecular Forces, Liquids, and Solids • Phase Diagrams • Triple Point – The combination of temperature and pressure that a substance exists as a solid, liquid, and a gas at the same time. • Critical point – The maximum temperature by which a substance can not longer be liquified

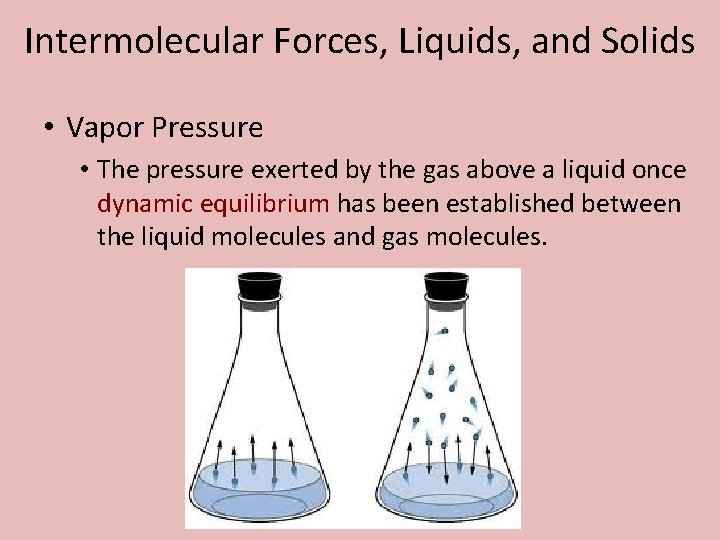
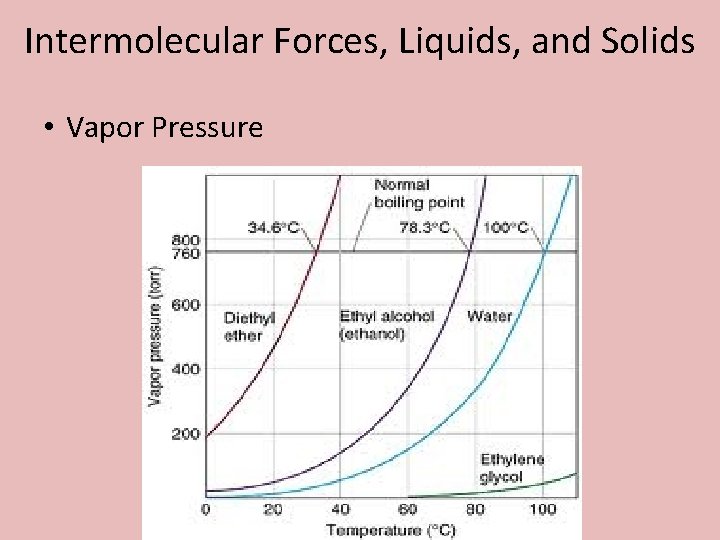
Intermolecular Forces, Liquids, and Solids • Vapor Pressure • The pressure exerted by the gas above a liquid once dynamic equilibrium has been established between the liquid molecules and gas molecules.

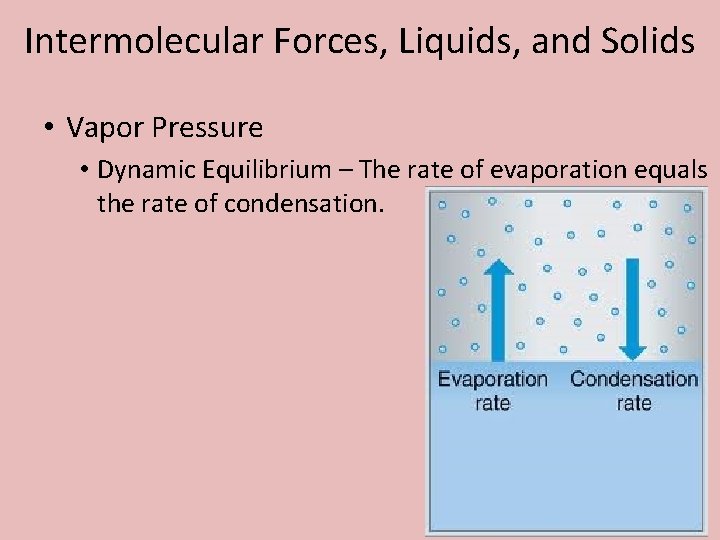
Intermolecular Forces, Liquids, and Solids • Vapor Pressure • Dynamic Equilibrium – The rate of evaporation equals the rate of condensation.

Intermolecular Forces, Liquids, and Solids • Vapor Pressure vs Intermolecular Forces • The stronger the IMF’s, the more difficult it is for a molecule to escape to the gas phase. Therefore its vapor pressure will be lower. • The weaker the IMF’s, the higher the vapor pressure.

Intermolecular Forces, Liquids, and Solids • Vapor Pressure

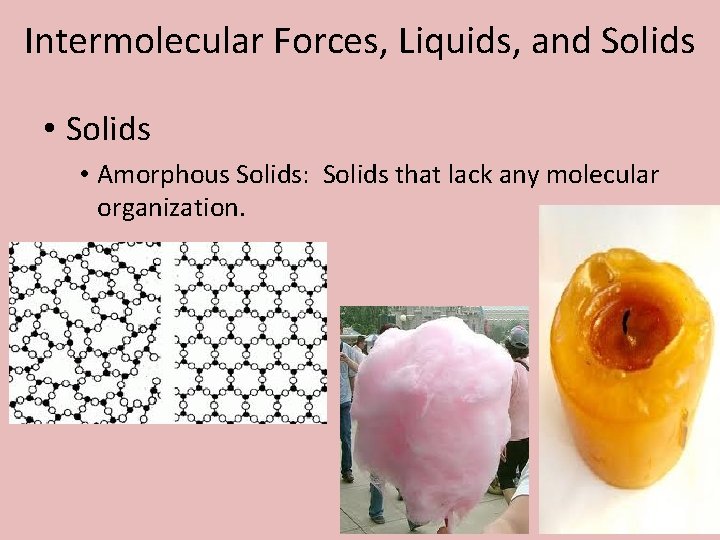
Intermolecular Forces, Liquids, and Solids • Amorphous Solids: Solids that lack any molecular organization.

Intermolecular Forces, Liquids, and Solids • Crystalline Solids: – Solids that have a high degree of molecular organization. – Crystals are three-dimensional shapes that have flat surfaces and angled corners.

Intermolecular Forces, Liquids, and Solids • Crystalline Solids: Geodes

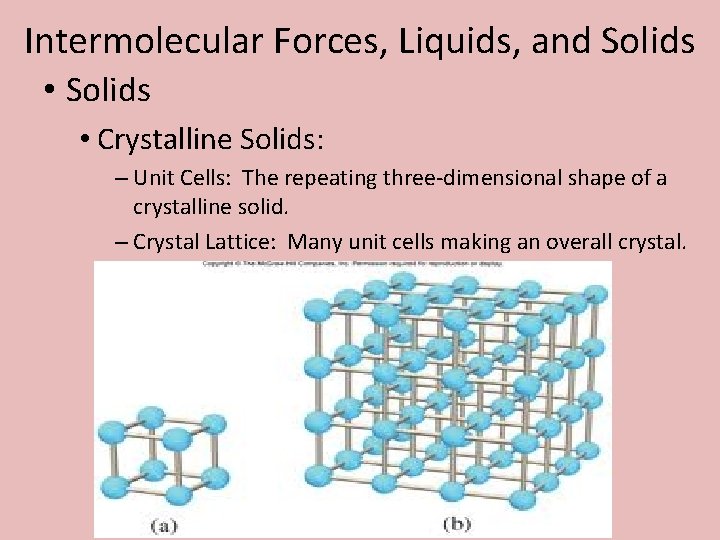
Intermolecular Forces, Liquids, and Solids • Crystalline Solids: – Unit Cells: The repeating three-dimensional shape of a crystalline solid. – Crystal Lattice: Many unit cells making an overall crystal.

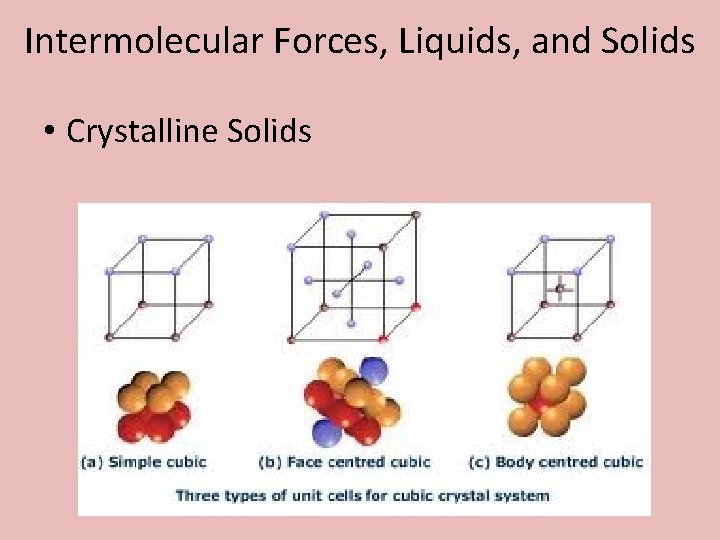
Intermolecular Forces, Liquids, and Solids • Crystalline Solids: – All ionic compounds can be explained with 7 different types of unit cells. – We will take a close look at 3 common types; primitive cubic, body-centered cubic, and face-centered cubic.

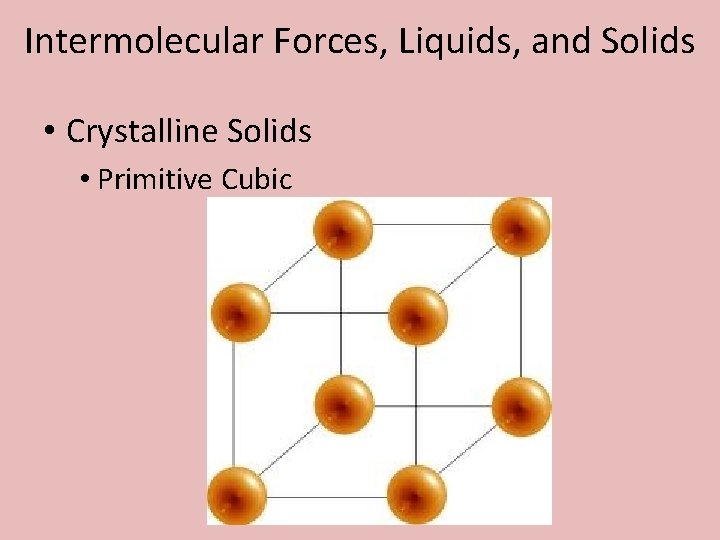
Intermolecular Forces, Liquids, and Solids • Crystalline Solids • Primitive Cubic

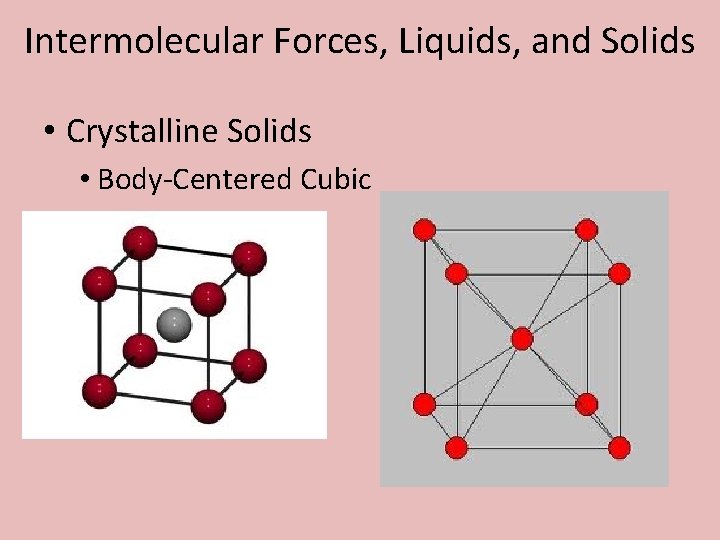
Intermolecular Forces, Liquids, and Solids • Crystalline Solids • Body-Centered Cubic

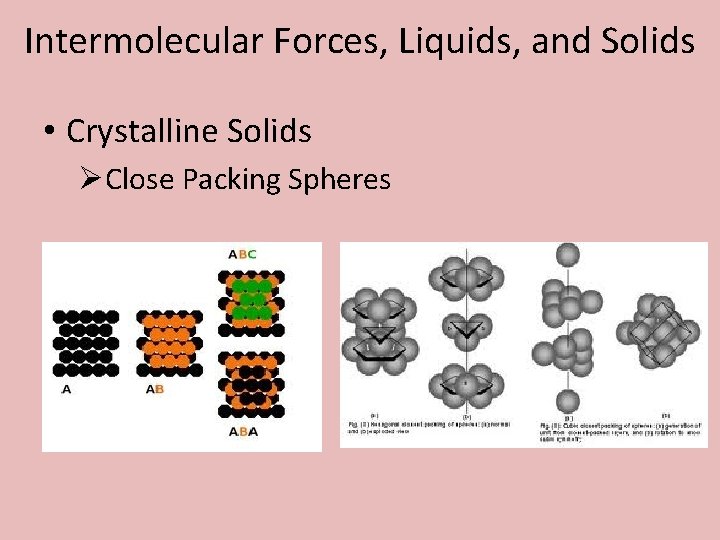
Intermolecular Forces, Liquids, and Solids • Crystalline Solids

Intermolecular Forces, Liquids, and Solids • Crystalline Solids ØClose Packing Spheres

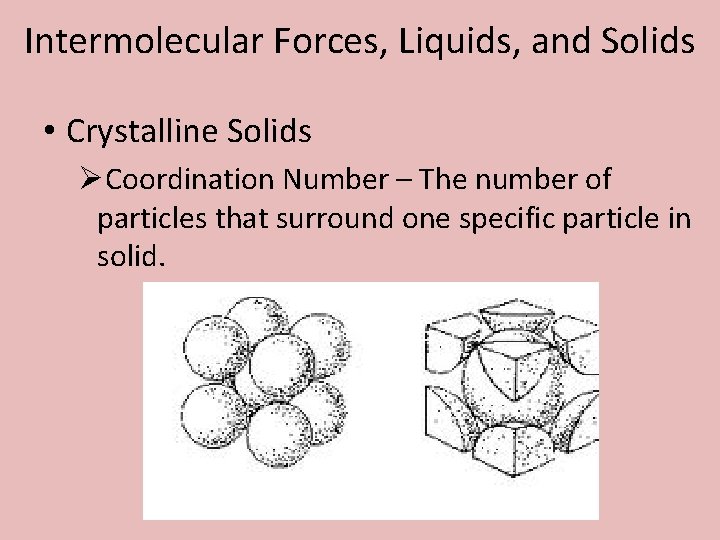
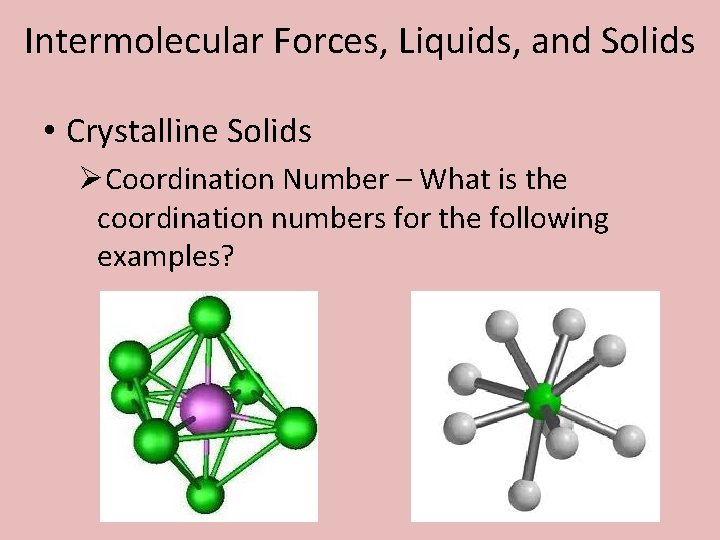
Intermolecular Forces, Liquids, and Solids • Crystalline Solids ØCoordination Number – The number of particles that surround one specific particle in solid.

Intermolecular Forces, Liquids, and Solids • Crystalline Solids ØCoordination Number – What is the coordination numbers for the following examples?

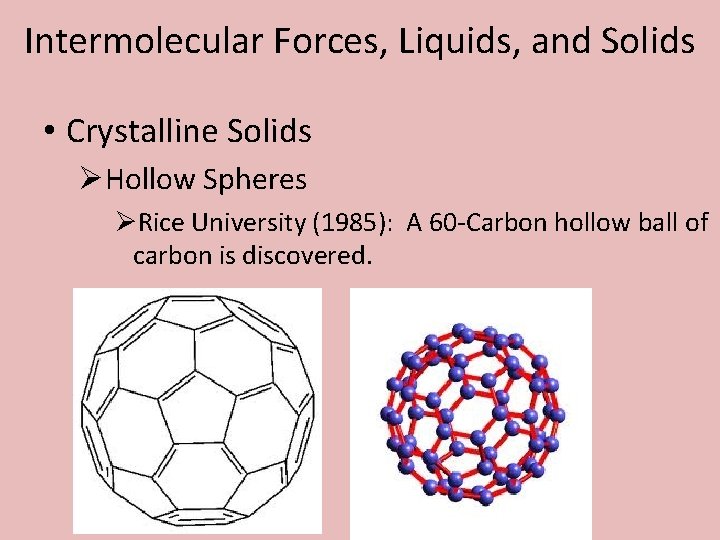
Intermolecular Forces, Liquids, and Solids • Crystalline Solids ØHollow Spheres ØRice University (1985): A 60 -Carbon hollow ball of carbon is discovered.

Intermolecular Forces, Liquids, and Solids • Crystalline Solids ØHollow Spheres ØThese structures were called buckminsterfullerenes or ‘buckyballs’. ØBuckminster Fuller: Architect who created geodome shapes.

Intermolecular Forces, Liquids, and Solids • Crystalline Solids ØHollow Spheres ØThese structures were called ‘buckyballs’. ØBuckminster Fuller: Architect who created geodome shapes.

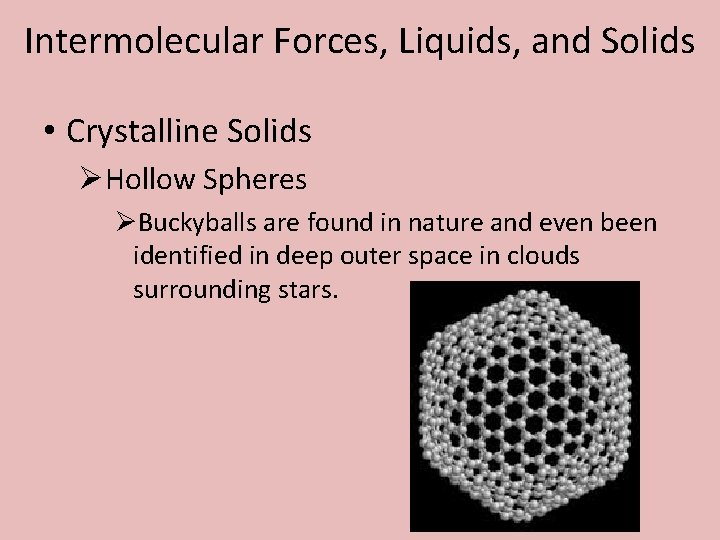
Intermolecular Forces, Liquids, and Solids • Crystalline Solids ØHollow Spheres ØBuckyballs are found in nature and even been identified in deep outer space in clouds surrounding stars.

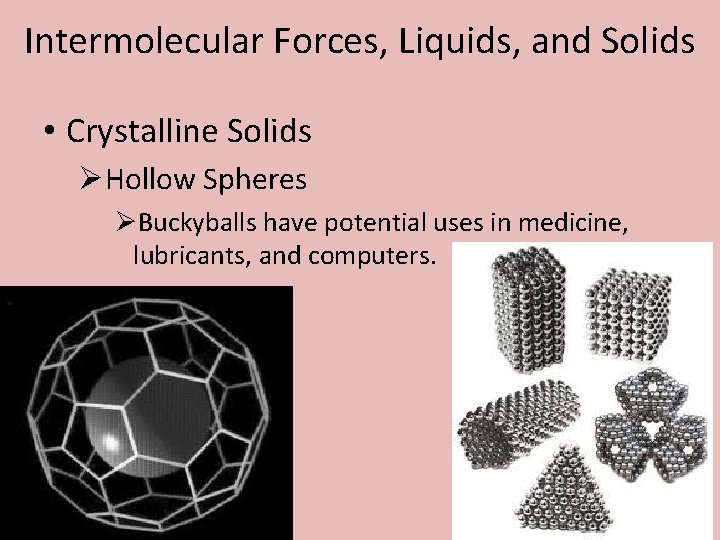
Intermolecular Forces, Liquids, and Solids • Crystalline Solids ØHollow Spheres ØBuckyballs have potential uses in medicine, lubricants, and computers.

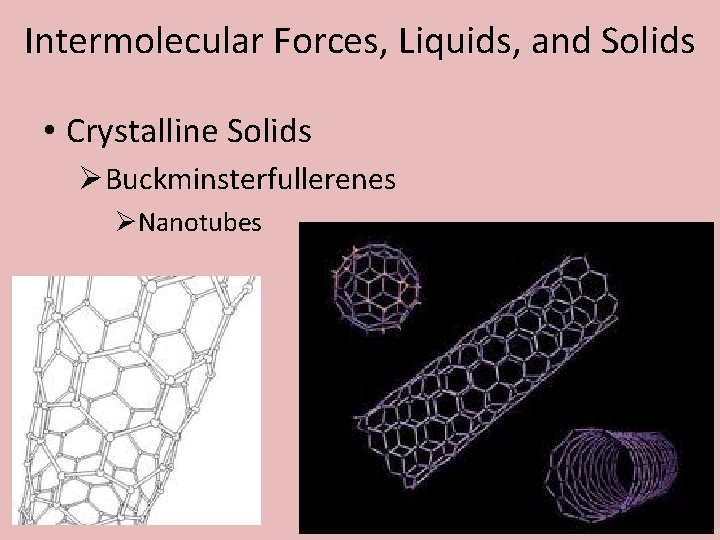
Intermolecular Forces, Liquids, and Solids • Crystalline Solids ØBuckminsterfullerenes ØNanotubes

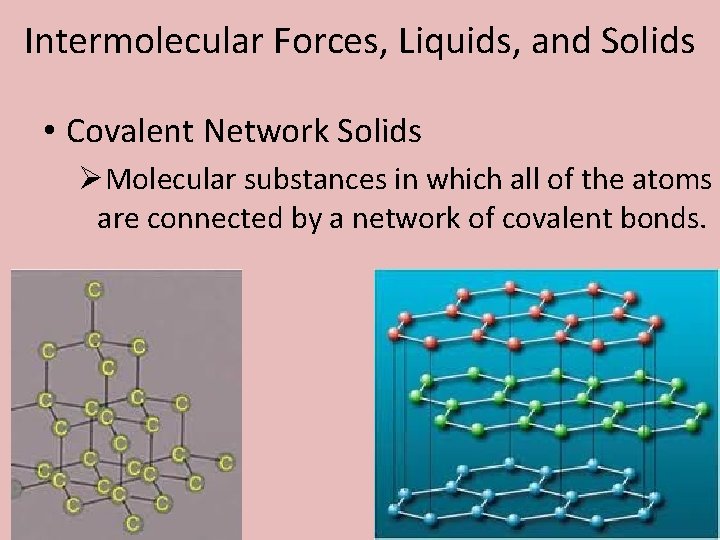
Intermolecular Forces, Liquids, and Solids • Covalent Network Solids ØMolecular substances in which all of the atoms are connected by a network of covalent bonds.

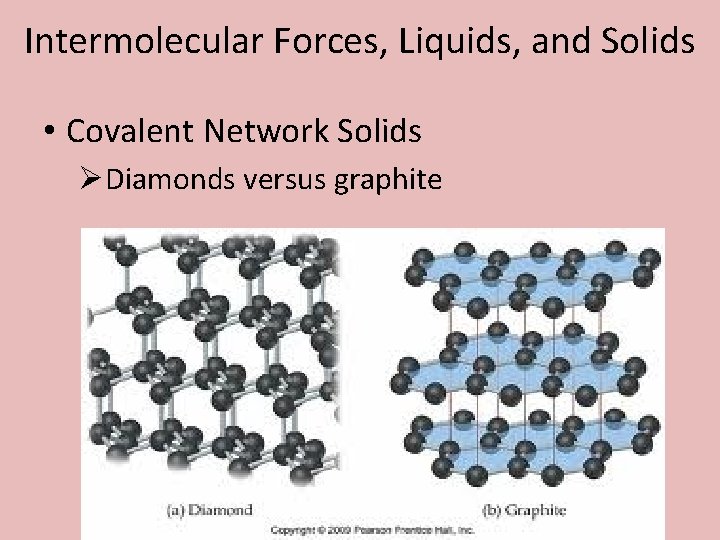
Intermolecular Forces, Liquids, and Solids • Covalent Network Solids ØDiamonds versus graphite

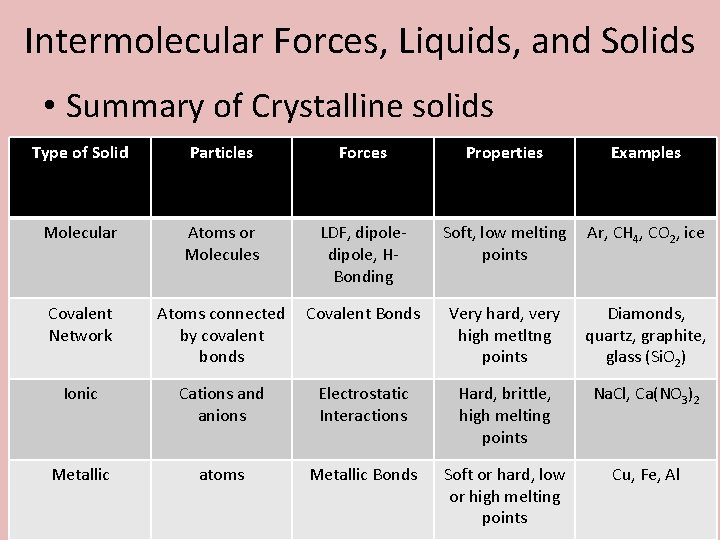
Intermolecular Forces, Liquids, and Solids • Summary of Crystalline solids Type of Solid Particles Forces Properties Examples Molecular Atoms or Molecules LDF, dipole, HBonding Soft, low melting points Ar, CH 4, CO 2, ice Covalent Network Atoms connected by covalent bonds Covalent Bonds Very hard, very high metltng points Diamonds, quartz, graphite, glass (Si. O 2) Ionic Cations and anions Electrostatic Interactions Hard, brittle, high melting points Na. Cl, Ca(NO 3)2 Metallic atoms Metallic Bonds Soft or hard, low or high melting points Cu, Fe, Al